Abstract
Management of small‐bowel fistulas which are in an open abdomen and have no soft tissue overlay or a fistula tract involves many complications and challenges. Controlling the local leakage of enteric contents has a central role in the success of medical treatment. There are several methods to deal with fistula discharge but unfortunately, the technical solutions only partially address such problems and a definitive management of fistula discharge still remains an insoluble challenge. We describe a simple and cheap method to control fistula leakage by using a percutaneous endoscopic gastrostomy tube.
Keywords: Abdominal sepsis, Negative pressure wound therapy, Open abdomen, Small‐bowel fistula, Temporary abdominal closure
Introduction
Enteroatmospheric fistula (EAF) is a severe disease following trauma or unsuccessful abdominal surgery. It is often related with an open abdomen, which holds an enteric opening to atmosphere and has no overlying soft tissue or fistula tract.
The management of EAF faces several challenges in the treatment of systemic and local complications. Besides sepsis control, nutrition support and clinical stabilisation, the local control of fistulae is the central point of the treatment 1, 2. The high output of enteric fluids causes severe dermatitis, limits their ability to maintain proper hygiene, prevents or limits oral feeding, delays or prevents wound healing, increases hospital costs and results in high mortality rates 3.
Several methods have been described in medical literature for the local control of high‐output atmospheric fistulae 4, 5, 6. Currently, negative pressure wound therapy has an essential role in the local control of the wound because it deals with leakage of enteric fluids and helps with the healing process. Unfortunately, vacuum therapy causes a waste of protein, volume and electrolytes and limits oral feeding, solving only some of the problems 6, 7, 8.
Here, we describe an easy, simple, efficient and cheap method for the local control of a high‐output EAF that facilitates oral feeding and home management.
Techniques
After the patient has been clinically stabilised, and the abdominal wound has granulated, the result is a large and open granulated wound that holds an EAF. Therefore, the leakage of intestinal fluids is managed using a standard percutaneous endoscopic gastrostomy tube (PEG) as described below:
A silicone dome‐bolstered PEG tube (standard PEG kit, order number M00568241, Boston Scientific, USA) is used like a plug to occlude the fistula. First, facilitated by a Kelly clamp, the silicone dome‐bolstered tube is gently placed inside the bowel lumen. Once inside the lumen, a plastic sheet obtained from a plastic urine drainage bag is cut into the shape of the wound and placed on top of the granulated wound. The next step is to make the PEG tube pass through the plastic sheet. Then, over the plastic sheet, we place the round external bolster of the PEG kit and gently adjust it to plug the fistula opening without excessive pressure (Figure 1). It is important to apply gentle pressure so as to prevent intestinal leakage. After the fistula has been plugged, a negative pressure curative is applied. Finally, outside the dressing, the PEG tube is cut short, and the external end is occluded by the Y‐port of the PEG kit.
Figure 1.
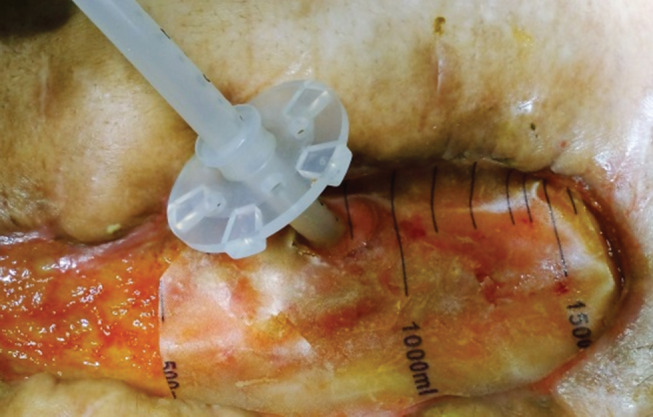
The silicone dome‐bolstered tube is inside the enteric lumen. A plastic sheet was placed on top of the granulated wound; the percutaneous endoscopic gastrostomy (PEG) tube was passed through the plastic sheet, and the round external bolster of the PEG tube is being adjusted to gently occlude the fistula opening.
It will be necessary to replace the dressing every 2 days because a small amount of enteric fluid, which should be cleaned by the vacuum system, is expected to leak over the wound. During this process, the PEG tube is left in place, and the round external bolster is gently adjusted. Once the enteric discharge is quickly controlled, the vacuum dressing could be replaced by a conventional dressing, leaving only the PEG tube in place, as described above, and the wound around the tube protected by the plastic sheet. Once leakage is under control, the patient can walk and be orally fed.
Because of the local wound control, oral feeding and free ambulation, the patient's clinical condition and nutrition quickly improves, and the abdominal wound naturally heals around the fistula opening. In a few days, the patients can be discharged under the supervision of a visiting nurse. Nevertheless, reconstructive abdominal surgery must be delayed until the inflammatory process is resolved, and the surgical risk is decreased (Figure 2).
Figure 2.
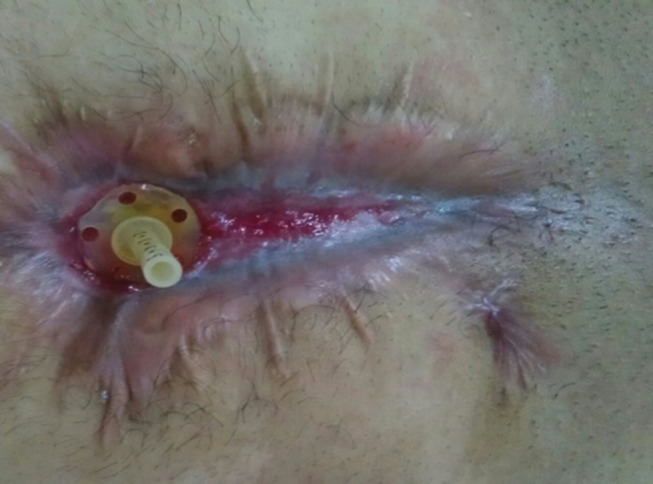
Wound healing after 3 months of treatment.
All procedures performed in this study were conducted in compliance with the standards of the institutional and/or national committee for research in humans.
Informed consent was obtained from all individual participants included in the study. All the individual rights of the patients were guaranteed and were in accordance with the ethical standards. Anonymity was particularly assured. Both patients gave their informed consent in writing prior to inclusion in this scientific communication. They consented to the proposed medical treatment, and they allowed their photographs to be published.
Discussion
In 2016, we have managed two patients with EAF using the PEG tube as described above. Both presented rapid control of enteric leakage. They were orally fed and eliminated the demand for parenteral nutrition. After a few days, they were managed in their homes and returned weekly for medical consultation. A 22‐year‐old boy with Crohn disease, who developed an EAF after an emergency laparotomy, has retained the PEG tube until a colostomy bag could be adapted in a near‐closed intestinal opening, and a 62‐year‐old depressed woman received a fistula‐special dressing for control of leakage after an incomplete abdominal closure. We would have liked to gather a more significant experience with the PEG tube for EAF before sharing our outcomes, but fortunately, patients like that are not recurrent in our surgical infirmary, and this initial experience was so satisfactory that we agreed it deserved publication.
The advantages of the described technique here are unquestionable: oral feeding and early ambulation increase the comfort of the patient, but perhaps one could argue that the main benefit is the possibility of early hospital discharge, which means lower hospitals costs.
To make this treatment option possible, the fistula opening diameter must be fit to the silicone dome‐bolstered tube. A large intestinal opening will make it difficult to fix the PEG tube, making it easier to expel it from the intestinal lumen.
Conclusion
Obviously, here, we described a handmade dressing, making use of cheap inputs layout available at general hospitals. Although a larger experience is necessary to confirm these initial results, we believe that in the future, when further studies confirm the present outcome, specific silicone devices working like the PEG tube plug may be manufactured by industry to systematise the method and properly treat EAF. The proposed idea could be easily reproduced as a reasonable alternative in selected patients in countries with low‐incoming rates because it uses cheap materials. Consequently, it could annually help hundreds of unfortunate people who have a severe disease and no access to suitable medical support.
References
- 1. Di Saverio S, Villani S, Biscardi A, Giorgini E, Tugnoli G. Open abdomen with concomitant enteroatmospheric fistula: validation, refinements, and adjuncts to a novel approach. J Trauma 2011;71:760–2. [DOI] [PubMed] [Google Scholar]
- 2. Yin J, Wang J, Yao D, Zhang S, Mao Q, Kong W, Ren L, Li Y, Li J. Is it feasible to implement enteral nutrition in patients with enteroatmospheric fistulae? A single‐center experience. Nutr Clin Pract 2014;29:656–61. [DOI] [PubMed] [Google Scholar]
- 3. Majercik S, Kinikini M, White T. Enteroatmospheric fistula. Nutr Clin Pract 2012;27:507–12. [DOI] [PubMed] [Google Scholar]
- 4. Meshikhes AW, Al‐Hariri A, Al‐Zahir AA, Al‐Nahawi M. A rare approach to entero‐atmospheric fistula. Am J Case Rep 2013;14:476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ozer MT, Sinan H, Zeubek N, Peker Y. A simple novel technique for enteroatmospheric fistulae: silicone fistula plug. Int Wound J 2014;11:22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terzi C, Egeli T, Canda AE, Arslan NC. Management of enteroatmospheric fistulae. Int Wound J 2014;11:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruhin A, Ferreira F, Chariker M, Smith J, Runkel N. Systematic review and evidence based recommendations for the use of negative pressure wound therapy in the open abdomen. Int J Surg 2014;12:1105–14. [DOI] [PubMed] [Google Scholar]
- 8. Yetişir F, Salman AE, Aygar M, Yaylak F, Aksoy M, Yalçin A. Management of fistula of ileal conduit in open abdomen by intra‐condoit negative pressure system. Int J Surg Case Rep 2014;5:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


