ABSTRACT
This test–retest pilot study investigated the intra‐rater reliability and reproducibility of non‐invasive technologies to objectively quantify morphological (colour, thickness and elasticity) and physiological (transepidermal water loss (TEWL), hydration, sebum and pH) skin properties in an aged care population. Three consecutive measurements were taken from five anatomical skin sites, with the mean of each measurement calculated. The intra‐class correlation coefficient (ICC) and the standard error of measurement (SEM) were used to examine the intra‐rater reliability and reproducibility of measurements. Non‐invasive technologies in this study showed almost perfect reliability for ultrasound measurements of the subepidermal low echogenicity band (SLEB) (ρ = 0·95–0·99) and skin thickness (ρ = 0·95–0·99) across all sites. The ICC was substantial to almost perfect for pH (ρ = 0·76–0·88) and viscoelasticity (ρ = 0·67–0·91) across all sites. Hydration (ρ = 0·53–0·85) and skin retraction (ρ = 0·57–0·99) measurements ranged from moderate to almost perfect across all sites. TEWL and elasticity were substantial to almost perfect across four sites. Casual sebum levels and most colour parameters showed poor ICC. The use of non‐invasive technologies in this study provided an objective and reliable means for quantifying ageing skin and may offer future studies a valuable option for assessing skin tear risk.
Keywords: Aged skin, Intra‐rater reliability, Non‐invasive technologies, Reproducibility, Skin properties, Test–retest
Introduction
The methodical and quantitative assessment of aged skin, using non‐invasive technologies, has the potential to objectively identify individuals at risk of sustaining skin tears. Current clinical practice for identifying at‐risk persons has generally been based on the subjective assessment of a broad range of individual and skin characteristics. A recent review found that common patient characteristics associated with skin tears included a history of skin tears, impaired mobility and cognition, while general skin characteristics comprised of visible signs of senile purpura, ecchymosis and oedema 1. A case–control study of 151 tertiary patients aged 50 and over with skin tears and 302 non‐matched controls aged over 50 years without skin tears found six variables; ecchymosis (bruising); senile purpura; haematoma; evidence of a previously healed skin tear; oedema; and the inability to reposition independently were predictors of skin tears in older patients 2. Despite the considerable advances over the last three decades in biophysical skin analysis and the availability of non‐invasive bioengineered technologies to obtain objective, quantitative and reproducible measurements, only one article by Koyano et al. 3 was found published in the English literature which reported using these technologies to objectively quantify skin properties associated with skin tears. The authors examined the dorsal forearm and reported that increased low‐echogenic pixels in the subepidermal low echogenicity band (SLEB) (measured by 20‐MHz ultrasonography), decreased type IV collagen, decreased matrix metalloproteinase‐2 (MMP‐2) and increased tumour necrosis factor‐a (quantified by nitrocellulose membranes from skin blotting) were related to skin tear occurrence in elderly Japanese patients 3.
The purpose of this study was to determine the intra‐rater reliability and test–retest of measuring the skin properties of long‐term aged care residents using non‐invasive technologies. This paper examines the use of non‐invasive technologies, from a diagnostic perspective, for characterising ageing skin. The potential novel applications of these technologies to inform diagnostic investigations will be relevant to both clinicians and those with a technological background. Those skin properties that recorded good reliability were applied in a subsequent study that examined skin characteristics associated with skin tear occurrence in a larger sample of aged care residents.
Materials and method
Study design and setting
This reliability study was conducted in two phases: the initial phase, which established the feasibility of a single investigator to use non‐invasive technologies to assess ageing skin properties, and the present test–retest pilot study. The competency of the investigator in the use of the devices and assessment of skin properties was confirmed against manufacturers' standards and international guidelines respectively 4, 5, 6, 7. The feasibility phase was undertaken on 10 independent non‐residential older volunteers (four males and six females), aged between 62–83 years (mean = 71·3, SD = 6·2). All assessments were undertaken in a university clinical environment under the supervision of a specialist clinician between November and December 2013, at two points in time, 4 weeks apart. The pilot test–retest reliability study was conducted at an 81‐bed residential aged care facility in Western Australia, between January and March 2014. The study investigated the intra‐rater reliability and reproducibility of using non‐invasive technologies to test–retest the morphological (colour, thickness and elasticity) and physiological (transepidermal water loss (TEWL), hydration, sebum and pH) skin properties of a subset population of 31 aged care residents. Bilateral toe brachial pressure index (TBPI) and venous photoplethysmography (PPG) were obtained to determine arterial and venous blood flow to residents' lower extremities. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the Australian Code for the Responsible Conduct of research 8, 9. Ethics approval for this study was obtained from Curtin University Human Research Ethics Committee (RD‐23‐13) and The Bethanie Group Inc for the study site.
Measurements and instruments
A data collection tool, which comprised of nine sections, was used to record residents demographic details; medical history; Braden Scale score; medications; skin care regime; common skin characteristics (purpura, ecchymosis, oedema and pseudoscars); Fitzpatrick skin type; and previous history of skin tears. The Fitzpatrick skin type assessment tool, which comprises of six characteristic groupings, was initially developed to predict skin reactivity to photochemotherapy and is also used as a reference standard for classifying a person's skin type 10. All information was obtained either from the resident, their legal guardian or the medical records.
This pilot study used three commercially available, non‐invasive instruments (DermaLab Combo®, Sebumeter® and Skin‐pH‐meter®) to assess ageing skin properties. The DermaLab Combo® (Cortex Technology, Hadsund, Denmark) is a multipurpose device and was used to assess skin colour, TEWL, hydration, skin thickness and elasticity. The Sebumeter® and Skin‐pH‐meter® (Courage + Khazaka, Cologne, Germany) devices were used to evaluate skin surface sebum and pH. A fourth device, the Hadeco Smartdop 30Ex® (Hayashi Denki, Kawasaki, Japan), was used to objectively evaluate lower extremity arterial and venous blood flow that can become sub‐optimal with ageing, leading to peripheral vascular disease and oedema, which has the potential to impact morphological and physiological skin properties of the lower limbs 11.
The DermaLab Combo® comprises of a central unit that supports multiple assessment probes. The TEWL probe, an open‐chamber device, measured vapour pressure gradient at the skin surface, with results reported in grams per square metre per hour (g/m2/hour) 12. The hydration probe evaluated the stratum corneum water‐binding capacity by measuring conductance with results present in micro‐Siemens (μS) per centimetre 13. Skin colour was assessed using a narrow‐band reflectance spectrophotometry. The 7‐mm diameter aperture of the optical focus measured melanin (pigmentation); erythema (vascularity); and the Commission Internationale d'Eclairage (CIE) colour space coordinates (L*a*b*) 13. The melanin index is reported as M = 100 × log (1/intensity of reflected red light) and the erythema index as E = 100 × log (intensity of reflected red light/intensity of reflected green light) 14. The CIEL*a*b* values permit colour to be reported in a three‐dimensional space, as depicted in Figure 1.
Figure 1.
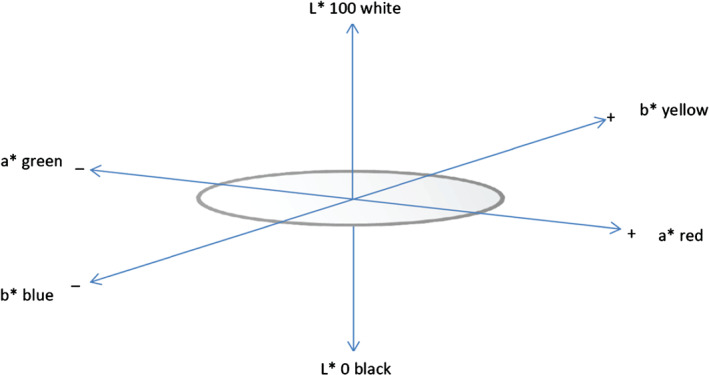
Depiction of CIEL*a*b* colour space. (Reprinted with permission from reference 15. Macmillan Publishers Ltd)
The vertical L* axis of the colour space represents lightness, with results ranging between 0 for black and 100 for white. The a* and b* axis are at right angles to one another across the horizontal axis, with the a* axis signifying green at the negative and red at the positive limit; and the b* axis denoting blue on the negative and yellow on the positive axis 16. There are no specified numerical limits for a* and b* values. Human skin, regardless of ethnicity, falls in the same yellow and red hue ranges of the a*and b* colour values 17. Colour parameter measurements were obtained within 1 second.
Skin thickness was determined using a pulsed 20 MHz B‐mode high frequency ultrasound, with a penetration depth of 3·7 mm that permitted the echogenic dermis to be differentiated from the hypoechoic subcutaneous tissue 18. An adjustable gain setting (±10 db) amplified reflection signals. The bi‐dimensional ultrasound skin image generates values for three separate variables: the SLEB, skin thickness and skin (structural) intensity 18. The SLEB value measures the extent of the dark low echogenicity band at the level of the papillary dermis, which is associated with photoageing and the degeneration of collagen fibres 19. The measure of skin thickness pertains to the width of the dermis, with negligible contribution of the viable epidermis to the score 13. Ultrasound frequencies with ranges between 50 and 150 MHz are needed to distinguish the epidermal layers 20. The SLEB and skin thickness values are expressed in micrometres (µm). Skin intensity quantifying the density of collagen signals in the skin is indicated by the colour scale in Figure 2 and is presented as an arbitrary score 13. Darker colours pertain to areas of low reflection or poor echogenicity between tissue structures, while higher densities, depicted by bright yellow, green or red pixels, signify strong reflection between structures and evidence of larger quantities of collagen 13. Aged and photodamaged skin are both associated with decreased collagen production that alters the intensity of dermal echogenicity 18. Figure 2 depicts the characteristics of an ultrasound (SLEB, skin thickness and intensity of collagen signals) image of the dorsal forearm of an 87‐year‐old female.
Figure 2.
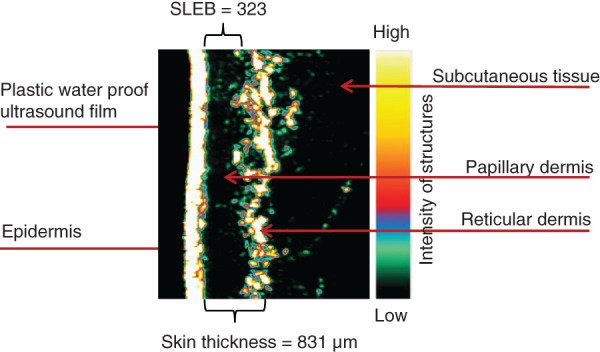
Ultrasound image of the dorsal forearm of an 87‐year‐old female.
The measurement of elasticity provides objective data about the mechanical properties of skin by quantifying the amount of force required to elevate skin 21. The elasticity probe measures three properties: viscoelasticity (VE), elasticity (E) and skin retraction (R) time 13. A lightweight plastic suction chamber with a 10‐mm aperture was attached to the skin surface using skin‐friendly double‐sided adhesive tape to form a closed chamber. Two narrow beams of parallel lights, situated 1·5 mm apart within the chamber, measure the height of skin deformation under suction. The E represents the amount of suction required to elevate skin from the first to the second beam, with results recorded in Mega Pascal (MPa) 21. The lower the E value, the more elastic the skin. The R is the time, in milliseconds (ms), for skin to retract 1·5 mm from full elevation. The VE combines both elevation and retraction phases. The effects of mechanical hysteresis that arises when skin is continually retracted were addressed in this study by taking repeated elasticity measurements 10 mm apart at each test site.
The Sebumeter® photometrically quantified casual skin surface sebum 22. In clinical practice, casual sebum levels are generally recorded 4 hours after the skin has been cleansed 22. Following contact of 30 seconds with the skin surface, a microprocessor evaluated the specialised plastic strip and reported results in units that range from 0 to 350 micrograms per square centimetre (µm/cm2) 23. The Skin‐pH‐Meter® PH 905 planar glass electrode evaluated the fine hydrophilic acidic film that covers the skin surface. pH measures energy changes from activity of hydrogen cations.* Prior to measuring the test site, the pH electrode was rinsed in distilled water and then gently applied to the skin surface for 10 seconds to stabilise the electrochemical potential and optimise skin surface contact 24.
The Hadeco Smartdop 30Ex® evaluated the TBPI and venous insufficiency of the lower extremities. The TBPI was used to assess the presence of peripheral arterial disease as toe vessels are less susceptible to medial arterial stenosis, which is associated with misleadingly elevated ankle brachial pressure index results 25. The TBPI measurements were taken with residents lying in the supine position and in accordance with clinical best practice 26. Bilateral systolic brachial pressures were measured. The TBPI results were manually calculated using the systolic toe pressure divided by the highest brachial result. Venous insufficiency was assessed by calculating the venous refill time in seconds 27. Individuals were required to sit at the side of the bed with their legs hanging down but not touching the ground. A sensory probe was placed over the posterior tibial vein using double‐sided adhesive tape. Individuals were required to perform five dorsiflexion's of the foot whilst sitting to partially empty the calf and skin venous reservoir. Results were obtained when the graph returned to the baseline amplitude on the monitor screen 28.
Participants
A convenient sample of all residents who resided in an aged care facility were invited to participate in this pilot reliability study to examine the utility of non‐invasive technologies for determining skin colour, TEWL, pH, hydration, thickness, elasticity, sebum production and lower extremity vascular status. An information sheet was mailed to all residents or to their legal guardian outlining the research protocol and data collection methodology and inviting their participation. Inclusion criteria comprised all residents aged 65 years or older with informed written consent. Residents were excluded from this study if they had a serious medical condition; a connective tissue disorder; were in pain; agitated; had an amputation; or were receiving palliative services. Data was collected from all residents at two points in time, over a 3‐month period from January to March 2014. A total of 2 hours was allocated for each assessment. All assessments were undertaken in consultation with the clinical nurses between the hours of 0700 and 1300 to ensure that measurements were standardised and to comply with the routine of residents and the aged care facility.
Data collection
Assessments of skin properties were undertaken at a residential aged care facility under standardised testing conditions and in accordance with international guidance for the assessment of skin properties 4, 5, 6, 7. The use of direct lighting and movement of air flow was avoided, with all measurements undertaken over the same season by a single assessor. A digital hydrothermograph sensor (Testo 608‐H2, Lenzkirch, Germany) measured the room temperature (test accuracy ± 0·5°C) and relative humidity (test accuracy ± 2%) prior to all assessments. Skin assessments were performed in the privacy of resident's room 15 minutes after they had acclimatised to the environment and the skin was preconditioned to obtain accurate recordings. Where practicable, residents laid supine with their arms positioned by their side with the dorsal area exposed for testing. Skin surface temperature was measured using an infrared non‐contact thermographic scanner (Exergen, Watertown, MA). The temperature of the skin was taken to evaluate any impact on the rate of TEWL 4
Measurements were taken at five anatomical test sites: bilateral mid‐dorsal forearms (midpoint between lateral epicondyle and radial styloid process), upper quartile of the lateral lower legs and midpoint between the umbilicus and the left iliac crest. The extremities were selected as skin tears primarily occur across these anatomical locations 29, 30, 31. While skin tears are not specific to the upper quartile of the lateral lower legs, this site was selected following technical difficulties experienced with lower leg oedema and the use of the elasticity probe that were identified amongst the volunteers who participated in the competency assessment phase of the study. The left lower abdominal region was selected as a control site as no previous studies have reported skin tears within this region; the area was generally not subjected to the effects of extrinsic ageing; and there is generally minimal scarring from surgical procedures. Three consecutive measurements were taken 10 mm apart at designated test sites, for all skin properties, with the combined mean used for calculation. All staff and residents were requested to avoid washing or applying moisturisers to the test sites for 24 hours prior to testing. Skin properties were assessed in a sequential order to minimise the impact on successive measurements.
Care was taken during the assessment to support the probes and avoid pulling cables that may alter biomechanical properties and influence results 21. Within this residential population, the gain setting that optimises the ultrasound image could not be standardised as adjustments were needed based on the extent of chronological or photoageing over the test site 32. Higher gains were necessary to penetrate photoaged skin, while lower gains were found to be more suitable for chronologically aged skin. A thin film of water was applied to the skin to form a coupling medium when taking ultrasound measurements. Elasticity measurements were recorded approximately 60 minutes into the assessment, with residents lying in the supine position. To minimise the impact of this ageing population needing to constantly turn, elasticity measurements of the lower extremities were taken using horizontal rather than vertical suction forces. Perpendicular forces are the manufacturers preferred option and are the most commonly used technique for assessing the skin's biomechanical properties 33. Elasticity measurements of the upper extremities and abdomen were assessed using the vertical suction technique. The elasticity probe has a relatively small 10‐mm diameter aperture that measures softer skin more readily than firmer tissue 34. To control for the effects of mechanical hysteresis from deformation, repeat elasticity measurements were taken 10 mm apart at each test site 35. Values obtained from the test–retest periods were compared to determine intra‐rater reliability.
Data analysis
The intra‐rater reliability of the mean for the three quantitative values recorded over both measurements periods were analysed using the intra‐class correlation coefficient (ICC) to assess the reliability of repeat measures 36. Lin's 37 concordance correlation coefficient (CCC) complemented the measurement of intra‐rater reliability. The ICC and Lin's CCC are designed to assess the consistency between two or more quantitative measurements. Both values range from 0 to 1, with a value of one signifying perfect agreement between repeat measurements, while an ICC value of zero denoted no agreement. Landis and Koch's benchmark scale was used to interpret the ICC, with ρ < 0 reflecting poor reliability, 0–0·20 slight, 0·21–0·40 fair, 0·41–0·60 moderate, 0·61–0·80 substantial and greater than 0·81 almost perfect reliability 38. In contrast to the ICC, Lin's CCC does not require analysis of variance (ANOVA) assumptions, which can differ according to the type of ANOVA model used 39. Lin's CCC is a more stringent measure of agreement for measuring identical continuous variables 40. McBride (2005) suggested that when interpreting the strength of agreement for Lin's CCC, values < 0·90 is poor; 0·90–0·95 is moderate; 0·95–0·99 is substantial; and > 0·99 is almost perfect 40. The standard error of measurement (SEM) quantifies the measurement error as it relates to the extent that measurements vary with repeated testing without any underlying changes to the individual 41.
Values obtained using the non‐invasive biophysiological technologies were continuous variables. The TBPI and PPG for venous blood flow values were ranked according to clinical best practice and normally reported values 26, 42. Differences for TBPI and PPG for venous blood flow repeat values were investigated using the Wilcoxon pair signed‐rank test. The Wilcoxon signed‐rank test is a non‐parametric test used to determine the median difference between paired observations of a single population 43. The level of statistical significance was set at the 95% confidence interval (CI) with P values > 0·05 indicating no difference between test–retest values. A Bland–Altman assessment for agreement compared test–retest measurements with the range of agreement defined as the mean bias ±2 standard deviations (95% CI). All statistical analyses were performed using SPSS (Version 22.0) 44.
Results
Thirty‐one residents (9 males and 22 females), aged between 74 and 95 years (Median = 88·3, SD = 4·23) agreed to participate into this test–retest reliability pilot study. All measurements were performed within the privacy of the resident's room with the ambient temperature and relative humidity recorded prior to completing the assessments. The mean test–retest results for room temperature and relative humidity are reported in Table 1.
Table 1.
Mean room temperature and relative humidity
| Room temperature and relative humidity | Test: x̄ (SD) | Retest: x̄ (SD) |
|---|---|---|
| Temperature | 23·1°C (1·2) | 22·9°C (1·1) |
| Relative humidity | 50·3% (4·7) | 49·9% (4·9) |
x̄ = mean; SD = standard deviation.
Baseline resident's characteristics are detailed in Table 2 including residents' age, sex, Fitzpatrick skin type, history of skin tears, medications and underlying comorbidities. Skin temperature was recorded at each test site prior to the assessments. The mean skin temperature remained relatively constant over both test periods with results ranging from 31·2 to 31·9°C for the extremities and 32·6 to 32·8°C for the abdomen. Variations in skin temperature influence the measurement of physiological skin properties 4, 6, 45.
Table 2.
Residents' baseline characteristics as a percentage
| Characteristic | Residents' values % (n = 31) |
|---|---|
| Median age | 88·3 years (inter‐quartile range 84·3–89·2) |
| Gender | |
| Males | 29·0 (9) |
| Females | 71·0 (22) |
| Fitzpatrick skin type | |
| Type 1 | 19·4 (6) |
| Type 2 | 32·3 (10) |
| Type 3 | 45·2 (14) |
| Type 4 | 3·2 (1) |
| Previous history of skin tears | |
| Males | 46·7 (7) |
| Females | 53·3 (8) |
| Body mass index | 27·0 (inter‐quartile range 20·42–33·58) |
| Medications | |
| Corticosteroid medication | 48·4 (15) |
| Anticoagulant therapy | 12·9 (4) |
| Antiplatelet medication | 54·8 (17) |
| Underlying comorbidities | |
| Heart disease | 74·2 (23) |
| Respiratory disease | 12·9 (4) |
| Dementia | 35·5 (11) |
Note. Number of residents' is reported in parentheses.
Non‐invasive skin assessments were well tolerated by residents with no reports of physical discomfort or skin trauma. The intra‐rater reliability using the ICC for TEWL and hydration showed substantial to almost perfect reproducibility across the bilateral extremities. The test‐retest reliability was fair for TEWL and moderate for hydration over the abdomen. The SLEB and skin thickness was almost perfect across all test sites. Skin retraction ranged from moderate to almost perfect across the five sites. The ICC for erythema and the skin intensity score showed moderate to substantial reproducibility across all sites. Test results for melanin and CIEL*a*b* varied across sites with the ICC displaying poor to moderate values as presented in Table 3. Intra‐rater reliability results for assessing sebum were poor to slight for sebum across all sites.
Table 3.
Intra‐class coefficient (ICC), standard error of measurement (SEM), and Lin's concordance correlation coefficient for skin colour parameters
| Skin properties | ICC (95% CI) | SEM of ICC (95% CI) | Lin's CCC |
|---|---|---|---|
|
Melanin* right arm Melanin* left arm Melanin* right leg Melanin* left leg Melanin* abdomen |
0·42 (−0·19, 0·72) 0·51 (−0·02, 0·76) 0·05 (−1·16, 0·49) −0·07 (−1·20, 0·48) −0·31 (−1·70, 0·37) |
6·21 (5·60–6·82) 6·51 (5·87–7·15) 6·37 (5·75–7·00) 6·56 (5·91–7·20) 5·89 (5·31–6·47) |
0·29 0·33 0·01 −0·02 −0·07 |
|
Erythema† right arm Erythema† left arm Erythema† right leg Erythema† left leg Erythema† abdomen |
0·71 (0·41, 0·86) 0·67 (0·33, 0·84) 0·63 (0·23, 0·82) 0·62 (0·22, 0·82) 0·41 (−0·22, 0·71) |
1·85 (1·67–2·03) 2·09 (1·89–2·30) 1·95 (1·76–2·14) 1·88 (1·69–2·06) 1·70 (1·53–1·87) |
0·57 0·54 0·50 0·48 0·30 |
|
CIE L right arm CIE L left arm CIE L right leg CIE L left leg CIE L abdomen |
0·71 (0·39, 0·86) 0·68 (0·36, 0·85) 0·47 (−0·09, 0·74) 0·32 (−0·40, 0·67) 0·35 (−0·34, 0·69) |
3·81 (3·44–4·19) 4·66 (4·20–5·12) 4·60 (4·15–5·05) 5·23 (4·71–5·74) 5·19 (4·68–5·70) |
0·54 0·51 0·30 0·18 0·22 |
|
CIE a right arm CIE a left arm CIE a right leg CIE a left leg CIE a abdomen |
0·17 (−0·72, 0·60) 0·01 (−1·07, 0·51) 0·32 (−0·39, 0·67) 0·31 (−0·43, 0·66) 0·37 (−0·30, 0·70) |
2·84 (2·56–3·12) 2·84 (2·56–3·12) 3·08 (2·78–3·39) 2·91 (2·62–3·19) 3·04 (2·74–3·34) |
0·19 0·09 0·28 0·27 0·28 |
|
CIE b right arm CIE b left arm CIE b right leg CIE b left leg CIE b abdomen |
0·21 (−0·62, 0·69) −0·03 (−1·13·0.50) 0·36 (−0·33, 0·69) 0·57 (0·12, 0·79) 0·19 (−0·67, 0·61) |
2·28 (2·06–2·51) 2·54 (2·29–2·79) 2·73 (2·46–3·00) 2·44 (2·20–2·68) 4·84 (4·36–5·32) |
0·14 0·07 0·28 0·44 0·10 |
|
Sebum†† right arm Sebum†† left arm Sebum†† right leg Sebum†† left leg Sebum†† abdomen |
0·34 (−0·36, 0·68) 0·26 (−0·52, 0·64) 0·63 (0·23, 0·82) 0·04 (−0·97, 0·54) −0·23 (−1·52, 0·41) |
0·40 (0·36–0·44) 0·44 (0·40–0·48) 0·13 (0·11–0·14) 0·0 (0·00–0·00) 0·25 (0·23–0·28) |
1·11 1·22 0·35 0·00 0·70 |
Note. Values reported in: * 100 × log (1/intensity of reflected red light); † 100 × log (intensity of reflected red light/intensity of reflected green light); seconds; ††µm/cm2.
CCC, concordance correlation coefficient.
Intra‐rater reliability
The ICC (including 95% CI), SEM of the ICC and Lin's CCC were calculated for skin colour (erythema, melanin and CIEL*a*b*), TEWL, hydration, pH, ultrasound (SLEB, skin thickness and intensity), elasticity (viscoelasticity, elasticity and retraction) and sebum. Intra‐rater reliability of the multipurpose device using the ICC was almost perfect for TEWL (ρ = 0·8–0·92) across the upper and lower limbs. A Bland–Altman plot for the intra‐rater agreement mean differences of the right dorsal forearm TEWL is presented in Figure 3. The 95% CI of the mean difference for right forearm TEWL, as demonstrated with the Bland–Altman analysis had an acceptable range of approximately ± 2·11 g/m2/hour.
Figure 3.
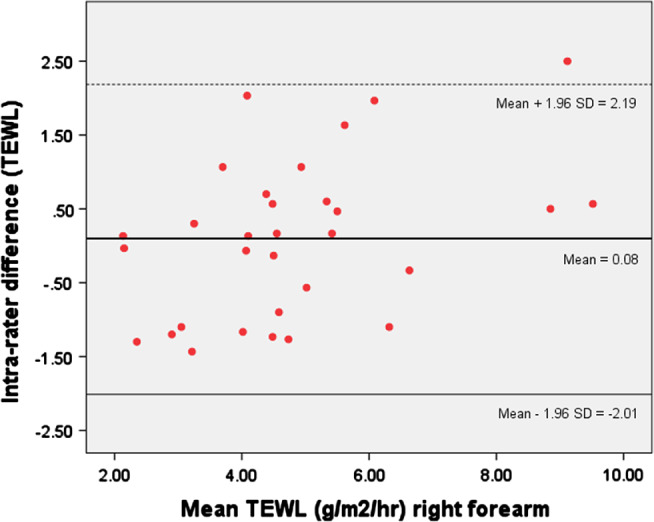
A Bland–Altman plot showing differences between test–retest measurements for TEWL right forearm.
The intra‐rater reliability for elasticity was substantial to almost perfect (ρ = 0·65–0·81) at all sites except the right forearm (ρ = 0·12). The test–retest reliability was almost perfect for SLEB (ρ = 0·95–0·99) and skin thickness (ρ = 0·95–0·99). The ICC was substantial to almost perfect for viscoelasticity (ρ = 0·67–0·91) and pH (ρ = 0·76–0·88) across all sites. Reliability for hydration (ρ = 0·53–0·85) and retraction (ρ = 0·57–0·99) were moderate to almost perfect across all test sites. Test–retest reliability was moderate to substantial for erythema (ρ = 0·41–0·71) and skin intensity score (ρ = 0·59–0·75) across all test sites. Skin colour parameters (melanin and CIEL*a*b*) and sebum measurement results demonstrated varied intra‐rater reliability across all sites as presented in Table 3. Reliability varied from poor to moderate for melanin (P = −0·31–0·51); slight to moderate for CIE a* (ρ = 0·01–0·37); fair to substantial for CIE L* (ρ = 0·32–0·71) and CIE b* (ρ = −0·03–0·57). The intra‐rater reliability scores for assessing sebum was poor to fair across (ρ = −0·23–0·34) across four sites (Table 3).
Results of the comparison between the test–retest TBPI and venous PPG values, according to the Wilcoxon signed‐rank test, indicated no statistically significant median difference (P > 0·05). Table 4 demonstrates the Wilcoxon P values for the right and left TBPI and venous PPG test–retest results.
Table 4.
Wilcoxon signed rank test–retest reliability results for vascular assessments
| Vascular assessment | z‐score (P value) |
|---|---|
|
Right TBPI Left TBPI Right venous PPG Left venous PPG |
−0·33 (0·75) 0·33 (0·74) 1·52 (0·13) 1·57 (0·12) |
Note. z‐score approximates data to the normal distribution.
TBPI, toe brachial pressure index; PPG, photoplethysmography.
Discussion
The pilot study was conducted in preparation for a larger which will investigate skin characteristics associated ageing and skin tears. The pilot study investigated the reliability of using non‐invasive technologies to test–retest the morphological (colour, thickness and elasticity) and physiological (TEWL, hydration, sebum and pH) skin properties and the vascular status (bilateral TBPI and venous reflux) of 31 aged care residents. The agreement between measurements and the reproducibility of pilot study results was established using the ICC and Lin's CCC for quantitative measurements, and the Wilcoxon signed rank test for arterial and venous measurements.
Three commercially available biophysical skin analysis instruments were used to non‐invasively quantify a range of morphological and physiological skin properties. In previous studies the DermaLab Combo® has been reported to be a reliable tool for measuring: scar tissue; radiation fibrosis; skin barrier function; biophysical differences between gender, age and skin location; breast elasticity and thickness; and topical anti‐wrinkle treatments 46, 47, 48, 49, 50, 51, 52. The Sebumeter® has previously been used to quantify sebaceous gland activity across different genders, ethnicities and a range of body sites 53, 54, 55. While the majority of these studies relate to sebaceous gland activity of the forehead, other assessment sites have included the neck, ventral forearm, dorsal hand and back 24, 56. The Skin‐pH‐meter® has been used by other researchers to measure acidity of the skin surface across various age groups, ethnicities and sex 24, 56, 57, 58.
Prior to this pilot study, only one other published research used non‐invasive technologies to objectively quantify skin properties associated with skin tears 3. No previous studies have reported the intra‐rater reliability and test–retest reliability of using non‐invasive technologies for quantifying skin properties, across five test sites, to determine their suitability for evaluating aged skin and clarifying any association with the occurrence of skin tears.
The intra‐rater reliability results of this study, using the multipurpose device was substantial to almost perfect for elasticity at all test sites, except the right forearm. The presence of intradermal oedema and the accumulation of elastotic material in photo‐damaged skin may have altered the reproducibility of elasticity parameters (viscoelasticity, elasticity and retraction). Despite the majority of assessments having been performed in the morning, variation in lower leg dermal oedema may have arisen from one test period to the other from differences between the time of assessments and the amount of time resident's feet were dependent prior to the assessment. The poor reproducibility of the right forearm elasticity results may in part be associated with the inability to precisely locate the initial test site, and elastosis, which arises when degenerated elastic fibres accumulate in the papillary dermis from chronic photoageing that leads to loss of functional elastin 59. In Australia, where motor vehicles are driven on the left‐hand side of the road, the right forearm is subjected to chronic sun exposure and is particularly susceptible to the influences of photoageing from the penetrating effects of ultraviolet radiation 60.
The test–retest reliability was substantial to almost perfect for ultrasound measurements of SLEB and skin thickness across all test sites. The SLEB, a hypoechoic band that forms in the papillary dermis, is associated with photoageing as it primarily occurs in those areas that are chronically exposed to UV radiation 61. The presence of oedema with its diurnal component varies according to the time of the day and season, can reduce skin echogenicity and increase the width of the SLEB as well as the thickness of skin 62, 63. The SLEB readily increases with oedema while dermal echogenicity is inversely correlated with the amount of tissue fluid 64, 65.
The intra‐rater reliability results were substantial to almost perfect for TEWL and hydration across the upper and lower extremities. The measurement of TEWL has been identified as a reliable means for evaluating the epidermal permeability barrier function under basal conditions 66. Conversely, hydration relates to the ability of the stratum corneum to retain water 67. The TEWL and hydration are readily influenced by a range of individual, environmental and assessment factors including the ambient temperature and relative humidity 4, 6. To minimise the impact of these factors, residents were requested to avoid washing or applying moisturisers to the test sites for 24 hours prior to the assessment, as cleansers increase TEWL values and moisturisers inflate hydration results 45. Prior to the assessments and to standardise the procedure, residents were acclimatised to their room with the test site exposed for a minimum of 15 minutes.
The poorer intra‐rater reliability of the TEWL and hydration at the left lower abdominal region compared to the extremities may in part relate to the use of absorbent aids to manage incontinence. Research has shown that where skin is covered with occlusive absorbent products, TEWL increases 68. Preconditioning of the abdominal skin may have cofounded the results with some residents reluctant to expose the test site for the entire duration of the procedure.
Reliability ranged from moderate to substantial for erythema and skin intensity score across all sites. The Skin‐pH‐meter® reliability was substantial to almost perfect for pH across all test sites. The intra‐rater reliability score for casual sebum levels were poor to slight across all test sites due to an inability of the instrument to measure sebum across non‐seborrheic skin surfaces. While there is widespread distribution of mature sebaceous glands across all skin areas, except for the palms and soles, there are fewer sebaceous glands in the extremities and abdomen compared with seborrheic sites such as the forehead, nose, chin, cheek and upper back 69, 70, 71. Despite the density of sebaceous glands remaining constant throughout the life span, the level of sebum secretion declines around 60 years of age 72, 73. The sebum results for this aged care population was therefore not unexpected as the extremities and abdomen are considered non‐seborrheic skin surfaces.
Skin colour parameters (erythema, melanin and CIEL*a*b*) varied across test sites. The reliability ranged from poor to moderate for melanin; slight to moderate for CIE a*; and fair to substantial for CIE L*and CIE b*. The inability to obtain a good level of agreement for colour may be a consequence of: sensitivity of the spectrophotometry; relatively small size of the probe head that limits analysis of surface area; imprecision identifying the initial test site at the mid‐dorsal forearm; lack of colour uniformity at test site from photoageing and resulted pigmented changes; or fluctuations in vascular perfusion that alter the degree of erythema 74, 75. Measurements of skin colour are generally undertaken on the volar aspect of the forearm to minimise the impact of photoageing 76 and the concomitant effect of mottled pigmentation 77. Stress or anxiety and the resultant increased blood flow from vasodilation can alter both erythema and CIE a* values. Conversely, vasoconstriction and local ischemia occurs where contact of the colour probe applies excessive pressure to the skin 75. Likewise, fluctuations in the ambient room temperature, relative humidity, air movement and lighting can vary results 78. While direct lighting was avoided and the time of repeat assessments were similar to the initial test, the ambient temperature and relative humidity of individual resident's rooms could not be regulated by the investigator. Nevertheless, baseline and retest room temperature and relative were relatively consistent, and readings fell within recommended limits 4, 45. The poor reproducibility of CIEL*a*b colour space may have resulted from metamerism, where repeat measurements of the test site exhibited colour changes under varying individual, environmental or assessment conditions 79.
The SEM estimates the standard deviation of errors of measurement with the magnitude of the error contingent upon the precise value of the variables measured 80. High SEM values were obtained for hydration (16·32–44·35), SLEB (13·53–28·50), skin thickness (33·51–77·90) and skin intensity (7·18–12·23) score result in wide CI measures. Hydration values were reported in micro‐Siemens (μS) while ultrasound (SLEB and skin thickness) values were measured in micrometres. The SEM for retraction values, which were initially reported in milliseconds (104·20–1663·07), was converted to seconds (0·10–1·66) for clinical interpretation. The SEM for pH (0·17–0·26) and viscoelasticity (0·24–0·87), measured respectively in mg/cm2 and MPa, were lower and therefore displayed narrower CI values.
There was no statistically significant median difference (P = > 0·05) between the test–retest measurements for TBPI and venous PPG according to the Wilcoxon signed‐rank test. The P values for the Wilcoxon signed rank test for venous PPG of the lower legs were smaller than the values obtained for the TBPI. The variation in P values may be associated with the individual factors as 55% (n = 17) of residents had a reported diagnosis of osteoarthritis that potentially reduced their reproducibility to dorsiflex their ankle and perform the procedure. The literature report the utility of PPG to investigate venous insufficiency is hampered amongst individuals with fixed or restricted joint movement 81.
A major strength of this study lies in the in vivo evaluation of non‐invasive technologies to quantify morphological and physiological skin properties of ageing skin according to international guidance for standardising individual, environmental and assessment conditions. The test–retest methodology research technique provided additional strength for determining the reliability of measurements by quantifying the intra‐rater reliability. The need for research to demonstrate reproducibility and consistency of clinical measurements is well‐documented 82.
Conclusion
The use of non‐invasive technologies in this test–retest reliability pilot study provided an objective and reliable means for quantifying morphological and physiological skin properties. The inability of the investigator to precisely remeasure colour across sun exposed, non‐uniformed highly pigmented, upper and lower extremities of ageing skin resulted in poor reproducibility. The decline in sebum secretion in this study population aged, 74–95 years and the inability of the device to measure sebum across non‐seborrheic skin sites also lead to poor reproducibility. The measurement of TEWL, elasticity, hydration, pH and skin thickness, however, is worth examining further in relation to skin tears. The results from this study demonstrate that non‐invasive technologies provide a safe, reliable and objective means for quantifying ageing skin properties and provide a suitable option for future research into the exploration of skin tears in this age group.
Acknowledgements
The authors wish to acknowledge the support and contributions of the following people: The Staff, Management and Residents of The Bethanie Group Inc., including Ms Amy Steers, Research & Report Coordinator and Ms Cate Maguire, Project Office. R. Rayner is a recipient of a 2013 Australian Postgraduate Award, Curtin University Postgraduate Scholarship and a Wound Management Cooperative Research Centre (CRC) PhD stipend. The authors also acknowledge the support of the Australian Government's Cooperative Research Centres Program. The authors have no conflicts of interest.
Footnotes
*Correction added on 20 February 2017, after first online publication: a sentence has been deleted and the previous sentence added in its place.
References
- 1. Rayner R, Carville K, Leslie G, Roberts P. A review of patient and skin characteristics associated with skin tears. J Wound Care 2015;24:406–14. [DOI] [PubMed] [Google Scholar]
- 2. Lewin GF, Newall N, Alan JJ, Carville KJ, Santamaria NM, Roberts PA. Identification of risk factors associated with the development of skin tears in hospitalised older persons: a case–control study. Int Wound J 2015:Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koyano Y, Nakagami G, Iizaka S, Minematsu T, Noguchi H, Tamai N, Mugita Y, Kitamura A, Tabata K, Abe M, Murayama R, Sugama J, Sanada H. Exploring the prevalence of skin tears and skin properties related to skin tears in elderly patients at a long‐term medical facility in Japan. Int Wound J 2014;13:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990;22:164–78. [DOI] [PubMed] [Google Scholar]
- 5. Piérard GE. EEMCO guidance for the assessment of skin colour. J Eur Acad Dermatol Venereol 1998;10:1–11. [DOI] [PubMed] [Google Scholar]
- 6. du Plessis J, Stefaniak A, Eloff F, John S, Agner T, Chou T‐C, et al. International guidelines for the in vivo assessment of skin properties in non‐clinical settings: Part 2. Transepidermal water loss and skin hydration. Skin Res Technol 2013;19:265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefaniak AB, Plessis JD, John SM, Eloff F, Agner T, Chou T‐C, et al. International guidelines for the in vivo assessment of skin properties in non‐clinical settings: Part 1. pH. Skin Res Technol 2013;19:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Medical Association . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 9. Australian Government National Health and Medical Research Council . Australian code for the responsible conduct of research 2007. URL http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/r39.pdf.
- 10. Dadzie OE. Defining ethnic dermatology: Challenges, limitations, and merits. In: Dadzie OE, Petit A, Alexis AF, editors. Ethnic dermatology: principles and practice. West Sussex: Wiley‐Blackwell, 2013:1–4. [Google Scholar]
- 11. Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging. Part 2 of a 2‐part review: clinical vascular disease in the elderly. Circulation 2011;123:1900–10. [DOI] [PubMed] [Google Scholar]
- 12. Grove GL, Zerweck C. Hardware and measuring principles: the computerized DermaLab transepidermal water loss probe. In: Fluhr J, Elsner P, Berardesca E, Maibach HI, editors. Bioengineering of the skin: water and the stratum corneum. Boca Raton: CRC Press LLC, 2005:275–86. [Google Scholar]
- 13. Cortex Techology . DermaLab® series skinlab combo. Instruction manual2012:[1–41 pp.]. URL http://www.deernz.org/sites/dinz/files/Instruction%20Manual%20SkinLab%20Z5010108%20UK.pdf.
- 14. Verhaegen PDHM, van der Wal MBA, Middelkoop E, van Zuijlen PPM. Scar assessment. In: Kamolz L‐P, Jeschke MG, Horch RE, Küntscher M, Brychta P, editors. Handbook of burns: reconstruction and rehabililitation. Vol. 2. New York: Springer‐Verlag/Wien, 2012:69–89. [Google Scholar]
- 15. Weatherall IL, Coombs BD. Skin color measurements in terms of CIELAB color space values. J Invest Dermatol 1992;99:468–73. [DOI] [PubMed] [Google Scholar]
- 16. Kaur A, Kranthi B. Comparison between YCbCr color space and CIELab color space for skin color segmentation. Int J Appl Inform Syst 2012;3:30–3. [Internet]. URL http://www.ijais.org/. [Google Scholar]
- 17. Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res 2012;21:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pellacani G, Giusti F, Seidenari S. Ultrasound assessment of skin aging. In: Serup J, Jemec G, Grove G, editors. Handbook of non‐invasive methods and the skin, 2nd edn. Boca Raton: CRC Press LLC, 2006:511–14. [Google Scholar]
- 19. Sandby‐Møller J, Wulf HC. Ultrasonographic subepidermal low‐echogenic band, dependence of age and body site. Skin Res Technol 2004;10:57–63. [DOI] [PubMed] [Google Scholar]
- 20. Jasaitiene D, Valiukeviciene S, Linkeviciute G, Raisutis R, Jasiuniene E, Kazys R. Principles of high‐frequency ultrasonography for investigation of skin pathology. J Eur Acad Dermatol Venereol 2011;25:375–82. [DOI] [PubMed] [Google Scholar]
- 21. Grove GL, Damia J, Grove MJ, Zerweck C. Suction chamber method for measurement of skin mechanics: the DermaLab. In: Serup J, Jemec G, Grove G, editors. Handbook of non‐invasive methods and the skin, 2nd edn. Boca Raton: CRC Press, 2006:593–9. [Google Scholar]
- 22. Piérard GE, Piérard‐Franchimont C, Marks R, Paye M, Rogiers V. EEMCO guidance for the in vivo assessment of skin greasiness. Skin Pharmacol Physiol 2000;13:372–89. [DOI] [PubMed] [Google Scholar]
- 23. Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydratation, sebum content and skin surface pH. Int J Cosmet Sci 2013;35:477–83. [DOI] [PubMed] [Google Scholar]
- 24. Gerhardt L‐C, Lenz A, Spencer ND, Münzer T, Derler S. Skin‐textile friction and skin elasticity in young and aged persons. Skin Res Technol 2009;15:288–98. [DOI] [PubMed] [Google Scholar]
- 25. Park SC, Choi CY, Ha YI, Yang HE. Utility of toe‐brachial index for diagnosis of peripheral artery disease. Arch Plast Surg 2012;39:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wound Ostomy and Continence Nurses Society . Toe brachial index: best practice for clinicians. Mt. Laurel: Wound Ostomy and Continence Nurses Society, 2008. p. 1–7. [Google Scholar]
- 27. Kelechi TJ, McNeil RB. A pilot study of venous photoplethysmography screening of patients with chronic venous disorders. Appl Nurs Res 2010;23:178–83. [DOI] [PubMed] [Google Scholar]
- 28. Hadeco® . Operating manual: Probe assemblies. In: Photoplethysmography (PPG) and pneumoplethysmography (PV). Models PG‐21, PV‐20, PGV‐20. Kawasaki: Hadeco, Inc., 2007:1–24. [Google Scholar]
- 29. Payne R, Martin M. The epidemiology and management of skin tears in older adults. Ostomy Wound Manage 1990;26:26–37. [PubMed] [Google Scholar]
- 30. LeBlanc KA, Christensen D, Cook J, Culhane B, Gutierrez O. Prevalence of skin tears in a long‐term care facility. J Wound Ostomy Continence Nurs 2013;40:1–5. [DOI] [PubMed] [Google Scholar]
- 31. Malone ML, Rozario N, Gavinski M, Goodwin J. The epidemiology of skin tears in the institutionalized elderly. J Am Geriatr Soc 1991;39:591–5. [DOI] [PubMed] [Google Scholar]
- 32. Ihnatsenka B, Boezaart A. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg 2010;4:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piérard GE, Lapiere CM. Physiopathological variations in the mechanical properties of skin. Arch Dermatol Res 1977;260:231–9. [DOI] [PubMed] [Google Scholar]
- 34. Serup J. Hardware and measuring principles: the Dermalab. In: Bioengineering of the skin: skin biomechanics. Linköping: Linköpings Universitet, 2002:117–21. [Google Scholar]
- 35. Malm M, Samman M, Serup J. In vivo skin elasticity of 22 anatomical sites. Skin Res Technol 1995;1:61–7. [DOI] [PubMed] [Google Scholar]
- 36. Gwet KL. Intrarater reliability. In: D'Agostino MA, Sullivan L, Massaro J, editors. Wiley encyclopedia of clinical trials. Hoboken: John Wiley & Co, 2008:1–14. [Google Scholar]
- 37. Lin LIK. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45:255–68. [PubMed] [Google Scholar]
- 38. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 39. Chen C‐C, Barnhart HX. Comparison of ICC and CCC for assessing agreement for data without and with replications. Comput Stat Data Anal 2008;53:554–64. [Google Scholar]
- 40. McBride GB. A proposal for strength‐of‐agreement criteria for Lin's concordance correlation coefficient. NIWA Client Report: HAM2005‐062. 2005. URL http://www.medcalc.org/download/pdf/McBride2005.pdf.
- 41. Harvill LM. Standard error of measurement. Educa Meas 1991;10:33–41. [Google Scholar]
- 42. Kelechi TJ, Bonham P. Measuring venous insufficiency objectively in the clinical setting. J Vasc Nurs 2008;XXVI:67–73. [DOI] [PubMed] [Google Scholar]
- 43. Rey D, Neuhäuser M. Wilcoxon‐signed‐rank test. In: Lovric M, editor. International encyclopedia of statistical science. Berlin and Heidelberg: Springer, 2014:1658–9. [Google Scholar]
- 44. IBM Corporation Released. IBM SPSS Statistics for Windows, Version 22.0. Armonk: IBM Corp, 2013. [Google Scholar]
- 45. Rogiers V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Physiol 2001;14:117–28. [DOI] [PubMed] [Google Scholar]
- 46. Waring M, Bielfeldt S, Matzold K, Wilhelm K, Butcher M. An evaluation of the skin stripping of wound dressing adhesives. J Wound Care 2011;20:421–2. [DOI] [PubMed] [Google Scholar]
- 47. Anthonissen M, Daly D, Fieuws S, Massagé P, Van Brussel M, Vranckx J, et al. Measurement of elasticity and transepidermal water loss rate of burn scars with the Dermalab® . Burns 2012;39:420–8. [DOI] [PubMed] [Google Scholar]
- 48. Nguyen N‐TA, Roberge D, Freeman CR, Wong C, Hines J, Turcotte RE. Skin elasticity as a measure of radiation fibrosis: is it reproducible and does it correlate with patient and physician‐reported measures? Technol Cancer Res Treat Express 2013;1:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sutradhar A, Miller MJ. In vivo measurement of breast skin elasticity and breast skin thickness. Skin Res Technol 2013;19:e191–9. [DOI] [PubMed] [Google Scholar]
- 50. Firooz A, Sadr B, Babakoohi S, Sarraf‐Yazdy M, Fanian F, Kazerouni‐Timsar A, et al. Variation of biophysical parameters of the skin with age, gender, and body region. Scientific World Journal 2012;386936:1–5. doi: 10.1100/2012/386936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gankande T, Duke J, Danielsen P, DeJong H, Wood F, Wallace H. Reliability of scar assessments performed with an integrated skin testing device – the DermaLab Combo® . Burns 2014;40:1521–9. [DOI] [PubMed] [Google Scholar]
- 52. Calabrò G, De Vita V, Patalano A, Mazzella C, Lo Conte V, Antropoli C. Confirmed efficacy of topical nifedipine in the treatment of facial wrinkles. J Dermatolog Treat 2014;25:319–25. [DOI] [PubMed] [Google Scholar]
- 53. Kleesz P, Darlenski R, Fluhr JW. Full‐body skin mapping for six biophysical parameters: baseline values at 16 anatomical sites in 125 human subjects. Skin Pharmacol Physiol 2012;25:25–33. [DOI] [PubMed] [Google Scholar]
- 54. Kim E, Cho G, Won NG, Cho J. Age‐related changes in skin bio‐mechanical properties: the neck skin compared with the cheek and forearm skin in Korean females. Skin Res Technol 2013;19:236–41. [DOI] [PubMed] [Google Scholar]
- 55. Mizukoshi K, Akamatsu H. The investigation of the skin characteristics of males focusing on gender differences, skin perception, and skin care habits. Skin Res Technol 2013;19:91–9. [DOI] [PubMed] [Google Scholar]
- 56. Yao L, Li Y, Gohel MDI, Chung WJ. The effects of pajama fabrics' water absorption properties on the stratum corneum under mildly cold conditions. J Am Acad Dermatol 2011;64:e29–36. [DOI] [PubMed] [Google Scholar]
- 57. Luebberding S, Krueger N, Kerscher M. Age‐related changes in skin barrier function – quantitative evaluation of 150 female subjects. Int J Cosmet Sci 2013;35:183–90. [DOI] [PubMed] [Google Scholar]
- 58. Luebberding S, Krueger N, Kerscher M. Age‐related changes in male skin: quantitative evaluation of one hundred and fifty male subjects. Skin Pharmacol Physiol 2014;27:9–17. [DOI] [PubMed] [Google Scholar]
- 59. Mironov S. Biochemical and molecular mechanisms and manifestations of connective tissue aging. In: Omelyanenko N, Slutsky L, Mironov S, editors. Connective tissue histophysiology, biochemistry, modecular biology. Boca Raton: CRC Press LLC, 2013:199–206. [Google Scholar]
- 60. Lewis KG, Bercovitch L, Dill SW, Robinson‐Bostom L. Acquired disorders of elastic tissue: Part I. Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol 2004;51:1–21. [DOI] [PubMed] [Google Scholar]
- 61. Gniadecka M, Gniadecki R, Serup J, Søndergaard J. Ultrasound structure and digital image analysis of the subepidermal low echogenic band in aged human skin: diurnal changes and interindividual variability. J Invest Dermatol 1994;102:362–5. [DOI] [PubMed] [Google Scholar]
- 62. El Gammal S, El Gammal C, Altmeyer P, Vogt M. Sonography of the skin. In: Berardesca E, Maibach HI, Wilhelm K‐P, editors. Non invasive diagnostic techniques in clinical dermatology. Berlin and Heidelberg: Springer, 2014:135–56. [Google Scholar]
- 63. Gniadecka M. Effects of ageing on dermal echogenicity. Skin Res Technol 2001;7:204–7. [DOI] [PubMed] [Google Scholar]
- 64. Seidenari S, Pellacani G. Ultrasound and water in the stratum corneum. In: Fluhr J, Elsner P, Berardesca E, Maibach HI, editors. Bioengineering of the skin: water and the stratum corneum, 2nd edn. Boca Raton: CRC Press LLC, 2005:77–82. [Google Scholar]
- 65. Serup J, Keiding J, Fullerton A, Gniadecka M, Gniadecki R. High‐frequency ultrasound examination of skin: introduction and guide. In: Serup J, Jemec G, Grove G, editors. Handbook of non‐invasive methods and the skin, 2nd edn. Boca Raton: CRC Press LLC, 2006:473–91. [Google Scholar]
- 66. Fluhr J, Darlenski R. Transepidermal water loss (TEWL). In: Berardesca E, Maibach HI, Wilhelm K‐P, editors. Non invasive diagnostic techniques in clinical dermatology. Berlin and Heidelberg: Springer, 2014:353–6. [Google Scholar]
- 67. Verdier‐Sevrain S, Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol 2007;6:75–82. [DOI] [PubMed] [Google Scholar]
- 68. Zimmerer RE, Lawson KD, Calvert CJ. The effects of wearing diapers on skin. Pediatr Dermatol 1986;3:95–101. [DOI] [PubMed] [Google Scholar]
- 69. Piérard‐Franchimont C, Quatresooz P, Piérard GE. Sebum production. In: Farage MA, Miller KW, Maibach HI, editors. Textbook of aging skin. Berlin and Heidelberg: Springer‐Verlag, 2010:343–52. [Google Scholar]
- 70. Sheu H‐M, Chao S‐C, Wong T‐W, Yu‐Yun LEE, Tsai J‐C. Human skin surface lipid film: an ultrastructural study and interaction with corneocytes and intercellular lipid lamellae of the stratum corneum. Br J Dermatol 1999;140:385–91. [DOI] [PubMed] [Google Scholar]
- 71. Rode B, Ivens U, Serup J. Degreasing method for the seborrheic areas with respect to regaining sebum excretion rate to casual level. Skin Res Technol 2000;6:92–7. [DOI] [PubMed] [Google Scholar]
- 72. Jacobsen E, Billings JK, Frantz RA, Kinney CK, Stewart ME, Downing DT. Age‐related changes in sebaceous wax ester secretion rates in men and women. J Invest Dermatol 1985;85:483–5. [DOI] [PubMed] [Google Scholar]
- 73. Pochi PE, Strauss JS. Endocrinologic control of the development and activity of the human sebaceous gland. J Invest Dermatol 1974;62:191–201. [DOI] [PubMed] [Google Scholar]
- 74. American Society for Testing Materials . Standard practice for obtaining spectrophotometric data for object‐color evaluation. ASTM E1164. Annual book of ASTM standards. West Conshohocken: American Society for Testing and Materials, 2009. [Google Scholar]
- 75. Balas C. An imaging colorimeter for noncontact tissue color mapping. IEEE Trans Biomed Eng 1997;44:468–74. [DOI] [PubMed] [Google Scholar]
- 76. Wee AG, Beatty MW, Gozalo‐Diaz DJ, Kim‐Pusateri S, Marx DB. Proposed shade guide for human facial skin and lip: a pilot study. J Prosthet Dent 2013;110:82–9. [DOI] [PubMed] [Google Scholar]
- 77. Bolognia JL. Aging skin. Am J Med 1995;98:S99–103. [DOI] [PubMed] [Google Scholar]
- 78. Taylor H. Instruments for measuring skin toxicity. In: Chilcott R, Price S, editors. Principles and practice of skin toxicology. West Sussex: John Wiley & Sons, Ltd, 2008:201–20. [Google Scholar]
- 79. Marcus RT. Colorimetry. In: Webster JG, editor. The measurement, instrumentation and sensors handbook. Boca Raton: CRC Press LLC, 1999:70‐58. [Google Scholar]
- 80. Cerin E. Standard error of measurement. In: Michalos A, editor. Encyclopedia of quality of life and well‐being research. Dordrecht: Springer, 2014:6318–19. [Google Scholar]
- 81. Lazarides M, Giannoukas A. The role of hemodynamic measurements in the management of venous and ischemic ulcers. Int J Low Extrem Wounds 2007;6:254–61. [DOI] [PubMed] [Google Scholar]
- 82. Kottner J, Audigé L, Brorson S, Donner A, Gajewski BJ, Hróbjartsson A, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol 2011;64:96–106. [DOI] [PubMed] [Google Scholar]


