Abstract
The conventional methods of treatment of pressure ulcers (PUs) by serial debridement and daily dressings require prolonged hospitalisation, associated with considerable morbidity. There is, however, recent evidence to suggest that negative pressure wound therapy (NPWT) accelerates healing. The commercial devices for NPWT are costly, cumbersome, and electricity dependent. We compared PU wound healing in traumatic paraplegia patients by conventional dressing and by an innovative negative pressure device (NPD). In this prospective, non‐randomised trial, 48 traumatic paraplegia patients with PUs of stages 3 and 4 were recruited. Patients were divided into two groups: group A (n = 24) received NPWT with our NPD, and group B (n = 24) received conventional methods of dressing. All patients were followed up for 9 weeks. At week 9, all patients on NPD showed a statistically significant improvement in PU healing in terms of slough clearance, granulation tissue formation, wound discharge and culture. A significant reduction in wound size and ulcer depth was observed in NPD as compared with conventional methods at all follow‐up time points (P = 0·0001). NPWT by the innovative device heals PUs at a significantly higher rate than conventional treatment. The device is safe, easy to apply and cost‐effective.
Keywords: Negative pressure wound therapy, Out patient department procedure, Pressure ulcer, Traumatic paraplegia
Introduction
Pressure ulcers (PUs) are wounds initiated by pressure on the skin that blocks circulation, causing the skin and underlying tissues to die. They are areas of necrosis where tissues are compressed between bony prominences and hard surfaces. The ulcers result from pressure alone or pressure in combination with friction, shearing forces or both. Management of PUs is an important clinical challenge 1. The first major study of PUs was carried out by Jean‐Martin Charcot, a clinician, in the 19th century 2, 3, 4. Without care, PUs continue to grow in diameter and depth, and are exceptionally difficult to heal. These wounds pose a serious health care problem, particularly for individuals with spinal cord injury living in developing countries where socio‐economic conditions often dictate treatment modalities 5, 6. Patients with spinal cord injury and associated comorbidity are at an increased risk for the formation of PUs, because of anaesthetic skin and prolonged bed rest 7. The precipitating factors of PUs in traumatic paraplegia patients are immobility, constant pressure, moisture and irritation to the skin.
Conventional methods of daily dressing and serial debridement require prolonged hospitalisation, and may lead to additional comorbidities and socio‐economic burden. Alternative methods, such as silver and hydrocolloid dressing, are cumbersome, expensive and not readily available.
Negative pressure wound therapy (NPWT) is a vacuum‐assisted method for wound care that imparts a negative pressure of −60 to −125 mm Hg on the wound bed. The mechanism by which NPWT promotes wound healing is unclear. It is believed that the negative pressure assists in removal of interstitial fluid, decreasing oedema, increasing blood flow and reducing tissue bacterial levels 8. In addition, mechanical deformation of cells is thought to result in protein‐ and matrix molecule synthesis, which increases the rate of cell proliferation and granulation tissue formation. This, in turn, may promote healing 1. NPWT is evolving and is under investigation for management of difficult, chronic and unrelenting wounds. Negative pressure devices (NPDs) (such as Info vac from KCI and others) for NPWT are costly and hard‐to‐afford for patients in developing countries. Another disadvantage is that these devices need to be attached to an electricity source for 22 hours each day and uninterrupted power supply is a challenge in a developing country. Considering these limitations of the currently available NPDs, this study was focussed on an innovative NPD. This study compared NPWT using our NPD to conventional wound dressing and specifically evaluated (i) reduction of wound surface area and depth, (ii) reduction of pathological organisms, (iii) removal of slough and formation of granulation tissue and (iv) safety and cost‐effectiveness.
Methods
This prospective, non‐randomised, controlled clinical trial was conducted in the spinal cord injury unit of the Department of Orthopaedic Surgery, in collaboration with the Department of Plastic Surgery, at King George Medical University (KGMU), Lucknow, from January 2011 to December 2012. The study was approved by the institutional ethics committee (IEC) of the University (KGMU). All participants signed an informed consent form. The inclusion criteria were (i) traumatic paraplegia, (ii) age 16–60 years, (iii) either gender, (iv) stages 3 and 4 PU as defined by the National Pressure Ulcer Advisory Panel (NPUAP) 9 and (v) subjects able to give informed consent. The exclusion criteria were (i) a wound with necrotic tissue unlikely to tolerate debridement; (ii) chronic osteomyelitis not treatable by antibiotics alone; (iii) comorbidities, such as diabetes mellitus, rheumatoid disease, vasculitis, neuropathy, chemotherapy and radiation therapy; (iv) poor nutritional status as determined by a Braden Scale nutritional assessment score of 2 or 1; (v) serum albumin <2·5 g/l and (vi) haemoglobin <9·0 g/l.
A total of 65 patients with PU undergoing treatment in the spinal cord injury unit were enrolled in the study. A total of 48 patients fulfilled the inclusion criteria and were equally divided into two groups – 24 cases (GpA) receiving NPD and 24 controls (GpB) receiving conventional dressing. The patients were followed up for 9 weeks. The investigators allocated participants to either group without attempting randomisation 10. The mean age was almost similar among the patients of both groups and the majority of patients in both the groups were males (group A: 79·2%, group B: 75%). Initial debridement of slough and necrotic tissue was performed in all patients at the time of admission and before their being allocated a group.
Methods of conventional wound care
The surface of the PU was cleaned with normal saline and packed with sterilised gauze to cover the wound. Dressing changes were performed once or twice daily depending on the soakage of the dressing.
Methods of NPWT
Our NPD was applied exclusively as a bedside procedure. NPD is a low‐cost device and comprises a low‐power continuous suction apparatus consisting of a bellow unit of 800 ml capacity, a connecting tube with clamp, a ‘Y’ connector, a curved needle with matching catheter and spare perforated catheter (Romo Vac Set®GS‐5002 Size‐10, Romsons Scientific & surgical Industries Pvt. Ltd., Agra, India), a sterilised piece of foam and a transparent polyurethane adhesive dressing (Opsite, G. Surgiwear Ltd., Shahjahanpur, India). The components of the device are easily available and are used for other surgical procedures in the country.
Method of application of NPD
The perforated end of the Romovac drainage tube was placed on the wound surface and its other end, placed 10 cm away from the wound margin (Figure 1A), was connected to the Romovac bellow. Sterilised foam was trimmed according to the size and geometry of the wound and placed on top as a cover (Figure 1B). Opsite finally covered the wound and the adjoining healthy skin with an airtight seal (Figure 1C). The Romovac bellow is charged to obtain appropriate cyclical negative pressure (Figure 1D).
Figure 1.
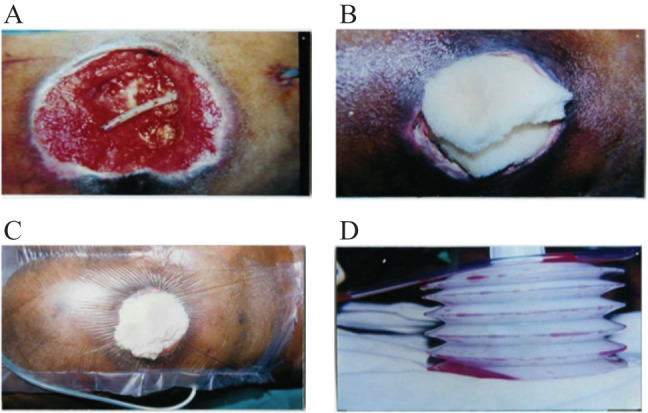
(A) The perforated end of the Romovac drainage tube is placed on the wound surface. (B) Sterilised foam is placed on top of the wound. (C) Opsite covers the wound with an airtight seal. (D) The other end of the drainage tube is connected to Romovac.
The patients were taught how to charge the Romovac and their attendants were advised to charge it every 5–6 hours. A mean pressure of −80 mmHg (range −60 to −120 mm Hg) was obtained on full charging. The pressure was measured by a pressure‐measuring device.
Following recharge of the Romovac bellow, fluid was drawn from the wound through the foam into the reservoir for subsequent disposal. The thin Opsite (plastic membrane) prevents the ingress of air and allows a partial vacuum. NPD is changed every week, or earlier if there is a soakage/leakage. The ulcer was evaluated and documented by clinical photography.
The outcome measures were wound surface area, depth of wound, discharge, conversion of slough into red granulating tissue and cost‐effectiveness at 9 weeks' follow‐up. Data was recorded every seventh day and analysed at weeks 3, 6 and 9 following intervention.
The ulcer was measured for its greatest length and width with flexigrid Opsite. The surface area was estimated from these values. Bedsores in both the groups were measured at each time point by a uniform procedure. Ulcer depth was measured with a sterilised cotton‐tipped applicator, which was inserted into the ulcer and marked at the deepest level. The amount of exudates was visualised as none, light, moderate or heavy after the dressing was removed in both NPWT and conventionally treated groups. Necrotic tissue, slough and formation of red granulation tissue were assessed by visual inspection at dressing changes.
The actual costs of all consumables required for NPWT by our NPD and for conventional dressing were calculated for two representative bedsores of similar size in each group.
Cost analysis
The costs were obtained from the hospital's central supply department but did not include sterilisation of materials, dressing forceps or scissors because these were common for both groups. All dressing materials were considered to be for single use. The total costs of consumables for one application in the NPWT group and one in the conventional dressing group were summed and the total daily cost calculated based on one or two daily dressing changes of the control group and every seventh day dressing change of the treatment (NPWT) group. The total NPWT and conventional treatment cost of one representative bedsore was determined by multiplying the daily cost by the number of days required to achieve wound granulation and split skin grafting (SSG).
Statistical analysis
Data were presented as mean ± standard deviation (SD) or numbers (%) as appropriate. Statistical differences were calculated using unpaired t‐test, McNemar's test and χ 2 test/Fisher exact test. P < 0·05 was considered as statistically significant. Data were analysed using Statistical Package for the Social Sciences (SPSS) version 16 (Chicago, IL).
Results
PUs of stage 4 were higher among patients in both the groups (Table 1). At week 3, wound bed slough (P = 0·0001) converted to granulation tissue in 33·3% patients in group A, whereas there was no change in slough status in the patients of group B. However, at week 9, slough converted to granulation tissue in all the patients of group A, while it was still present in 10 (41·7%) patients of group B (Table 2).
Table 1.
| Group A (n = 24) | Group B (n = 24) | P‐value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age in years, (mean ± SD) | 53·5 ± 12·5 | 54·34 ± 14·32 | 0·82 |
| Male gender | 19 (79·2) | 18 (75·0) | 0·73 |
| Pressure ulcer stage | |||
| Stage 3 | 9 (37·5) | 10 (41·7) | 0·76 |
| Stage 4 | 15 (62·5) | 14 (58·3) | |
| Clinical signs of active infection at the wound site | 21 (87·5) | 20 (83·3) | 0·68 |
| Presence of comorbid conditions such as UTI and URTI | 20 (83·3) | 19 (79·2) | 0·71 |
SD, standard deviation; URTI, upper respiratory tract infection; UTI: urinary tract infection.
Values were presented as mean ± SD and number (%).
Statistical difference between the two groups has been calculated using χ 2 test for numbers and student's unpaired t‐test for mean ± SD.
Table 2.
Evaluation of tissue type (slough to red granulation tissue) in both the groups at different time intervals*
| Group A (n = 24) | Group B (n = 24) | |||
|---|---|---|---|---|
| Time of evaluation | n | % | n | % |
| 0 week | 24 | 100·0 | 24 | 100·0 |
| Week 3 | 16† | 66·7 | 24 | 100·0 |
| Week 6 | 7† | 29·2 | 15† | 62·5 |
| Week 9 | 0† | 0·0 | 10† | 41·7 |
Group A: negative pressure wound therapy, group B: conventional methods of dressing.
P = 0·0001 (McNemar's test); statistical difference between week 0 to subsequent follow‐ups. P < 0·05 considered as statistically significant.
All the PUs in both the groups were found to be culture‐positive for pathogenic organisms at the time of enrollment. The conversion of culture from positive to negative was higher among group A patients than among group B patients. At week 9, the culture was negative in all the patients of group A, while it was positive in 10 (41·6%) patients of group B (Figure 2).
Figure 2.
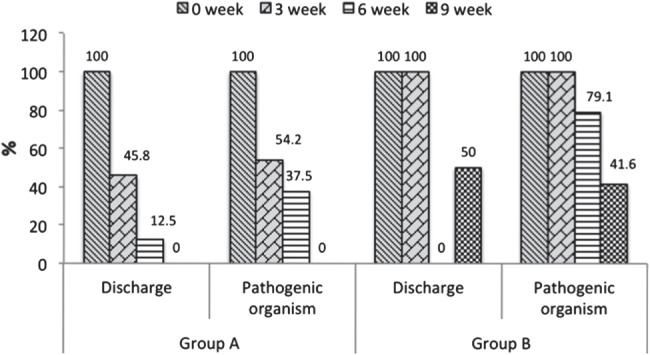
Evaluation of discharge and pathogenic organisms (culture positive to culture negative) in both groups at different time intervals [significant reduction (P = 0·0001) from 0 week to weeks 3, 6 and 9 in group A, McNemar's test]. Group A, negative pressure wound therapy; group B, conventional methods of dressing.
The wound discharge became minimal at weeks 3–6 and negligible at week 9 in the patients of group A; this was statistically significant (P = 0·0001, Figure 2). In group B patients, the wound discharge continued till week 9.
Intra‐group analysis during 9 weeks of therapy showed that the ulcer size and depth decreased significantly (P = 0·0001) from 0 week to weeks 3, 6 and 9 in patients of group A, while there was a minimal decrease in wound size and depth in patients of group B, and this was statistically insignificant (Table 3).
Table 3.
| Group A | Group B | P‐value | |
|---|---|---|---|
| Surface area (cm2) | |||
| 0 week | 13·22 ± 4·57 | 15·10 ± 2·12 | 0·07 |
| Week 3 | 9·12 ± 3·69§ | 14·12 ± 3·15 | 0·0001* |
| Week 6 | 5·12 ± 1·12§ | 12·14 ± 1·45 | 0·0001* |
| Week 9 | 0·21 ± 0·01§ | 11·13 ± 4·45 | 0·0001* |
| Depth (mm) | |||
| 0 week | 21·12 ± 9·31 | 22·23 ± 10·78 | 0·78 |
| Week 3 | 12·23 ± 3·24§ | 20·21 ± 3·39 | 0·0001* |
| Week 6 | 7·23 ± 1·14§ | 18·12 ± 3·94 | 0·0001* |
| Week 9 | 0·11 ± 0·02§ | 16·12 ± 3·37 | 0·0001* |
Group A: negative pressure wound therapy, group B: conventional methods of dressing. Values were presented as mean ± standard deviation (SD).
Statistical difference between the groups has been calculated using student's unpaired t‐test and within the group using paired t‐test.* P < 0·05, considered as statistically significant.
P = 0·0001* (paired t‐test, from 0 to subsequent follow‐ups).
Inter‐group analysis showed a significant reduction in ulcer size and depth (P = 0·0001) in patients of group A as compared with group B at all the follow‐up points (Table 3).
The quality of healed scar with NPD has been satisfactory on follow‐up and there has been no incidence of scar breakdown at the end of follow‐up. However, the quality of scar cannot be compared with that of a full thickness skin graft, as grafting is one of the established definitive procedures for wound closure.
Cost
The total cost of a 9‐week treatment of one PU was approximately 46% less than the costs of conventionally treated comparable ulcer (Table 4).
Table 4.
Evaluation of cost‐effectiveness between the two groups at week 9
| Cost in US $ | Conventional group (n = 24) | NPWT group (n = 24) |
|---|---|---|
| NPD related products – (Romovac, Opsite, Dynaplast, sterilised piece of foam) | 0 | 117 |
| Bandages and dressing – hydrogen peroxide, chlorine water, Betadine lotion | 218 | 0 |
| Personal cost, nurse cost | 0 | 0 |
| Total cost | 218 US$ | 117 US$ |
NPD, negative pressure device; NPWT, negative pressure wound therapy.
Discussion
PUs are complex and chronic wounds, and no gold standard has yet been established for their prevention and treatment. Several attempts at developing guidelines have been undertaken by different organisations. PUs are difficult to prevent or manage and lead to devastating comorbidities in patients 11.
In developing countries, SCI patients often seek hospital care late and present with PUs of moderate to large size. The conventional methods of treatment require prolonged hospitalisation with considerable morbidity in terms of pain, discomfort and economic burden. NPWT is a recent technical innovation in wound care, with a growing number of applications. In NPWT, the application of topical negative pressure (TNP) removes blood and serous fluid, reduces infection and increases localised blood flow by neovascularisation, thereby supplying the wound with oxygen and nutrition to promote accelerated healing 12.
We found that, in a primary care setting, PU treatment with our innovative device led to accelerated ulcer healing in the majority of cases in group A. Our study shows that ulcer treatment with NPD can be used as a manageable method in primary care for stages 3 and 4 PUs. The bacterial colonisation of a wound is a recognised detrimental factor in the multi‐factorial process of wound healing. While some studies have found that tissue bacterial counts significantly decreased after four days of NPWT application 13, 14, other studies have shown that bacterial colonisation increased significantly with NPWT and remained in the range of 104–106. In our study, no patients were culture‐positive in group A at the end of week 9, while 41·6 % patients in group B remained culture‐positive at week 9. This may have been the result of the cyclical negative pressure provided by our innovative device, which might have activated a signalling pathway, resulting in an increased rate of cell division and granulation, as discussed later.
Trauma as the common cause of SCI in our study is similar to studies carried out by Yarkony et al. 15 In our study, wound closure in group A was faster than in the conventional treatment group; this was in agreement with the study of Mody et al. 16, who conducted a randomised controlled trial comparing a locally constructed TNP device with wet‐to‐dry gauze dressings on varied wound aetiologies, including diabetic foot ulcers, PUs, cellulitis/fasciitis and other types of ulcers. Except in a sub‐set of PUs, the authors did not find any statistically significant differences in the time to closure between the two treatment groups. In agreement with our study, their study found a significant difference in time to closure of PUs (mean 10 ± 7·11 days) between the treatment and control groups (27 ± 10·6 days, P = 0·05).The direct costs of closing a PU were also lower in the TNP compared with the control group 16.
In earlier studies on NPWT, the researchers have concluded that this technology should be considered ‘the treatment of choice’ for chronic (hard‐to‐heal) ulcers because of its significant advantages of time for wound healing and ‘wound bed preparation’ compared with conventional therapy.
NPWT was originally utilised to speed bedside debridement of wounds 16. By the end of week 9, slough was converted to red granulation tissue in all group A patients. Negative pressure has become a popular wound closure option in the management of PU despite the paucity of well‐designed randomised controlled trials.
In an experimental study in rabbits, it was found that VAC increased capillary calibre and blood volume, stimulating angiogenesis and thereby improving the blood circulation in wounds. Further, it narrowed endothelial spaces and restored the integrity of capillary basement membranes, causing a decrease in the permeability of blood vessels and wound oedema by removing excess fluid from the wound bed 17. Significantly, an increased rate of granulation tissue formation was also shown to occur with both continuous and intermittent VAC application. The cyclical application of sub‐atmospheric pressure alters the cytoskeleton of the cells in the wound bed, triggering a cascade of intracellular signals that increases the rate of cell division and subsequent formation of granulation tissue.
Our innovative device provides intermittent/cyclical negative pressure with an average 80 mm Hg pressure (−60 to −120 mmHg) when fully charged. With time, the negative pressure is gradually lost, requiring periodic recharge of the device. This by itself provides an intermittent negative pressure as suggested by Morykwas and Argenta 13. They compared the commercial V.A.C.® device to standard wound dressings on acute wounds in animal models and reported that negative pressure (−125 mm Hg) improved wound blood flow, particularly after intermittent cessation of pressure. Our device produced similar results as commercial electricity‐driven devices. These results help set current recommendations for commercial NPD settings, although data comparing healing rates in different regimens are lacking.
Many patients in both groups in our study had urinary tract infection (UTI) as a comorbid condition. According to a study by Hirsch et al. 18 increased risk and frequent recurrence of UTI is the most common cause of anaemia in persons with SCI 18. Allman et al. reported that infection is the major complication associated with PUs 19.
In our study, pain was not a predominant feature although some patients complained of discomfort during initial application of NPD; when the Romovac is fully charged, it attains its maximum pressure, which gradually decreases to its lowest level in 5–6 hours.
In all our patients in whom our device was used, the wound‐care protocol was simplified and healing accelerated. The NPD allowed optimal wound closure by preparing the wound for skin grafting or flap closure as and when required. The results of this study suggest that wound healing outcomes using NPWT made from indigenously available resources are similar to those reported using commercially available NPWT dressings (KCI and others) 15. PUs that healed during the study took less time with our NPD than when conventional dressings were used.
There are some limitations to our study. (i)This procedure is ineffective in low sacral ulcers in which the ulcer involves the area close to the natal cleft because the adhesive dressing (Opsite) cannot be properly applied to obtain an airtight seal. (ii)The sterile foam used in the negative pressure apparatus has a tendency to disintegrate and make the secretions viscous, thus clogging the drain. (iii) Previous and current NPWT studies have used wet‐to‐dry dressings as a control treatment. Although both may achieve mechanical debridement and keep the wound healing environment moist, there is evidence to support a better healing environment by the use of advanced materials (including hydrocolloids and silver alginates) in chronic and acute wound healing.
Conclusions
(i) The procedure is a bedside procedure that is easy to apply, and patient compliance was good to excellent. It can be promoted as an out patient department (OPD) procedure and domiciliary treatment for bed sore management, requiring only weekly follow‐up. (ii) Because of the airtight seal, there is minimal discharge, soakage and soiling of clothes and bed sheets. The airtight seal was very effective in the social acceptance of these patients by minimising the ‘characteristic odour’ of a bed sore discharge. The near‐absence of this characteristic odour meant that the procedure was also more readily accepted by the patients. (iii) Our apparatus is financially viable in settings where resources are limited, and is effective in providing intermittent negative pressure at the wound site. It is more efficient and less painful for removing slough, preventing fluid collection, controlling bacterial growth and decreasing purulence in absolute terms. (iv) The procedure was well tolerated by the patients in our study and had minimal side effects.
In future, a larger study may have to be undertaken to provide further evidence of the safety, efficacy and cost‐effectiveness of this device before its use as an OPD procedure can be universally adopted.
Acknowledgements
The authors are thankful to Dr Divya Sanghi and Dr Rajendra P Mishra, and Ms Kavita Baghel for their kind support. They are also thankful to the Council of Science and Technology, Uttar Pradesh, for the financial assistance.
This work was conducted at Spinal cord injury unit, Department of Orthopaedic Surgery, King George's Medical University (KGMU), Lucknow, Uttar Pradesh, India
References
- 1. Kuffler DP. Techniques for wound healing with a focus on pressure ulcers elimination. Open Circ Vasc J 2010;3:72–84. [Google Scholar]
- 2. Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg 1999;38:34–40. [DOI] [PubMed] [Google Scholar]
- 3. Levine JM. Historical perspective: the neurotrophic theory of skin ulceration. J Am Geriatr Soc 1992;40:1281–3. [DOI] [PubMed] [Google Scholar]
- 4. Levine JM. Historical perspective on pressure ulcers: the decubitus ominosus of Jean‐Martin Charcot. J Am Geriatr Soc 2005;53:1248–51. [DOI] [PubMed] [Google Scholar]
- 5. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC). Am J Clin Dermatol 2005;6:185–94. [DOI] [PubMed] [Google Scholar]
- 6. Stover SL, Fine PR. Spinal cord injury. The facts and figures. Birmingham: University of Alabama at Gary M. Yarkony, David Chen. Rehabilitation of patients with spinal cord injuries. Randall LB. Text book of physical medicine and rehabilitation. Philadelphia: WB Saunders; 1996:1149–79. [Google Scholar]
- 7. Basson MD, Burney RE. Defective wound healing in patients with paraplegia and quadriplegia. Surg Gynecol Obstet 1982;155:9–12. [PubMed] [Google Scholar]
- 8. Plikaitis CM, Molnar JA. Subatmospheric pressure wound therapy and the vucuum‐assisted closure device: basic science and current clinical successes. Expert Rev Med Devices 2006;3:175–84. [DOI] [PubMed] [Google Scholar]
- 9. National Pressure Ulcer Advisory Panel . Pressure ulcers, incidence, economics, risk assessment. West Dundee: National Pressure Ulcer Advisory Panel, 2009. [Google Scholar]
- 10. Deeks JJ, Dinners J, D'Amico R, Swoden AJ, Sakarovitch C, Song F, Pettricrew M, Altman DG. Evaluating non randomized interventional studies. Health Technol Assess 2003;7:P‐2. [DOI] [PubMed] [Google Scholar]
- 11. Thomas DR. Prevention and treatment of pressure ulcers. J Am Med Dir Assoc 2006;7:46–59. [DOI] [PubMed] [Google Scholar]
- 12. Genecov DG, Schneider AM, Morywas MJ. A controlled subatmospheric dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg 1998;40:219–25. [DOI] [PubMed] [Google Scholar]
- 13. Morykwas MJ, Argenta LC, Shelton Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 14. Moues CM, Vos MC, Van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–7. [DOI] [PubMed] [Google Scholar]
- 15. Yarkony GM, Chen D. Rehabilitation of patients with spinal cord injuries. In: Randall LB, editor. Text book of physical medicine and rehabilitation. Philadelphia: WB Saunders, 1996:1149–79. [Google Scholar]
- 16. Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy Wound Manage 2008;54:36–46. [PubMed] [Google Scholar]
- 17. Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum‐assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28:211–7. [DOI] [PubMed] [Google Scholar]
- 18. Hirsch GH, Menard MR, Anton HA. Anemia after traumatic spinal cord injury. Arch Phys Med Rehabil 1991;72:195–201. [PubMed] [Google Scholar]
- 19. Allman RM. Pressure ulcers among the elderly. N Engl J Med 1989;320:850–3. [DOI] [PubMed] [Google Scholar]


