Abstract
Chronic venous ulceration represents a very common event. Current standard treatment includes local wound care with the application of compression. We report the effects of platelet‐rich plasma in patients with chronic venous ulcers, which is able to stimulate fibroblasts, macrophages and mesenchymal cells and growth factors in order to achieve re‐epithelialisation and neovascularisation within the microenviroment of the wound. We also documented the efficacy of this method as the sole treatment without surgical procedures.
Keywords: Chronic venous ulceration, Growth factors, Platelet‐rich plasma
Introduction
Chronic venous ulceration (CVU) may complicate the clinical course of chronic venous disease (CVD) 1, 2 in which several pathophysiological events are involved, that is inflammation, activation of polymorphonucleocytes and activation of proteases secretion 3. We have previously published an involvement of several inflammatory mediators 4. Current standard of care 5 for CVU is local wound care with the application of compression therapy. We have also shown that doxycycline and minocycline as well as nutraceutical substances are able to improve CVUs through the action on inflammatory mediators 6, 7, 8, 9. Recently, we reported the effects of topical application of platelet gel and skin grafting in the improvement of CVU disease 2. In this report, the successful use of fibrin membranes as a biological wound dressing in the treatment of chronic venous ulcers is discussed.
Methods
Patients with CVUs were treated using a fibrin membrane treatment along with the application of concentrated growth factors (CGFs) as described by Rodella et al. 10.
Nine millilitres of blood was drawn in sterile Vacuette tubes (Greiner Bio‐One, GmbH, Kremsmunster, Austria) without anticoagulant and was immediately centrifuged (Medifuge MF200, Silfradent srl, Forlì, Italy) at non‐constant velocities: 30 seconds acceleration, 2 minutes at 872 g, 4 minutes at 689 g, 4 minutes at 872 g, 3 minutes at 1077 g and 36 seconds deceleration and stopped. Three blood fractions were obtained: (i) the upper platelet‐poor plasma (PPP) layer; (ii) the middle fibrin‐rich gel with aggregated platelets and CGFs; (iii) the lower red blood cell (RBC) layer.
Before the application of fibrin membrane, ulcers were cleansed and cefepime was topically applied for 30 minutes, after which the fibrin‐rich gel (fraction 2) was applied. Next, the PPP (fraction 1) was applied and the wound was protected with an occlusive dressing for 7 days, following which ulcer examination was performed. After resolution of healing and pain, the application of compression therapy was recommended.
Case 1
An 84‐year‐old female presented with chronic venous ulcers on the left leg. Past medical history included hypertension and hypercholesterolaemia, both well controlled with pharmacological treatment (enalapril 20 mg/day, atorvastatin 20 mg/day, aspirin 100 mg/day and omeprazole 20 mg/day). She also took acetaminophen occasionally for joint pain. No other significant systemic findings were noted. The venous leg ulcer measured an area of 10·9 cm2 (Figure 1A) with intense pain [visual analogical scale (VAS): 8]. Doppler ultrasound imaging revealed superficial venous incompetence. Considering the age of the patient, a novel treatment was proposed and was begun after signed informed consent in the presence of her family was obtained. Fibrin membrane treatment with the application of CGFs was performed using heterologous blood taken from the patient's adult sons. The donated blood was evaluated for microbiological and biochemical sterility, prior to use. During the early follow‐up stage, significant improvement of ulcer area was observed, as well as significant pain reduction (VAS: 4). Full resolution of symptoms and complete ulcer healing was achieved in just under 1 year (Figure 1B).
Figure 1.
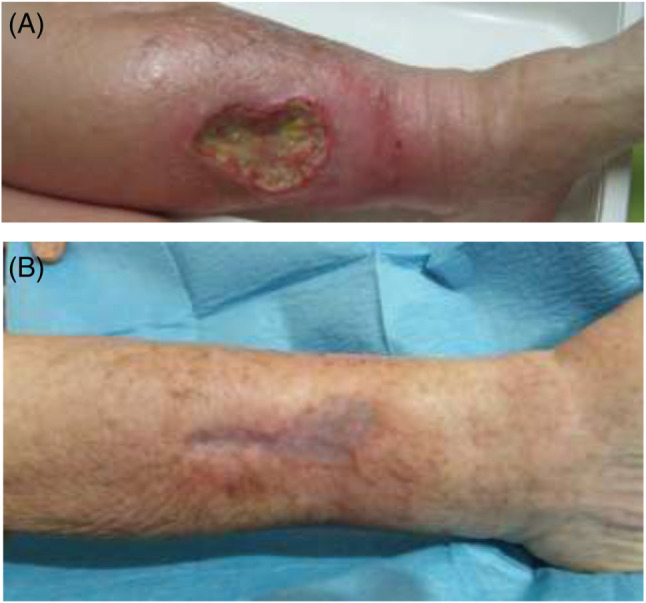
Chronic venous ulcers of the left leg in an 84‐year‐old female at the time of first presentation (A) and at the end of treatment (B).
Case 2
A 65‐year‐old woman with history of arterial hypertension and severe articular pain presented for CVU of the left leg (ulcer area 11·3 cm2; Figure 2A) with intense pain (VAS: 9). Doppler ultrasound imaging revealed incompetence of the superficial and perforator veins. After patient counselling and obtaining informed consent, fibrin membrane treatment with application of CGFs using autologous blood was started with a near total resolution of symptoms (VAS: 1) and complete ulcer healing was obtained at 2 months (Figure 2B).
Figure 2.
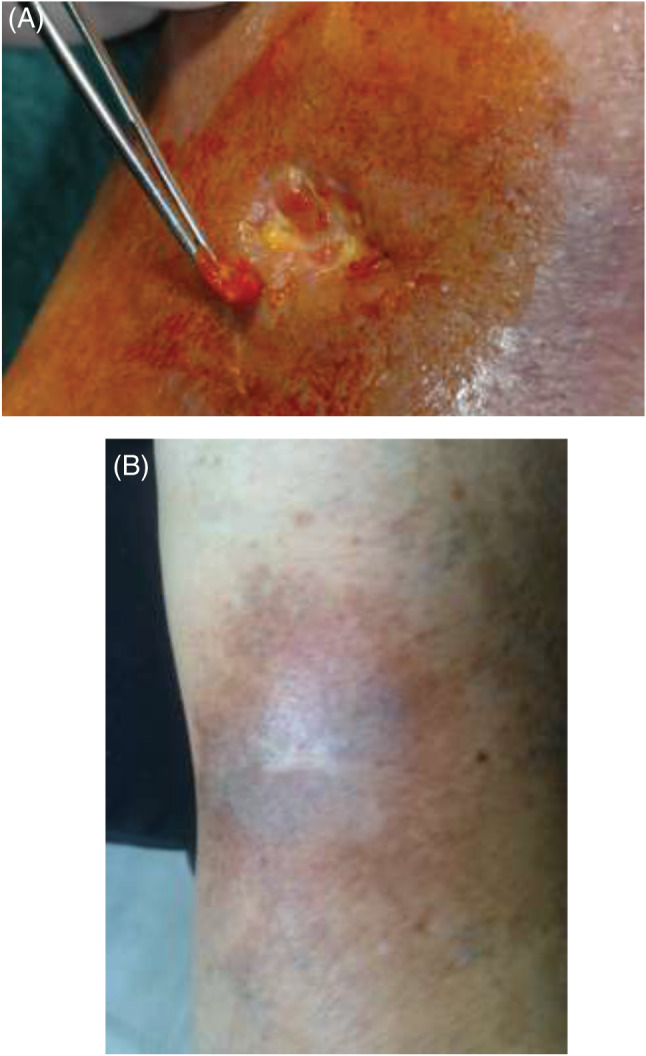
Chronic venous ulcers of the left leg in a 65‐year‐old female at the time of first presentation (A) and at the end of treatment (B).
Case 3
A 57‐year‐old man, cigarette smoker, with a hip replacement presented for evaluation of a chronic mixed arterial and venous ulcer of the left leg (ulcer area: 11·1 cm2; Figure 3A) with intense pain (VAS: 8). Doppler ultrasound imaging revealed superficial vein incompetence and peripheral arterial insufficiency. After treatment counselling and informed consent, fibrin membrane treatment with application of CGFs using autologous blood was initiated. Significant pain reduction was achieved (VAS: 1) with complete ulcer healing in 1 month (Figure 3B).
Figure 3.
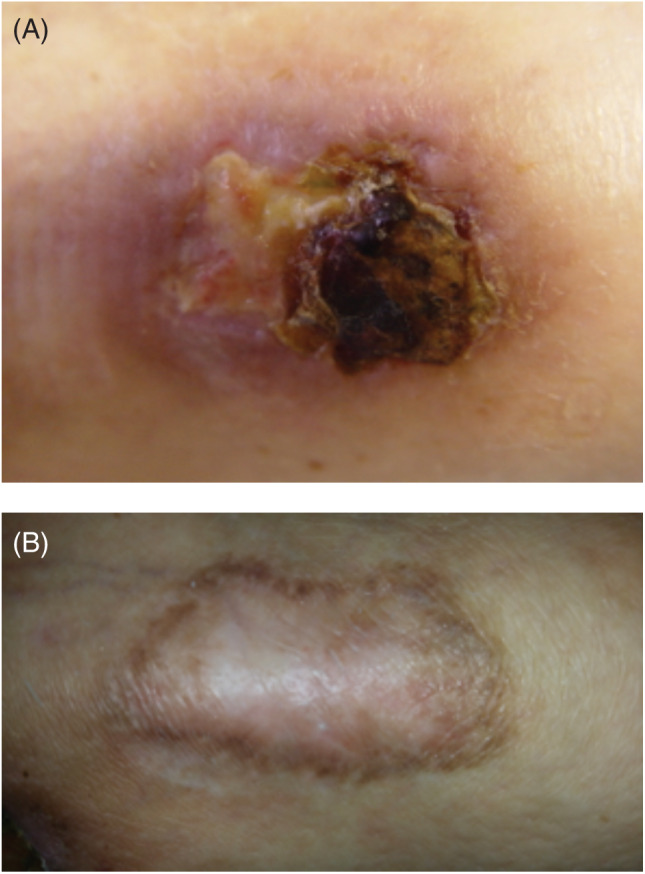
Chronic mixed arterial and venous ulcers of the left leg in 57‐year‐old man at the time of first presentation (A) and at the end of treatment (B).
Discussion
Wound healing is a complex process involving many cell types, cytokines and growth factors, for example platelet‐derived growth factors, fibroblast growth factors and granulocyte–macrophage colony‐stimulating factor. We report the effective use of fibrin membranes as a biologic wound dressing in the treatment of chronic venous ulcers obviating the need for surgical intervention.
Previously, we reported a series of 87 patients with lower extremity ulcers in which topical application of platelet gel resolved the clinical symptoms and accelerated healing time 1, 9. We now report the effects of growth factors, CD34+ cells and fibrin in the management of refractory chronic venous ulcers.
Using the method of Rodella 10, we isolated three blood sample fractions:
Fraction 1: upper PPP;
Fraction 2: fibrin‐rich gel with aggregated platelets and CGFs;
Fraction 3: RBC.
After topical application of cefepime to inhibit infection, fraction 2 was applied to the prepared wound followed by fraction 1.
Both fractions 1 and 2 play a central role in ulcer healing. Fraction 2 is crucial to stimulate fibroblasts, macrophages and mesenchymal cells. Fraction 1 (platelets) is rich in granules containing growth factors involved in re‐epithelialisation and neovascularisation.
Innovative methodology combining platelet gel, skin graft and fibrin glue may represent a novel treatment of chronic venous ulcers 2, 11, thereby reducing surgical complications and sometimes the need for surgery.
The treatment used in this study was demonstrated to be effective as the sole treatment, without skin grafting, and was also shown to be effective in case 3 of our case series, which was a mixed arterial and venous ulcer that is a particular form of hard‐to‐heal wound, nowadays seen as an emerging and serious challenge for physicians 8, 12
We encourage other investigators to validate our results in caring for their own patient populations and look forward to sharing their reported clinical trial results.
References
- 1. de Franciscis S, De Sarro G, Longo P, Buffone G, Molinari V, Stillitano DM, Gallelli L, Serra R. Hyperhomocysteinaemia and chronic venous ulcers. Int Wound J 2015;12:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serra R, Grande R, Butrico L, Montemurro R, De Caridi G, Fugetto F, Dominijanni A, Gallelli L, Greto Ciriaco A, Vitagliano T, Greco M, de Franciscis S. Skin grafting and topical application of platelet gel in the treatment of vascular lower extremity ulcers. Acta Phlebol 2014;15:129–36. [Google Scholar]
- 3. Abd‐El‐Aleem SA, Morgan C, Ferguson MW, McCollum CN, Ireland GW. Spatial distribution of mast cells in chronic venous leg ulcers. Eur J Histochem 2005;49:265–72. [PubMed] [Google Scholar]
- 4. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen 2013;21:395–401. [DOI] [PubMed] [Google Scholar]
- 5. Weller C, Evans S. Venous leg ulcer management in general practice – practice nurses and evidence based guidelines. Aust Fam Physician 2012;41:331–7. [PubMed] [Google Scholar]
- 6. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2015;12:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serra R, Grande R, Butrico L, Buffone G, Calio FG, Squillace A, Rizzo BA, Massara M, Spinelli F, Ferrarese AG, de Caridi G, Gallelli L, de Franciscis S. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J 2014. DOI: 10.1111/iwj.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serra R, Gallelli L, Conti A, De Caridi G, Massara M, Spinelli F, Buffone G, Caliò FG, Amato B, Ceglia S, Spaziano G, Scaramuzzino L, Ferrarese AG, Grande R, de Franciscis S. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des Devel Ther 2014;8:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Acta Phlebol 2013;14:99–107. [Google Scholar]
- 10. Rodella LF, Favero G, Boninsegna R, Buffoli B, Labanca M, Scari G, Sacco L, Batani T, Rezzani R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech 2011;74:772–7. [DOI] [PubMed] [Google Scholar]
- 11. Chen TM, Tsai JC, Burnouf T. A novel technique combining platelet gel, skin graft, and fibrin glue for healing recalcitrant lower extremity ulcers. Dermatol Surg 2010;36:453–60. [DOI] [PubMed] [Google Scholar]
- 12. De Caridi G, Massara M, Stilo F, Spinelli F, Grande R, Butrico L, de Franciscis S, Serra R. Effectiveness of prostaglandin E1 in patients with mixed arterial and venous ulcers of the lower limbs. Int Wound J 2014. DOI: 10.1111/iwj.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]


