Abstract
To investigate whether diabetes mellitus affects the wound‐healing‐promoting potential of adipose tissue‐derived stem cells, we designed a wound‐healing model using diabetic mice. We compared the degree of wound healing between wounds treated with normal adipose tissue‐derived stem cells and wounds treated with diabetic adipose tissue‐derived stem cells. We evaluated the wound‐healing rate, the epithelial tongue distance, the area of granulation tissue, the number of capillary and the number of Ki‐67‐stained cells. The wound‐healing rate was significantly higher in the normal adipose tissue‐derived stem cells group than in the diabetic adipose tissue‐derived stem cells group; it was also significantly higher in the normal adipose tissue‐derived stem cells group than in the control group. Although the diabetic adipose tissue‐derived stem cells group showed a better wound‐healing rate than the control group, the difference was not statistically significant. Similar trends were observed for the other parameters examined: re‐epithelisation and keratinocyte proliferation; granulation tissue formation; and dermal regeneration. However, with regard to the number of capillary, diabetic adipose tissue‐derived stem cells retained their ability to promote neovasculisation and angiogenesis. These results reflect the general impairment of the therapeutic potential of diabetic adipose tissue‐derived stem cells in vivo.
Keywords: adult stem cell, adipose tissue, cell therapy, diabetes mellitus, wound healing
Introduction
Various types of treatment are used to promote wound healing in diabetic patients currently; surgical debridement, control of infection, arterial reconstruction, use of advanced dressing materials, growth factor therapy, bioengineered tissues, negative pressure therapy and hyperbaric oxygen therapy 1, 2, 3. However, none of these is sufficient to guarantee wound healing, and the treatment of diabetic ulcers remains an unsolved problem. Stem cell applications are emerging as a new alternative for reversing impaired wound healing in diabetic patients. Numerous studies have reported the potency of bone marrow‐derived mesenchymal stem cells (BM‐MSCs), adult murine bone marrow stromal progenitor cells (BM‐SPCs), and adipose tissue‐derived stem cells (ADSCs). Among these, ADSCs are considered the optimal solution, because they can easily be obtained by liposuction of adipose tissue, they can be obtained in large quantities, and they display multi‐lineage developmental plasticity 4, 5. Many researchers have shown that ADSC therapy can provide systemic improvements in wound healing, including enhanced cell proliferation, ECM synthesis, growth factor release and neovascularisation 6, 7.
Despite the ability of ADSCs to promote wound healing, wound healing remains delayed in diabetic patients because of the functional impairment of resident and recruited cells 1, 6, 8. Thus, many studies are being conducted to explore the differences in the abilities of normal and diabetic ADSCs to promote wound healing. In this study, we hypothesised that diabetic and normal ADSCs would show functional differences in their ability to promote wound healing. We used a new model of diabetic wound healing that simultaneously employs artificial dermis and a silicone splint to prevent wound healing by skin contraction, and designed a study to explore the functional differences between normal and diabetic ADSCs.
Materials and methods
Animals
Ten‐week‐old C57BL/6 mice (OrientBio, Sungnam, Korea) were used in this study. The mice were maintained under specific pathogen‐free conditions in an animal facility with controlled humidity (55–65%), light (12/12 hour light/dark), and temperature (22 ± 1°C). The air in the facility was passed through a high‐efficiency particulate arrestance (HEPA) filtration system designed to exclude bacteria and viruses. Animals were fed mouse chow and tap water ad libitum.
Isolation and culture of ADSCs
Two diabetic mice (C57BL/6 db/db) for obtaining diabetic ADSCs and four normal mice (C57BL/6 m+/m+) for obtaining normal ADSCs were sacrificed, and epididymal fat pads were obtained. Tissues were processed to isolate ADSCs as described previously 9. In brief, fat tissues were washed at least three times with phosphate‐buffered saline (PBS; pH 7·4; Sigma‐Aldrich, St. Louis, MO) to remove contaminating blood and debris. Fat tissues were finely minced and digested with collagenase type I (0·05%; Sigma–Aldrich) in PBS for 30 min at 37°C with constant agitation. Cell suspension was centrifuged at 2094 g for 3 minutes and filtered through a 100‐µm nylon mesh filter to remove floating adipocytes from the stromal–vascular fraction. The isolated ADSCs were cultured in Dulbecco's modified eagle's medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gibco), 100 U/ ml penicillin (Gibco), and 0·1 mg/ml streptomycin (Gibco) at 37°C in a humidified atmosphere of 5% CO2. After an overnight incubation, nonadherent cells were removed by changing the medium. The adherent cells were further expanded through three passages. A flow cytometry was performed to confirm the characteristics of the cultured ADSCs. The cultured ADSCs were harvested by trypsin, washed twice with PBS, incubated with fluorescently labelled monoclonal antibodies, including CD90, CD73, CD34, CD45 and CD146.
Experimental design
The dorsum of 12 anaesthetized diabetic mice (C57BL/6 db/db) was shaved. To prevent wound closure by contracture, a 3 × 3 cm2 silicone sheet (Bioplexus, Ventura, CA) was used as a splint. Using a 6‐mm‐diameter biopsy punch, we made four holes in each silicone sheet. Then, the silicone sheet was secured to the dorsum of the mice using 5–0 nylon sutures. A 6‐mm‐diameter full‐thickness skin defect was made using a biopsy punch in the same position as the hole. Immediately after wounding, an artificial dermis (Terudermis, Olympus Terumo Biomaterials, Japan) was applied to the wound by suturing using 5–0 nylon sutures. We simultaneously sutured the artificial dermis, the edge of the wound, and the silicone sheet. As Figure 1 shows, four wounds were made in each mouse.
Figure 1.
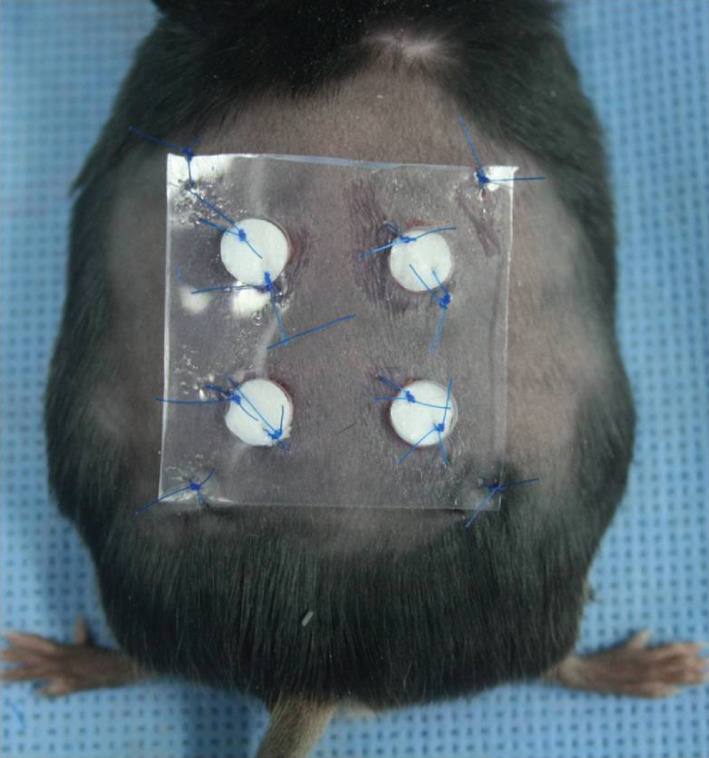
The wound healing model. The authors made four wounds at dorsum of each mouse. Among these four wounds, one was used as the control group and the remaining three wounds were used as the normal adipose tissue‐derived stem cells (ADSCs) group or the diabetic ADSCs group. We simultaneously sutured the artificial dermis, the edge of the wound, and the silicone sheet to minimise skin contraction around the wound site, allowing the wound healing via granulation and re‐epithelisation.
Among these four wounds, one was used as a control and the remaining three wounds were used as the normal ADSCs group or the diabetic ADSCs group. Consequently, the control group included 12 wounds, the normal ADSCs group included 18 wounds, and the diabetic ADSCs group included 18 wounds. Immediately after securing the artificial dermis, 1 × 106 cultured normal or diabetic ADSCs resuspended in 0·05 cc of medium (DMEM; Gibco) were administered over the three wounds. As a control, the remaining wound site on each mouse was treated with and an equivalent volume of medium. After the procedure, a film dressing (Tegaderm, 3M, London, ON, Canada) was applied to the wound site over the silicone sheet. Five mice were sacrificed at 1 week after the treatment and the remaining mice were sacrificed at 2 weeks.
Wound healing rate analysis
Digital photographs of the wounds were captured at 1 and 2 weeks after treatment. Wound area was measured by tracing the wound margin and the area was calculated using an image analysis program (IMT i‐Solution; IMT i‐Solution Inc. Vancouver, BC, Canada). The investigators who measured the wounds were unaware of the treatment group of the subjects. The wound healing rate was calculated as follows: (area of original wound – area of remaining wound)/area of original wound × 100 (Figure 2).
Figure 2.
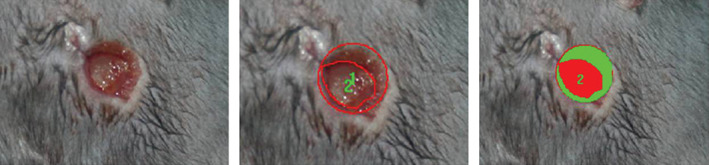
Photographs showing the method used for calculating the wound healing rate. Using an image analysis program, we defined the area of the original wound (outer red line) and the area of the remaining wound (inner red line). The wound healing rate was calculated as follows: (area of original wound – area of remaining wound)/area of original wound × 100
Histological examination
Skin samples including the wound and 2 mm surrounding skin were harvested at 1 and 2 weeks after treatment. The specimens were fixed in 10% neutral buffered formalin for at least 25 hours at room temperature. After fixation, perpendicular sections to the anterior‐posterior axis of the wound were embedded in paraffin, and sectioned at 4 µm‐thickness. The sections on glass slides were dried at 60°C for 3 hours and then were deparaffinised and stained with haematoxylin and eosin (H&E) by incubating the tissues in Harris haematoxylin (Sigma–Aldrich) followed by serial eosin (Sigma–Aldrich) and graded ethanol steps. For each section, a randomised area (magnification, 40×) showing re‐epithelisation and granulation tissue formation was photographed. We measured the distance between the nearest two points of both epithelial tongues. We refer to this measure as ‘epithelial tongue distance’. We also measured the area of granulation tissue. At both wound edges, we marked the boundary of the area of granulation tissue and calculated the gross area using an image analysis program (IMT i‐Solution; IMT i‐Solution Inc.). Four randomised areas (magnification 100×) showing capillary formation were photographed. The number of capillary lumens was counted and averaged. Only mature vessels containing erythrocytes were included.
Immunohistochemical examination
The 4‐µm paraffin sections were deparaffinised in xylene and rehydrated in graded alcohol series (100, 90, 80 and 70%). The sections were then washed in distilled water and heated in a microprobe 15 minutes for citrate buffer (pH 6·0) for antigen retrieval. Endogenous peroxidase was inhibited using 3% H2O2 for 20 minutes. Non‐specific binding was blocked by incubation in normal horse serum. The sections were then incubated with relevant primary antibody Ki‐67(BD, pharmingem, Franklin Lakes, NJ, 1:100) at 4°C for 16 hours. The sections were exposed to a streptavidin‐biotin‐peroxidase complex (Invitrogen Corporation, Carlsbad, CA) and color was developed with 3,3‐diaminobenzidine hydrochloride (Thermo Fisher Scientific, Fremont, CA). Counterstaining was perfomed with hematoxilin. Three randomised areas of the epithelial tongue (magnification 100×) were photographed. We counted the number of cells positive for Ki‐67‐staining (brown stain) in the basal cell layer, and calculated an average.
Ethical considerations
The protocols used in the present study were approved by the Animal Care and Use Committee of The Catholic University of Korea (Permit Number: YEO20131001FA).
Statistical analysis
Nonparametric statistical methods were used for all analyses. The authors used the Kruskal–Wallis test and Mann–Whitney test to compare the differences in the wound healing rate, the epithelial tongue distance, the area of granulation tissue, the number of capillaries, and the Ki‐67‐stained cells between the three groups. All statistical analyses were performed using the SPSS software package (Version 21.0; SPSS, Chicago, IL). A P < 0·05 was considered statistically significant in Kruskal–Wallis test and P < 0·017 (0·05/3) was considered statistically significant in Mann–Whitney test.
Results
Of the six mice with one control wound and three diabetic ADSCs wounds, one mouse died of unknown causes. Consequently, this study included 11 wounds in the control group, 18 wounds in the normal ADSCs group, and 15 wounds in the diabetic ADSCs group. The authors obtained specimens from five wounds in the control group, nine wounds in the normal ADSCs group, and six wounds in the diabetic ADSCs group at 1 week after treatment, and six wounds in the control group, nine wounds in the normal ADSCs group and nine wounds in the diabetic ADSCs group at 2 weeks after treatment.
Characterisation of stem cells
The cells took a spindle like shape throughout the culture period, and there were no morphological differences between the normal ADSCs and diabetic ADSCs. We confirmed the plastic‐adherent when maintained in standard culture conditions. To characterise the cell surface marker profile, some fractions of cultured cells were analysed. They were positive for CD90 and CD73, and were negative for CD34, CD45 and CD146.
Wound healing rate
At 1 week, the wound healing rate was 54·74 ± 6·67% in the control group, 84·34 ± 4·81% in the normal ADSCs group, and 61·13 ± 7·35% in the diabetic ADSCs group. At 2 week, the wound healing rate was 84·15 ± 11·12% in the control group, 96·76 ± 5·08% in the normal ADSCs group, and 82·89 ± 14·71% in the diabetic ADSCs group (Figure 3).
Figure 3.
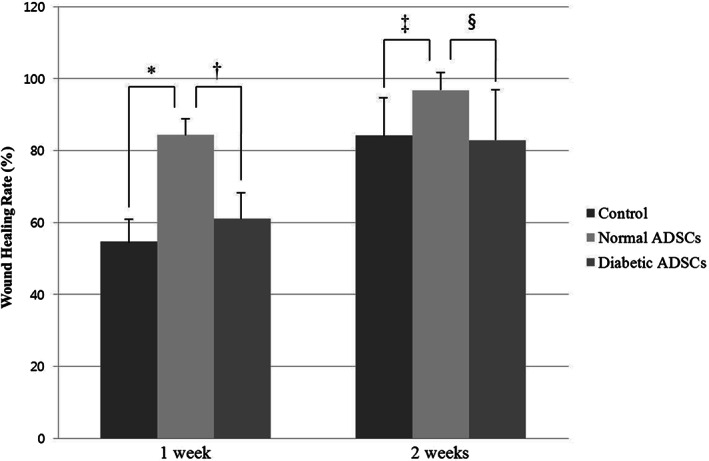
The wound healing rate of the control, normal adipose tissue‐derived stem cells (ADSCs), and diabetic ADSCs groups at 1 and 2 weeks. At 1 week, the wound healing rate was significantly higher in the normal ADSCs group than the control group (*P = 0·001), also it was significantly higher in the normal ADSCs group than the diabetic ADSCs group († P < 0·001). There was no significant difference between the control group and the diabetic ADSCs group. At 2 weeks, the wound healing rate was significantly higher in the normal ADSCs group than the control group (‡ P = 0·005), also it was significantly higher in the normal ADSCs group than the diabetic ADSCs group (§ P = 0·006). There was no significant difference between the control group and the diabetic ADSCs group.
At 1 week, the wound healing rate was significantly higher in the normal ADSCs group than the diabetic ADSCs group (P < 0·001), also it was significantly higher in the normal ADSCs group than the control group (P = 0·001). However, there was no significant difference between the control group and the diabetic ADSCs group. At 2 weeks, the wound healing rate was significantly higher in the normal ADSCs group than the diabetic ADSCs group (P = 0·006), also it was significantly higher in the normal ADSCs group than the control group (P = 0·005). However, there was no significant difference between the control group and the diabetic ADSCs group. There were significant increase of the wound healing rate in the control group (P = 0·004), the normal ADSCs group (P < 0·001) and the diabetic ADSCs group (P = 0·026) from 1 to 2 weeks.
Epithelial tongue distance
At 1 week, the epithelial tongue distance was 259·62 ± 97·71 µm in the control group, 153·65 ± 69·83 µm in the normal ADSCs group, and 387·11 ± 48·99 µm in the diabetic ADSCs group. At 2 weeks, the epithelial tongue distance was 95·85 ± 71·7 µm in the control group, 134·39 ± 66·79 µm in the normal ADSCs group, and 227·39 ± 123·73 µm in the diabetic ADSCs group (Figures 4 and 5).
Figure 4.
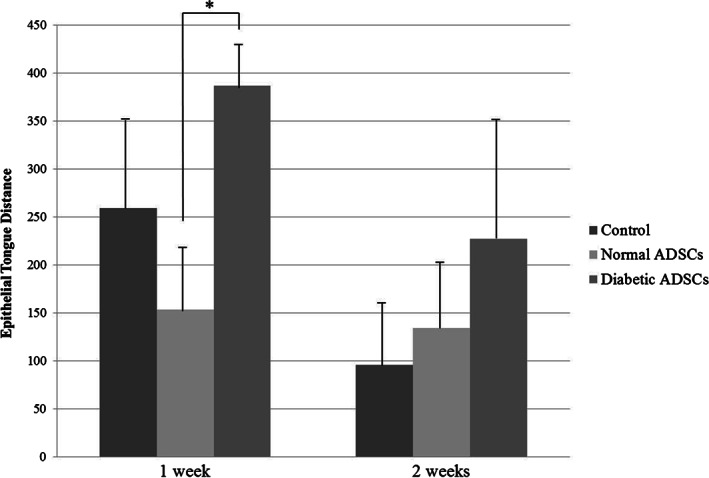
The epithelial tongue distance of the control, normal adipose tissue‐derived stem cells (ADSCs), and diabetic ADSCs groups at 1 and 2 weeks. At 1 week, the epithelial tongue distance was significantly lesser in the normal ADSCs group than in the diabetic ADSCs group (*P < 0·001). There was no significant difference between the control group and the normal ADSCs group and between the control group and the diabetic ADSCs group. At 2 weeks, there were no statistical significant differences of the epithelial tongue distance between the three groups.
Figure 5.
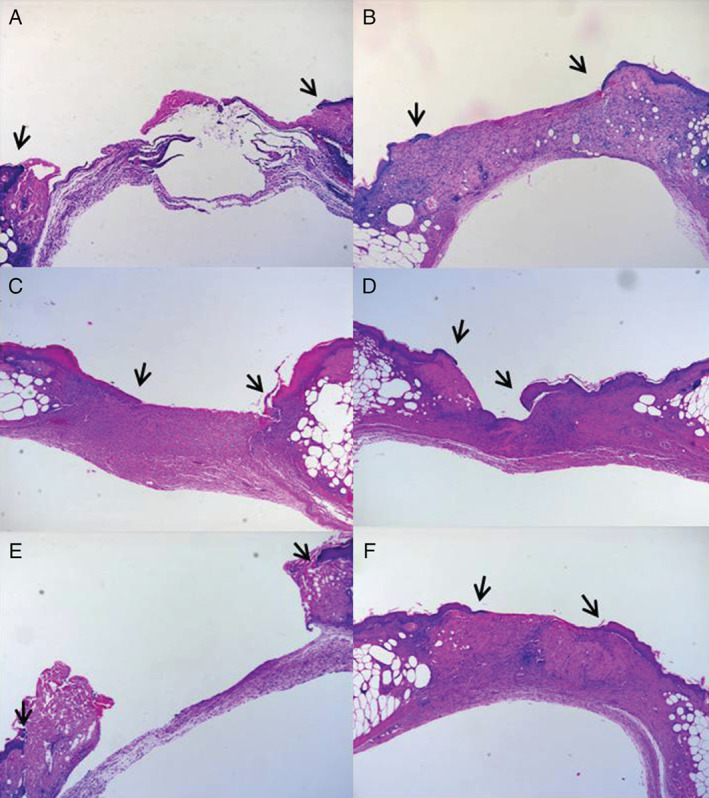
Micrographs of wound beds and wound margins in the control, normal adipose tissue‐derived stem cells (ADSCs), and diabetic ADSCs groups at 1 (left) and 2 weeks (right). Histological sections (H&E stain, 40×) showing both epithelial tongues (black arrows) at 1 week and 2 weeks after treatment of the control group (A) and (B), the normal ADSCs group (C) and (D), and the diabetic ADSCs group (E) and (F). Wounds in the normal ADSCs group showed a shorter epithelial tongue distance than those in the control or diabetic ADSCs group at the same time points.
At 1 week, it was significantly lesser in the normal ADSCs group than in the diabetic ADSCs group (P < 0·001). There was no significant difference between the control group and the normal ADSCs group and between the control group and the diabetic ADSCs group. At 2 weeks, there were no statistical significant differences of the epithelial tongue distance between the three groups. There were significant decreases of the epithelial tongue distance in the control group (P = 0·017) and the diabetic ADSCs group (P = 0·005) from 1 to 2 weeks. However, there was no significant difference of the epithelial tongue distance in the normal ADSCs group from 1 to 2 weeks.
The area of granulation tissue
At 1 week, the area of granulation tissue was 1·87 ± 0·37 × 103 µm2 in the control group, 2·88 ± 0·67 × 103 µm2 in the normal ADSCs group, and 2·14 ± 0·21 × 103 µm2 in the diabetic ADSCs group. At 2 weeks, the granulation tissue thickness was 0·74 ± 0·18 × 103 µm2 in the control group, 0·50 ± 0·33 × 103 µm2 in the normal ADSCs group, and 0·71 ± 0·19 × 103 µm2 in the diabetic ADSCs group (Figure 6).
Figure 6.
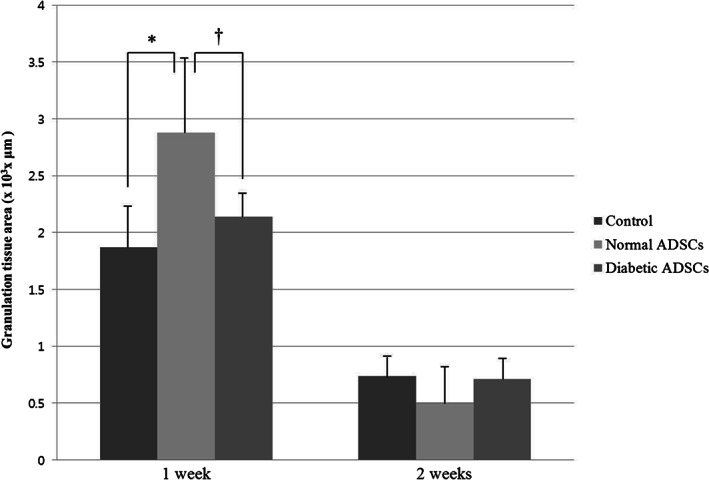
The area of granulation tissue of the control, normal ADSCs, and diabetic ADSCs groups at 1 and 2 weeks. At 1 week, the normal ADSCs group showed the significantly greater area of granulation tissue than control group (*P = 0·007), also it was significantly higher in the normal ADSCs group than the diabetic ADSCs group († P = 0·012). However, there was no significant difference between the control group and the diabetic ADSCs group. At 2 weeks, there were no significant differences of the area of granulation tissue between the three groups.
At 1 week, the normal ADSCs group showed the significantly greater area of granulation tissue than the the diabetic ADSCs group (P = 0·012), also it was significantly higher in the normal ADSCs group than the control group (P = 0·007). However, there was no significant difference between the control group and the diabetic ADSCs group. At 2 weeks, there were no significant differences of the area of granulation tissue between the three groups.
The number of capillary
At 1 week, the number of capillary was 3·90 ± 2·82 in the control group, 38·75 ± 12·12 in the normal ADSCs group, 22·00 ± 13·44 in the diabetic ADSCs. At 2 weeks, the number of capillary was 12·38 ± 5·03 in the control group, 27·97 ± 15·16 in the normal ADSCs group, 19·81 ± 6·64 in the diabetic ADSCs (Figure 7).
Figure 7.
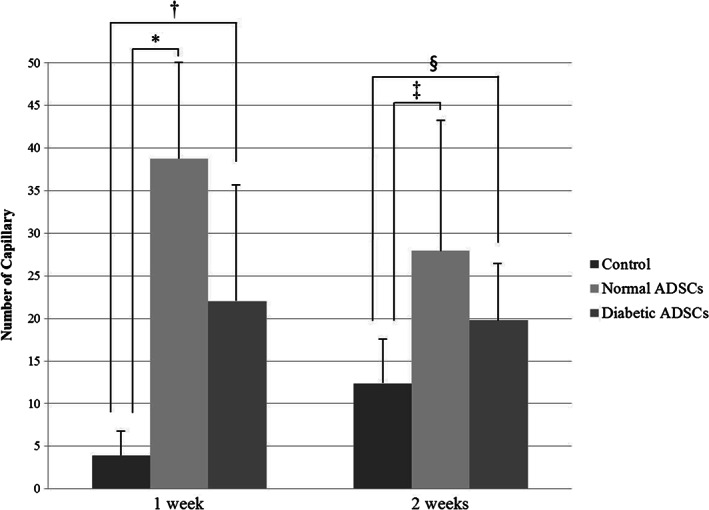
The capillary number of the control, normal adipose tissue‐derived stem cells (ADSCs), and diabetic ADSCs groups at 1 and 2 weeks. The normal ADSCs group (*P = 0·001 at 1 week, ‡ P = 0·005 at 2 weeks), and the diabetic ADSCs group († P = 0·004 at 1 week, § P = 0·012 at 2 weeks) showed the significantly greater mean capillary count than the control group at each time point, there was no significant difference between the normal ADSCs group and the diabetic ADSCs group at either time point.
The normal ADSCs group showed the significantly greater mean capillary count than the control group at 1 week (P = 0·001), also at 2 weeks (P = 0·005). And the diabetic ADSCs group showed the significantly greater mean capillary count than the control group at 1 week and 2 weeks (P = 0·004, P = 0·012, respectively). However, there was no significant difference between the normal ADSCs group and the diabetic ADSCs group at 1 week, also at 2 weeks.
The number of Ki‐67‐stained cell
At 1week, the number of Ki‐67‐stained cell was 1·6 ± 0·95 in the control group, 3·7 ± 1·87 in the normal ADSCs group, 1·28 ± 0·65 in the diabetic ADSCs. At 2 weeks, the number of capillary was 1·06 ± 0·71 in the control group, 2·59 ± 1·46 in the normal ADSCs group, 0·96 ± 0·7 in the diabetic ADSCs (Figures 8 and 9).
Figure 8.
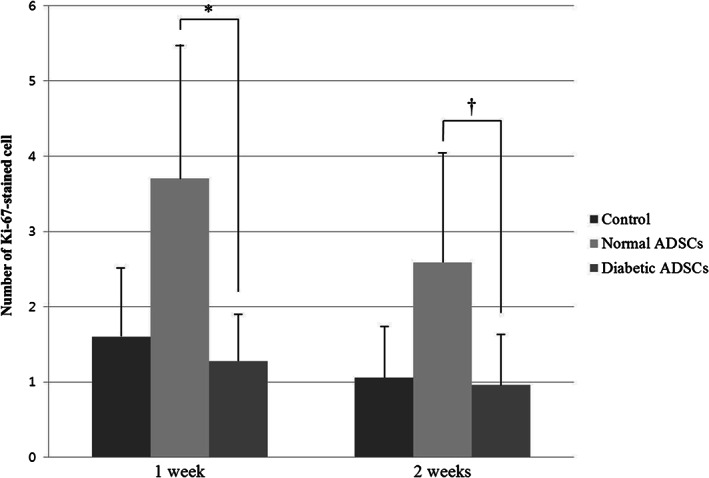
The number of Ki‐67‐stained cell of the control, normal adipose tissue‐derived stem cells (ADSCs), and diabetic ADSCs groups at 1 and 2 weeks. At 1 week, the number of Ki‐67‐stained cell was significantly greater in the normal ADSCs group than in the diabetic ADSCs group (*P = 0·005). Also at 2 weeks, the number of Ki‐67‐stained cell was significantly greater in the normal ADSCs group than in the diabetic ADSCs group († P = 0·014). There was no significant difference between the control group and the normal ADSCs group at 1 week, also at 2 weeks. There were no statistical significant differences of the number of Ki‐67‐stained cell between the control group and the diabetic ADSCs group at 1week and at 2weeks.
Figure 9.
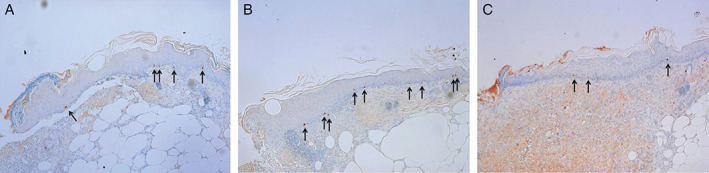
Histologic section (Ki‐67, 100×) showing cell proliferation in the basal layer of epithelial tongue of the control group (A), the normal ADSCs group (B), and the diabetic ADSCs group (C) at 1 week after treatment. The normal ADSCs group shows greater number of Ki‐67‐stained cells (black arrow) than the control group or the diabetic ADSCs group.
At 1 week, the number of Ki‐67‐stained cell was significantly greater in the normal ADSCs group than in the diabetic ADSCs group (P = 0·005). Also at 2 weeks, the number of Ki‐67‐stained cell was significantly greater in the normal ADSCs group than in the diabetic ADSCs group (P = 0·014). There was no significant difference between the control group and the normal ADSCs group at 1 week, and at 2 weeks. There were no statistical significant differences of the number of Ki‐67‐stained cell between the control group and the diabetic ADSCs group at 1 week and at 2 weeks.
Discussion
Although administration of autologous ADSCs has been reported to improve healing 7, 9, 10, 11, 12, some investigators have recently demonstrated that diabetic ADSCs exhibit lower abilities than normal ADSCs 1. ADSCs derived from diabetic mice exhibit significantly reduced baseline levels of cellular proliferation, VEGF expression, and tubulisation 1. Higher levels of cellular senescence and apoptosis induced via the intrinsic pathway have also been shown, along with reduced osteogenic and chondrogenic potential 6. Therefore, for clinical application of ADSCs in human diabetic wounds, it is important to evaluate the impact of the DM on the ablity of ADSCs to promote wound healing and prove that in vivo study.
To confirm that DM indeed affects the ability of ADSCs to promote wound healing, and to evaluate the underlying mechanisms, we designed a wound healing model by using diabetic mice. We used normal ADSCs and diabetic ADSCs to evaluate whether a diabetic wound would respond differently to normal ADSCs and diabetic ADSCs. Previous wound healing models have been used to evaluate the wound healing properties of ADSCs using an atelocollagen matrix 7 and a donut‐shaped silicone splint 9. We have performed some animal studies using the silicone splint previously, and we observed that skin contraction around the wound is not completely blocked by use of the splint alone 9, 13. Therefore, we designed a new wound healing model combining these two ideas to minimise skin contraction. We used an artificial dermis which includes a silicone layer on its surface, and secured the artificial dermis to the wound margin with another silicone sheet. The volume of artificial dermis occupied the entire space created by wounding. These measures were effective to minimise skin contraction around the wound site, allowing the wound healing via granulation and re‐epithelisation.
At 1 week, the wound healing rate was significantly higher in the normal ADSCs group than in the diabetic ADSCs group and the control group. The diabetic ADSCs group showed a better wound healing rate than the control group, but the difference was not significant. Similar patterns were observed at 2 week. We conclude that these results reflect a general impairment of the therapeutic potential of diabetic ADSCs in vivo, as some authors who have conducted in vitro studies have reported 1, 6. Despite of a reduction in function, diabetic ADSCs do retain a certain degree of their ability compared to the control group.
Re‐epithelisation, granulation tissue formation, neovascularisation and angiogenesis are all crucial steps in wound healing. ADSCs are thought to improve the wound healing by affecting these processes. Many previous studies have demonstrated that ADSCs promote wound healing in diabetic models via incorporation into epithelial tissue and by affecting keratinocyte migration 7, 10, 11. Notably, administering ADSCs to diabetic wounds stimulates the dermal fibroblasts and enhances the formation of ECM for dermal regeneration 5, 14. ADSCs enhance secretion of type I collagen by human dermal fibroblasts by regulating the mRNA levels of extracellular matrix proteins and have a stimulatory effect on chemotaxis of fibroblasts 15, 16. Neovascularisation and angiogenesis are necessary to maintain newly synthesised granulation tissue and ensure the survival of keratinocytes 9. Cultured ADSCs secrete a number of angiogenic cytokines such as basic fibroblast growth factor (b‐FGF), platelet‐derived growth factor bb (PDGF‐bb), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) 7. In this study, we compared the epithelial tongue distance and the number of cells stained for Ki‐67, a well‐known proliferation marker, to evaluate whether DM alters the ability of ADSCs to stimulate re‐epithelisation and keratinocyte proliferation. At 1 week, the epithelial tongue distance was significantly shorter in the normal ADSCs group than in the diabetic ADSCs group, but no significant difference was observed between the control group and the diabetic ADSCs group. Comparison of number of Ki‐67‐stained cell produced similar results. Collectively, these results suggest that the intrinsic ability of diabetic ADSCs to stimulate re‐epithelisation and keratinocyte proliferation may be blunted.
Evaluation of the granulation tissue area showed a similar pattern. At 1 week, the normal ADSCs group showed a significantly greater area of granulation tissue than the diabetic ADSCs group, or the control group. However, no significant difference was observed between the control group and the diabetic ADSCs group. These results indicate that DM impairs the ability of ADSCs to enhance formation of ECM for dermal regeneration.
El‐Ftesi et al. previously reported on the intrinsic neovascular potential of ADSCs in the context of advanced age and diabetes 1. They evaluated VEGF expression, proliferation, and tubulisation of ADSCs in normoxia and hypoxia in vitro, and demonstrated that the neovascularisation potential of aged and diabetic ADSCs is impaired in comparison with young controls. In the present study, both the normal and diabetic ADSCs groups showed a significantly greater number of capillary than the control group. In the normal ADSCs group, the number of capillary was greater than in the diabetic ADSCs group, but this difference was not significant. In contrast to our other results, diabetic ADSCs retained their ability to promote neovascularisation and angiogenesis, contrary to previously published study. This discrepancy may be attributed to age of cells, or it may be that the degree of the effects induced by DM are not detectable in vivo.
This study reveals that DM affects the ability of ADSCs to promote wound healing, which could influence their therapeutic potential. Therefore, prior to clinical use of ADSCs in diabetic patients, a solution is required to overcome their blunted ability. It is possible that an additional treatment will be required to augment the regeneration and differentiation of transplanted ADSCs, for example, growth factors. Alternatively, multiple repeated applications of ADSCs may produce beneficial effects. According to previous studies, ADSCs have immunosuppressive properties and show potential for allogenic cell treatment. ADSCs did not provoke in vitro alloreactivity of incompatible lymphocytes and suppressed mixed lymphocyte reaction and lymphocyte proliferative response to mitogens 17, similar findings have also been demonstrated in vivo 18. Given this data, one may assume that it may be better to employ allogenic normal ADSCs rather than autologous, diabetic, and impaired ADSCs in cell therapy for promotion of wound healing in diabetic patients.
The present study demonstrated that diabetic ADSCs are impaired compared to normal ADSCs in promoting wound healing in a diabetic setting. The possible mechanisms underlying this phenomenon are: (i) reduction of re‐epithelisation and keratinocyte proliferation; (ii) decreased formation of granulation tissue slows dermal regeneration; (iii) decreased neovascularisation and angiogenesis. In spite of decrease of ability, diabetic ADSCs retain a certain degree of their ability in comparison to the control group, especially with regard to the ability to promote neovascularisation and angiogenesis. Further large‐scaled in vivo studies to evaluate the exact mechanisms underlying the impaired ability of the ADSCs and considerations of their therapeutic uses are certainly warranted prior to clinical use of ADSCs in patients with diabetic ulcers.
Acknowledgements
None.
References
- 1. El‐Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose‐derived stromal cells. Plast Reconstr Surg 2009;123:475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim SG, Kim EY, Kim YJ, Lee SI. The efficacy and safety of ablative fractional resurfacing using a 2,940‐Nm Er:YAG laser for traumatic scars in the early posttraumatic period. Arch Plast Surg 2012;39:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS. Diabetic foot ulcers: part II. Management. J Am Acad Dermatol 2014;70:21.e1–4; quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 4. Jeon MK, Kang SJ, Sun H. Platysma flap with z‐plasty for correction of post‐thyroidectomy swallowing deformity. Arch Plast Surg 2013;40:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim WS, Park BS, Sung JH. The wound‐healing and antioxidant effects of adipose‐derived stem cells. Expert Opin Biol Ther 2009;9:879–87. [DOI] [PubMed] [Google Scholar]
- 6. Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 2010;19:1875–84. [DOI] [PubMed] [Google Scholar]
- 7. Nambu M, Kishimoto S, Nakamura S, Mizuno H, Yanagibayashi S, Yamamoto N, Azuma R, Nakamura S, Kiyosawa T, Ishihara M, Kanatani Y. Accelerated wound healing in healing‐impaired db/db mice by autologous adipose tissue‐derived stromal cells combined with atelocollagen matrix. Ann Plast Surg 2009;62:317–21. [DOI] [PubMed] [Google Scholar]
- 8. Kim HK, Kim YJ, Kim JT, Kwon CH, Kim YK, Bae YC, Kim DH, Jung JS. Alterations in the proangiogenic functions of adipose tissue‐derived stromal cells isolated from diabetic rats. Stem Cells Dev 2008;17:669–80. [DOI] [PubMed] [Google Scholar]
- 9. Lim JS, Yoo G. Effects of adipose‐derived stromal cells and of their extract on wound healing in a mouse model. J Korean Med Sci 2010;25:746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maharlooei MK, Bagheri M, Solhjou Z, Jahromi BM, Akrami M, Rohani L, Monabati A, Noorafshan A, Omrani GR. Adipose tissue derived mesenchymal stem cell (AD‐MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract 2011;93:228–34. [DOI] [PubMed] [Google Scholar]
- 11. Nambu M, Ishihara M, Kishimoto S, Yanagibayashi S, Yamamoto N, Azuma R, Kanatani Y, Kiyosawa T, Mizuno H. Stimulatory effect of autologous adipose tissue‐derived stromal cells in an atelocollagen matrix on wound healing in diabetic db/db mice. J Tissue Eng 2011;2011:158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue‐derived stromal cells. Arterioscler Thromb Vasc Biol 2005;25:2542–7. [DOI] [PubMed] [Google Scholar]
- 13. Yoon JW, Lim JS, Kim JN, Woo G. Effect of allogenic adipose‐derived stromal cells on wound healing in BALB/c mice. J Korean Soc Plast Reconstr Surg 2010;37:323–8. [Google Scholar]
- 14. Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue‐derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen 2013;21:545–53. [DOI] [PubMed] [Google Scholar]
- 15. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24. [DOI] [PubMed] [Google Scholar]
- 16. Sarojini H, Estrada R, Lu H, Dekova S, Lee MJ, Gray RD, Wang E. PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J Cell Biochem 2008;104:1793–802. [DOI] [PubMed] [Google Scholar]
- 17. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue‐derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–29. [DOI] [PubMed] [Google Scholar]
- 18. Yanez R, Lamana ML, Garcia‐Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue‐derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft‐versus‐host disease. Stem Cells 2006;24:2582–91. [DOI] [PubMed] [Google Scholar]


