Abstract
The increasing occurrence of hospital‐acquired infections and the emerging problems posed by antibiotic‐resistant microbial strains have both contributed to the escalating cost of treatment. The presence of infection at the wound site can potentially stall the healing process at the inflammatory stage, leading to the development of a chronic wound. Traditional wound treatment regimes can no longer cope with the complications posed by antibiotic‐resistant strains; hence, there is a need to explore the use of alternative antimicrobial agents. Pre‐antibiotic compounds, including heavy metal ions and essential oils, have been re‐investigated for their potential use as effective antimicrobial agents. Essential oils have potent antimicrobial, antifungal, antiviral, anti‐inflammatory, antioxidant and other beneficial therapeutic properties. Similarly, heavy metal ions have also been used as disinfecting agents because of their broad spectrum activities. Both of these alternative antimicrobials interact with many different intracellular components, thereby resulting in the disruption of vital cell functions and eventually cell death. This review will discuss the application of essential oils and heavy metal ions, particularly tea tree oil and silver ions, as alternative antimicrobial agents for the treatment of chronic, infected wounds.
Keywords: Antimicrobial, Silver, Tea tree oil, Wound infection
Introduction
The use of metal ions and essential oils as antimicrobial agents is of particular interest in topical wound management. Management of chronic wounds, such as varicose skin ulcers and burns, aims to induce rapid healing and minimise the extent of scarring. Infection of such wounds can not only delay the healing process but also lead to the development of a chronic wound with increased potential to develop into a systemic infection. Topical application of antimicrobial agents is common since effective concentrations may be difficult to achieve with systemic drugs as the effects of the wound trauma may impede delivery of the agent into the wound 1, 2. The reduced concentration may also create selective pressure for antibiotic resistance.
The current problems posed by increasing antibiotic resistance in Gram‐positive bacteria (methicillin‐resistant Staphylococcus aureus, MRSA, Vancomycin‐resistant enterococci, VRE) and Gram‐negative bacteria (New Delhi metallo‐β‐lactamase‐1 (NDM‐1) positive Escherichia coli, multidrug‐resistant Acinetobacter baumannii, ciprofloxacin‐resistant Pseudomonas aeruginosa) 3, 4 have renewed interest in pre‐antibiotic antibacterial agents, such as metal ions and essential oil [tea tree oil (TTO)] 5, 6. Alternative, non‐antibiotic‐based treatments are attractive due to their decreased side effects compared with synthetic drugs and their multiple target sites within the microorganisms, which may contribute to reduced development of resistant strains 7. Despite their usefulness and long history of use, these agents are mainly restricted to topical applications against infected wounds, skin burns, ulcers and fungal infections of the skin. Despite their effectiveness in treating topical infections, the complete healing of wounds, especially slow/non‐healing wounds, may require repeated application or use of high concentrations that may have adverse effects on the patient. Current developments in wound treatment focus on approaches that will allow reduced concentration (using combined agents) or delivery via controlled release delivery systems to minimise potential side effects whilst maintaining bioavailability to achieving therapeutic effects 8, 9, 10, 11.
The useful properties of alternative antimicrobial agents, together with advances in drug‐delivery technologies, may be able to enhance and expand the medical applications of these agents 9, 10, 12. Combining these alternative antimicrobial agents with advanced drug‐delivery systems aims to:
Promote bioavailability of agent at microbiocidal concentrations.
Reduce drug concentration to enhance safety and practicality of application.
Minimise scarring and promote wound‐healing processes.
Reduce discomfort and pain in consideration of the patient's psychological needs.
Decrease the frequency of dressing changes.
These aims would increase convenience, provide less opportunity for infection and/or reinfection of the wounds and ultimately reduce treatment costs.
Microorganisms and wound management
In healthy individuals, the skin supports a natural microflora comprising a balanced community of microorganisms, which rarely cause infection. However, a disturbance to the normal ratio of microflora or an exposure of subcutaneous tissue due to trauma may result in the pathogenic invasion by these microorganisms 7. Colonisation of wounds by these opportunistic pathogens is usually polymicrobial 12. The diversity and proliferation of the pathogens is influenced by various factors including the type, depth and location of the trauma as well as the host immune system response 7. The presence of microorganisms at a wound site does not confirm infection 13. Infection only occurs when the host immune system can no longer cope with the virulence factors expressed by the colonising microorganisms, thus triggering a series of systemic responses that delay the healing process 7, 13.
The increasing occurrence of hospital infections and widespread emergence of resistant microorganisms contribute to escalating treatment costs. In addition, hypersensitivity reactions to antibiotics and the lack of access to new treatments within the health care industry makes the provision of sufficient support and care for patients difficult. Modern lifestyles that frequently lack physical activity increase the possibility of developing various life‐long (interconnected) health conditions, for example, diabetes, obesity and hypertension in old age. These underlying health conditions may influence the complexity and severity of wound healing. In addition, the growing size and longevity of the elderly population has increased the prevalence of wounds associated with these conditions, including slow/non‐healing ulcers. The improvement in medical facilities has increased the number of patients surviving from complicated wounds, such as those caused by burns. Although rates of survival have improved, severely burned patients usually require extended stays in hospital, suffer from lowered immunity and extensive loss of skin. Such patients are prone to infection by both common wound pathogens as well as antibiotic‐resistant microorganisms, which may further complicate the treatment regime 1.
The nature of burn wounds, and varicose ulcers in particular, may involve relatively lengthy treatment with antibiotics, which carries the attendant risk of selecting drug‐resistant bacteria. Various approaches have been conducted to find the best method to treat and overcome this problem. The increasing incidence and broadening spectrum of pathogens resistant to antibiotics has refocused scientific interest on the use of alternative antimicrobial compounds 6, 14. Alternative, non‐antibiotic‐based treatments are attractive because of their decreased side effects compared with synthetic drugs and their multiple target sites within the microorganisms, which may contribute to reduced development of resistant strains 7. Despite their usefulness and long history of use, these agents are mainly restricted to topical applications against infected wounds, skin burns, ulcers and fungal infections of the skin.
Microorganisms and the wound environment
In healthy individuals, the skin supports a natural microflora comprising a balanced community of microorganisms, which rarely cause infection. However, a disturbance to the normal ratio of microflora or an exposure of subcutaneous tissue because of trauma may result in pathogenic polymicrobial invasion by these microorganisms 7. The diversity and proliferation of the pathogens is influenced by various factors, including the type, depth and location of the trauma as well as the host immune system response 7. The presence of microorganisms at a wound site does not confirm infection 13. Infection only occurs when the host immune system can no longer cope with the virulence factors expressed by the colonising microorganisms, thus triggering a series of systemic responses that delay the healing process 7, 13. Besides patient microflora, other sources of infection include those acquired directly or indirectly via air, other infected patients, health care workers, contaminated medical devices, hospital environment and external sources, such as visitors 15.
Wound healing mechanism
Wound healing is a complex cascading event involving cellular, enzymatic and biochemical pathways, spanning from the time the skin is damaged until the wound is completely healed 16, 17. Maintenance of healthy wound site homeostasis is thus important to maximise wound healing capacity, especially in immuno‐compromised patients or those with underlying chronic health conditions. The events in wound healing have been divided into four main stages 10, 18, 19:
Exudative – Haemostasis and clot formations occur, release of inflammatory mediators including cytokines, chemokines and growth factors
Resorptive – Inflammation occurs, signs of redness, swelling, heat, pain and possible loss of function, immune system reacts by sending neutrophils and macrophages into the wound cite to remove any foreign body (e.g. bacteria) and inflammatory debris to facilitate conditions for angiogenesis
Proliferative – Wound closure is initiated by the migration of epithelial cells from the wound edge, angiogenesis occurs, simultaneously, a provisional matrix is formed and granulation tissue is also deposited
Regenerative – Re‐epithelialisation occurs to allow full wound closure, development of scar, tissue remodelling occurs to re‐establish functionality
In general, wound healing follows this sequence but, being non‐linear, the stages may move forwards and backwards, based on intrinsic and extrinsic factors as well as the severity of the wound 19. The rate of wound healing differs between individuals and is influenced by factors such as type and depth of wound, age, nutrition, immune status, underlying health conditions as well as local microbial burden.
Microbial infection in particular retards wound healing by increasing the bio‐burden at wound sites, thus stalling the normal process at the inflammatory phase. It is common for chronic wounds to become contaminated and colonised by microorganisms. Such colonisation by microorganisms, especially common skin commensals such as Staphylococcus epidermidis and Corynebacterium species, does not usually impair wound healing, provided the population remains below the critical threshold of approximately 105 cells per gram of wound tissue 20. Their presence and production of proteolytic enzymes may help to stimulate the activity of neutrophils, thereby encouraging an inflammatory response which may facilitate wound healing 21. Critical colonisation is defined as a condition in which bacterial infection exceeds the ability of the host's immune system to eliminate further proliferation and colonisation (20).
Cutaneous wounds offer a favourable (moist, warm, nutritious) environment to support bacterial growth and proliferation. Heavy microbial infection (above critical colonisation) retards wound healing by increasing the bio‐burden at wound sites, which stalls the normal process at the inflammatory phase. When acute wounds become infected and reach critical colonisation by pathogenic microorganisms, the stimulated pro‐inflammatory environment (due to microbial production of toxins, proteases or pro‐inflammatory molecules) will stop the process of wound healing, and the site develops into a chronic wound 7, 10, 12, 20.
Alternative antimicrobial agents
Although conventional antibiotics are regarded as effective antimicrobial agents, there is concern about their side effects and the increasing incidence of microbial resistance to them 22. Antimicrobial agents are only effective until resistant strains of the target microorganisms begin to emerge 6, 23. With conventional antibiotics, the emergence of resistance is mainly because of their action against a single target. This has led to re‐examination of the use of other antimicrobial agents, such as metal ions and plant extracts, which often attack multiple target sites 6, 22, 24, 25.
The application of essential oils to reduce bacterial growth and prevent decay is not a new idea. Plants synthesise aromatic secondary metabolites to protect against predation and prevent colonisation by plant pathogens 24. These aromatic compounds are divided into classes, including essential oils (primarily phenolics and/or terpenoids), alkaloids, lectins, polypeptides and polyacetylenes, all of which have different mechanisms of antimicrobial activity 24. Some examples of essential oils or plant extracts commonly used for their antimicrobial properties are TTO, ylang ylang, betel pepper, manuka, eucalyptus, arnica, lemon verbena, rosemary, green tea extract and cadendula 23, 24. Although extensively practiced since ancient times, the use of natural extracts from plants as antimicrobial compounds declined after the development of synthetic antibiotics.
Metals (especially heavy metals) were used as disinfecting agents since ancient times. Silver, copper and gold, for example, have been used to treat diseases, disinfect wounds and water. Examples of metals commonly used in these applications include zinc, iron, bismuth, cobalt, magnesium, titanium, copper and the more extensively used heavy metal, silver. Despite having useful properties, treatment using metals is limited because excessive concentrations of metals, especially heavy metals, are toxic to human cells. Amongst the heavy metals, silver has a long history of use as an antimicrobial agent because of its relatively lower toxicity to human cells 26, 27. Recently, in response to issues surrounding antibiotic resistance, topical application of silver compounds has increased in popularity 28.
Plant products as antimicrobial agent
Plants with medicinal properties have been used for the treatment of various diseases both in traditional and modern medicine. When faced with microbial invasion or attack leading to infection, plants have their own defence mechanisms, which rely on the production of compounds that interfere with the cellular and intracellular structure of microorganisms 29. These antimicrobial plant compounds that show effective antimicrobial activity are often secondary metabolites formed in aromatic plants. These aromatic compounds give the plants their characteristic strong odour 29 that can repel insects or herbivores. In addition, certain compounds give plants pigment or flavour; these may be irritants to other organisms and may flow out from injured plants to prevent colonisation by microorganisms 30, 31. These secondary metabolites are mainly present as volatile compounds such as phenols or their oxygen‐substituted derivatives, which are categorised into five major classes detailed in Table 1 24. All plant organs, including buds, flowers, leaves, stems, seeds, fruits, twigs and branches, are able to synthesise these compounds.
Table 1.
Summary of the major classes of active antimicrobial compounds from plants [summarised from references 24, 29, 39, 99, 100, 101, 102]
| Class and description | Subclass | Example | Mechanism | Structure |
|---|---|---|---|---|
|
Phenolics • Simple phenolic ring structures that may exist in their highest oxidation state. For example, cinnamic and caffeic acid, which are phenylpropene‐derived compounds. • Number and site of hydroxyl group(s) present on the compound may indicate the relative toxicity to microorganisms. • Highly oxidised phenols or those with increased hydroxylation have increased microbial inhibitory effects. • Active against various types of bacteria, viruses and fungi. • May cause iron deprivation, reaction with sulphydryl groups, protein‐related polyamide polymers via non‐specific interaction and hydrogen bonding to inactivate proteins or enzymes in the cell. |
Simple phenol and phenolic acid | Catechol, epicatechin and caffeic acid | Substrate deprivation and membrane disruption |
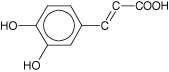
|
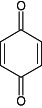
| Quinone | Hypericin | Binds to adhesins, complex with cell wall and inactivate enzyme |
|
|
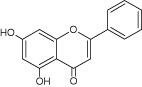
| Flavonoids | Chrysin | Binds to adhesins |
|
|
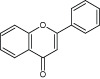
| Flavones | Abyssinone | Complexes with cell wall and inactivates enzymes |
|
|
| Tannins | Ellagitannin | Binds to proteins, adhesins, inhibits enzymes, causes substrate deprivation, complexes with cell wall, membrane disruption and metal ion complexation |
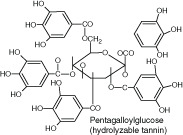
|
|
| Coumarins | Warfarin | Interactions with eukaryotic DNA and has antiviral properties |
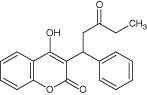
|
|
|
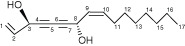
Terpenoids • Actively disrupts various membranes of bacteria, fungi, protozoa and enveloped viruses via its lipophilic properties. |
Monoterpenes | Terpinen‐4‐ol | Membrane disruption |
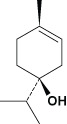
|
|
Alkaloids • Secondary plant metabolites that have antimicrobial activity. |
N/A | Berberine and piperine | Intercalate into cell wall or DNA, differentially inhibit sterol and chitin biosynthesis |
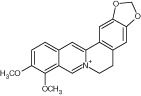
|
|
Polyacetylenes • Has antibacterial, antimycobacterial, anti‐inflammatory, anti‐platelet‐aggregatory effects. |
N/A | Falcarinol | Mechanism unknown |
|
|
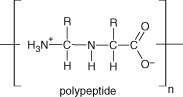
Lectins and polypeptides • Inhibition of membrane function and integrity via interaction with proteins, ion channels and displacement of lipids. • Presence of cholesterol and increasing ionic strength in the target membrane reduces the antimicrobial activity due to stabilisation of the lipid bilayer as well as the weakened electrostatic interaction to encourage binding to target membrane. Note: interaction with membrane peptides or weakening of electrostatic charge interactions required for antimicrobial activity. |
N/A | Mannose‐specific agglutinin, fabatin, defensins and thionins | Inhibit adhesion and fusion interactions between virus and host cell. May form ion channels in microbial membranes or reduce adhesion of microbial proteins to host receptors via competitive inhibition |
|
The amount of active compound present in botanical extracts varies depending on the main adaptation of the plant to its environment, harvesting period, the extraction process, dehydration procedures, purification and storage methods 25. The extraction of secondary metabolites from plants is usually by distillation (water or steam) to produce a volatile essential oil. Essential oils are aromatic compounds synthesised by plants as secondary metabolites and are well known for their antibacterial, antifungal and antiviral properties 6, 29, in addition to anti‐cancer, anti‐diabetic, anti‐inflammatory and antioxidant activities 29, 32. Essential oils are multi‐component compounds, usually with terpenes and their derivatives (terpeniods) as the major components. After extraction, they present as clear, almost colourless volatile liquids, soluble in organic solvents and lipids in addition to some hydrophilic components 29. Due to the versatility and wide‐ranging properties of essential oils, they are used not only in the pharmaceutical industry but also incorporated into the cosmetics, agriculture, sanitation, disinfection, food preservation and manufacturing industries 25, 29. Examples of the various uses of essential oils are summarised in Table 2.
Table 2.
Common essential oils extracted from plants and their medicinal and/or antimicrobial activity
| Common name | Scientific name | Active compound | Activity or therapeutic properties | Application/product | References |
|---|---|---|---|---|---|
| Aloe | Aloe barbadensis, Aloe vera | Anthraquinones (aloin, emodin and resistanol), β‐sitosterol and gibberellins |
• Antimicrobial • Increases wound healing • Induces hypoglycaemic effect • Lowers blood cholesterol |
• Used on burns and wounds • Treatment of psoriasis and genital herpes • Mediate insulin production |
42, 103, 104, 105, 106, 107 |
| Arnica | Arnica montana | Sesquiterpene and lactones | • Anti‐inflammatory |
• Applied to bruises and swelling • Osteoarthritis treatment |
108 |
| Basil | Ocimum basilicum | Linalool, methylchavicol, epi‐α‐cadinol, α‐bergamotene, γ‐cadinene and germacrene D |
• Antimicrobial • Antioxidant |
• Antimicrobial activity in food preservation and packaging industry | 109, 110 |
| Calendula (Marigold) | Calendula officinalis | Triterpenoid |
• Antimicrobial • Anti‐inflammatory • Anti‐tumorogenic |
• Herbal antimicrobial mouthwash • Treatment against athletes foot, ringworm and candidal infections |
111, 112, 113 |
| Cinnamon | Cinnamomum zeylanicum | Cinnamaldehyde |
• Analgesic • Antiseptic |
• Inhibits biofilm formation by clinical strains of Staphylococcus epidermidis • Active against common food spoilage microbes |
114, 115 |
| Clove | Syzygium aromaticum | Eugenol |
• Antibacterial • Antifungal |
• Used to treat toothache and skin sores | 104, 116, 117 |
| Eucalyptus | Eucalyptus globulus | 1,8‐cineole, Tannin |
• Antimicrobial • Insect repellent • Skin penetration enhancer for drugs |
• Herbal antimicrobial mouthwash • Inhibits growth of food pathogen • Fragrant in pharmaceuticals, soaps, detergents and food • Natural pesticide/insecticide |
118, 119, 120, 121 |
| Garlic | Allium sativum | Allicin |
• Antimicrobial • Potential anti‐cancer agent • Lowers cholesterol and triglycerides |
• In vitro antimicrobial activity against Helicobacter pylori • Food preservative • Reduce risk of hypertension |
32, 104, 122, 123 |
| Green tea extract | Camellia sinensis | Catechins, tannins, caffeine |
• Anti‐inflammatory • Antimicrobial • Anti‐cancer • Antioxidant |
• Reduces dental caries • Lowers risk of Helicobacter infection |
111, 124, 125, 126 |
| Lemon verbena | Aloysia triphylla or Lippia citriodora | Verbacoside |
• Antimicrobial, • Anti‐cancer agent • Antioxidant • Anti‐inflammatory • Antispasmodic • Antipyretic |
• In vitro antimicrobial activity against H. pylori, Escherichia coli, Mycobacterium tuberculosis, Staphylococcus aureus and Candida albicans • Treatment of asthma, cold, fever and indigestion |
127, 128, 129, 130 |
| Manuka | Leptospermum scoparium | α‐terpineol, cineole, β‐triketone |
• Antimicrobial • Anti‐inflammatory |
• Aids wound healing • Relieve fevers, inflammation and pain • Respiratory ailments |
23, 111 |
| Oregano | Origanum vulgare | Thymol, carvacol, ρ‐cymene, γ‐terpinene, Thymoquinone |
• Antimicrobial • Antioxidative |
• Used in food preservation | 131, 132 |
| Rooibos | Aspalathus linearis | Flavonoids, aspalathin, iso‐orientin, orientin and rutin |
• Antimicrobial • Antioxidant activity |
• In vitro antimicrobial and antifungal activity against Bacillus cereus, Micrococcus luteus and C. albicans, • In vitro anti‐HIV activity |
104, 133, 134, 135 |
| Rosemary | Rosmarinus officinalis | α‐pinene, bornyl acetate, eucalyptol, camphor, 1,8‐cineole |
• Antimicrobial • Antioxidant • Anti‐cellulitis |
• In vitro antifungal activity, particularly against Malassezia (Pityrosporum) spp. • Food preservation |
131, 136, 137, 138, 139 |
| Tea Tree Oil | Melaleuca alternifolia | Terpinen‐4‐ol, α‐terpineol, 1,8‐cineole |
• Antimicrobial • Anti‐cancer • Anti‐inflammatory |
• Treatment of wounds • Pharmaceutical cosmetics and cleaning product applications |
32, 33, 111 |
Tea tree essential oil as an antimicrobial
History, background of use and production of TTO
Bundjalung Aborigines of northern New South Wales traditionally used crushed tea tree leaves as a treatment for coughs and colds (inhalation), a herbal infusion for sore throats and also sprinkled them directly onto skin wounds to promote healing 33. The use of TTO as an antimicrobial agent became a common practice after Penfold published reports on its antimicrobial properties in the 1920s and 1930s 33. In modern society, the useful properties of TTO are made commercially available, either as the essential oil per se or alternatively formulated into various products, including antiseptic creams, soaps, shampoo, anti‐acne creams, toothpaste and household cleaning agents.
TTO is recognised as having broad spectrum antibacterial, antifungal, antiviral, antimycoplasmal and antiprotozoal activity as well as anti‐inflammatory and anti‐cancer properties 33, 34. There are over one hundred different components in whole TTO, of which its major component, terpinen‐4‐ol, is primarily responsible for its active antimicrobial properties 33. TTO is extracted from the leaves and terminal branches of the Melaleuca alternifolia (M. alternifolia) plant via steam distillation and condensed to yield a pale yellow oil 33. The potential variation in the composition of TTO has lead to the classification of six chemotypes of M. alternifolia, based on the amount of terpinen‐4‐ol (one type), terpinolene (one type) and 1,8‐cineole (four types) 33. Commercially acceptable grade of TTO are classed in the terpinen‐4‐ol chemotype and have to comply with ISO4730:2004 standards for 'Oil of Melaleuca, terpinen‐4‐ol type', which defines the maximum and minimum level for the 14 major components of the essential oil, including terpinen‐4‐ol 33.
Mode of action
The physicochemical properties of the oil include those from hydrophilic hydrocarbon compounds with sufficient lipophilicity and allow the oil to partition preferentially into biological membranes, causing bilayer expansion 33. Thus, TTO components diffuse easily through the hydrophobic lipid bilayer of the microbial cell membrane, causing disruption to integrity and function, increased fluidity, loss of permeability and inhibition of embedded membrane enzymes. Consequently, the cell loses essential metabolites and repair enzymes, ultimately resulting in cell death 33, 35. The microbiocidal properties of active monoterpenes in particular can mainly be attributed to disruption of the cell membrane's barrier function; cells are thus unable to establish control over membrane‐coupled energy‐transducing processes, solute transport, regulation of metabolism and maintenance of turgor pressure 35. However, when a compound is highly lipophilic, its low solubility in aqueous media hinders its ability to contact with and permeabilise cell membranes 36.
Cox et al. (2000) examined membrane disruption of E. coli, S. aureus and Candida albicans by TTO via the leakage of potassium ions, materials that absorb at 280 nm (proteins) and uptake of fluorescent nucleic acid stain, propidium iodide 35. All three microorganisms showed decreased microbial viability (inhibition of respiration), increased uptake of propidium iodide and increased leakage of 280 nm absorbing material. Leakage of potassium ions was prominent in E. coli and S. aureus but less so in C. albicans 35, 37. Carson et al. (2002) assessed the release of 260 nm absorbing materials (nucleic acids) from S. aureus after treatment with whole TTO, terpinen‐4‐ol, 1,8‐cineole and α‐terpineol 38. These results showed significant leakage of nucleic acids, suggesting extensive damage to the cytoplasmic membrane.
Applications
Preparations containing TTO are commonly used as antiseptic agents with antimicrobial, cleansing, healing and itch relieving properties 39. For example, creams containing 5% TTO have been used to treat acne and toenail onychomycosis. A 6% gel formulation was shown to have antiherpetic effects; and TTO has been used as an antiseptic agent in handwash soaps and mouthwashes as well as for the treatment of microbial infections such as folliculitis and vaginitis 39, 40, 41, 42, 43. Whole TTO essential oil applied over 12 days successfully and permanently removed skin warts, whereas salicylic acid (12% w/w) and lactic acid (4% w/w) only resulted in temporary removal of the warts 44. Treatment with 4% TTO nasal ointment together with a 5% TTO body wash performed better at eradicating MRSA from patients than 2% mupirocin nasal ointment together with 2% triclosan body wash 45. Similarly, a 10% TTO cream and 5% TTO body wash was more effective at clearing MRSA on skin compared with 4% chlorhexidine gluconate soap and 1% silver sulfadiazine cream 46. In contrast, treatment of MRSA in the nasal cavity alone, even with 10% TTO cream, showed only 47% eradication 46.
TTO has been widely used in the management of wounds because of its antimicrobial and therapeutic properties. Burnaid® (Mundipharma Pty Limited, Sydney, Australia) is a commercial hydrogel dressing impregnated with TTO for the treatment of burns 47. Other studies have reported the enhancement of antimicrobial activity when using TTO in combination with other antimicrobial agents such as chlorhexidine 8, 23, tobramycin 48 and silver ions 34.
Wound‐healing benefits
In addition to antimicrobial activity, TTO also plays a role in wound healing and modulation of the inflammatory response 6, 49, 50. Water soluble components of TTO, especially terpinen‐4‐ol, contribute to inflammatory regulation by suppressing monocyte production of superoxide ions 50 as well as inflammatory‐inducing mediators, for example, TNFα, IL‐1β, IL‐8, IL‐10 and PGE2 49. This in turn limits further production of other inflammatory cytokines 49 and reduces oxidative damage to cells 6, thereby enhancing wound healing.
The aromatic vapours and analgesic properties of TTO may promote wound healing by providing temporary relief to patients 47, 51. Burnaid® reduces skin temperature at the burn site by approximately 2°C within 20 min, providing localised soothing and cooling effects 47, and may improve the patients' ability to cope with the treatment. Using TTO to treat patients with malodorous skin ulcers showed a significant reduction of the malodour as well as of infection and pus secretion 51, 52. The TTO compounds therefore improved the patient's well‐being by reducing social isolation associated with the malodour.
Resistance
Despite the popularity of TTO in many applications, concerns of microorganisms developing resistance have not been totally neglected. In vitro exposure of S. aureus to stepwise increasing concentrations of TTO resulted in a selection of TTO‐resistant sub‐populations 53.
Although there is little evidence of cross‐resistance to conventional antibiotic‐resistant strains with TTO 33, mutant strains of S. aureus with reduced susceptibility to household cleaning agents containing plant extracts were less susceptible to TTO 54. Based on the available data, the activity of TTO may not favour spontaneous development of resistance; however, it is still important to minimise exposure to sublethal concentrations to limit the possibility of resistance development 33, 53.
TTO resistance is also noted in gram‐negative bacteria because of the nature of the outer membrane, which is composed of lipopolysaccharide, proteins and phospholipids. This membrane provides a hydrophilic permeability barrier, which is an essential factor in the tolerance of P. aeruginosa to membrane‐damaging agents, such as TTO 36.
Toxicity concerns
As with most drugs, overdose or extended exposure induces toxic side effects. Evidence from several reports has shown that toxicity of TTO when ingested is rarely, if ever, fatal 33. In general, the symptoms of oral toxicity of TTO vary according to age and dose ingested. Reported symptoms arise from effects on the central nervous system, resulting in changes in respiration rate, oxygen saturation levels, pupil reactivity, electrolyte and blood glucose concentration, development of systemic hypersensitivity, ataxia, muscle weakness, unconsciousness and hallucinations 55, 56. Similar toxic symptoms including lack of coordination, muscle tremors, dehydration, hypothermic, ataxic effects and, in more severe cases, death have been observed in animals 57.
Dermal toxicity of TTO has been reported to cause irritation and allergic reactions 33. The localised cooling effect on treated burn wound sites may lead to triggering hypothermia when using TTO‐based dressings on large areas of the skin 47.
Heavy metals as antimicrobial agents
Heavy metals have a density of at least 5 g/cm3 and are located centrally in the periodic table, along with all the other transition elements, because of their ability to form complexes via their partially filled d‐orbitals 58. These d‐orbitals allow the heavy metals to accept electrons to form complexes, either by redox or other biochemical reactions in the cell. At low concentrations, some heavy metals may serve as essential trace elements to maintain normal cell function. However, at high concentrations, cell toxicity may result from the formation of unspecified complexes within the cell 58, 59. Three classes of heavy metals have been proposed by Nies, 1999 58:
low toxicity and play an important role as trace elements, for example, iron (Fe), molybdate (Mo), manganese (Mn),
toxic but high to moderate importance as a trace element, for example, zinc (Zn), nickel (Ni), copper (Cu), vanadium (V), cobalt (Co), tungsten (W), chromium (Cr); and
toxic with little or no beneficial action, for example, arsenic (As), silver (Ag), antimony (Sb), cadmium (Cd), mercury (Hg), lead (Pb) and uranium (U).
Amongst the various biological functions of heavy metals, their antimicrobial activity will be discussed in further detail. The sequence of antimicrobial toxicity is reported as follows: Ag > Hg > Cu > Cd > Cr > Pb > Co > Au > Zn > Fe > Mn > Mo > Sn 60 The mechanism of antimicrobial action of heavy metal cations varies because of differences in chemical characteristics and their effect on different biochemical pathways in the cell (Table 3). These effects result in disruption of the microorganisms' normal cell function, leading to irreversible damage and cell death 58, 61.
Table 3.
Examples of heavy metals with antimicrobial properties
| Metal cations | Activity towards microorganism | References |
|---|---|---|
| Cadmium, Cd2+ | Reacts with sulphdryl groups on various intracellular proteins | 140 |
| Copper, Cu2+ | Membrane‐bound copper ions may undergo Cu(I) to Cu(II) redox cycle, catalysing formation of highly toxic hydroperoxide radicals (R‐OOH) | 58, 141, 142 |
| Cobalt, Co2+ | Competes with zinc for the active site of urease, thus inhibiting growth of Helicobacter pylori | 143 |
| Silver, Ag+ | Interacts with intracellular components to suppress expression of enzymes and proteins essential for ATP production and condenses DNA, thus impairing replication | 73 |
| Zinc, Zn2+ | Inhibits nutrient uptake, acid production and glycolysis in oral pathogens and rhinoviral replication. Interferes with proton transfer | 65, 144, 145 |
Metal cations interact with ionisable intracellular groups such as carboxylates and phosphates in the lipopolysaccharide layer of gram‐negative bacterial cells, peptidoglycan and teichoic acids of gram‐positive bacterial cells 62. Metal cations may get incorporated into the cell membrane, causing loss of fluidity, followed by further transport into the cell cytoplasm to inhibit vital biochemical processes, hence disturbing the normal growth 58, 63. This transport is usually achieved via an unspecified system using a chemiosmotic gradient between the cell and its environment or, alternatively, an ATP‐dependant specific transport system 58, 61.
Within the cytoplasm, metal cations may affect various cell processes 62; for example, they may bind to sulfhydryl or thiol groups, leading to inhibition of various enzymes 58. Some examples of toxic activities of metal cations towards microorganisms are detailed in Table 3.
Despite their usefulness as antimicrobial compounds, the intensity of exposure (duration and concentration applied) should be carefully considered. Cobalt, cadmium and mercury are too toxic to be used clinically as antimicrobial agents. Copper, zinc and silver are less toxic to human cells. These are often incorporated into antiseptic creams or cleaning agents as well as surfaces of medical devices surfaces, including hospital taps and door handles 64, 65. Examples of applications using heavy metal ions and their mechanism of action are detailed in Table 4.
Table 4.
Applications of heavy metals with lower toxicity to human cells
| Heavy metal ions | Antimicrobial mechanism and uses | References |
|---|---|---|
| Copper, Cu2+ |
• Inhibition of bacterial growth or bactericidal activity at 50–250 ppm. • Substitutes essential ions, blocks protein functional groups, inactivates enzymes, weakens membrane integrity and produces hydrogen peroxide‐free radicals when membrane bound. • For example, antimicrobial hand rub, coated contact surfaces (copper toilet seats, brass taps, door handles, door push plates) and cleaning materials (ultra‐microfibre cloths and mops). |
64, 146, 147, 148, 149 |
| Silver, Ag+ |
|
5, 28, 65, 150, 151 |
| Zinc, Zn2+ |
• Inhibits replication of rhinovirus at <0·1 mmol/l (equivalent to <6·539 ppm). At 0·01–0·1 mM (equivalent to 0·6539–6·539 ppm), inhibits acid production by oral plaque bacteria, effective against plaque and gingivitis when combined with trichlosan. Inhibits nutrient uptake, proton transfer and sugar transport. • Incorporated into polyalkenoate dental cements, stainless steel surface coatings, air conditioning ventilation, intake and exhaust ducts. |
65, 144, 145, 150, 152, 153 |
Silver as an antimicrobial
History and background of the uses of silver
The antimicrobial properties of silver, including its use as an active water disinfectant, have been documented since 1000 B.C. 66. The use of silver as an antimicrobial has been reported since ancient Greek and Roman times, in which silverware was used to store perishable food and drinks. By the 19th century, the use of silver ions, Ag+ (as silver nitrate), in medical applications became more widespread, with records of it being used to treat venereal diseases, salivary gland fistulae, bone and perianal abscesses and removal of granulation tissue prior to epithelialisation 66, 67. After the discovery of penicillin and the rapid expansion in the number and use of antibiotics 54, 66, 68, the antimicrobial use of silver declined. Recent widespread emergence of antibiotic‐resistant microorganisms has resulted in Ag+‐based agents regaining popularity as their multi‐target action in microbial cells is less likely to lead to silver resistance 60, 69.
Silver in its non‐ionised form is an inert metal that does not react with human cells 70. Compared with other heavy metals, Ag+ has relatively low toxicity towards human cells at concentrations that are still highly effective against microbial pathogens 71, 72, 73.
The reactivity of Ag+, even at concentrations as low as 10−9 to 10−6 mol/l (equivalent to 0·000108–0·108 ppm), has shown broad spectrum antibacterial, antiviral, antiprotozoal and antifungal activity 74. In addition, the antimicrobial activity and low toxicity of silver towards human cells is also accompanied by wound‐healing properties 67. Silver is able to treat infections while enhancing wound healing, which could be especially useful in the management of severe wounds such as burns and slow/non‐healing ulcers.
Mode of action
Ag+ is classified as highly reactive moieties, which readily bind anions formed by electron donor groups containing sulphur (thiols), oxygen and nitrogen 74. Ag+ demonstrates broad spectrum antimicrobial activity at concentrations as low as 0·05 ppm in phosphate‐buffered saline or between >50 and 60·5 ppm in complex organic biological fluids 5, 75, 76, 77. Ag+ expresses its antimicrobial activity initially by binding to cell surface proteins and enzymes, resulting in morphological cellular changes, inhibition of cell replication 75, 78, 79 and impairment of solute and electron transport systems, leading to reduced production of vital cell components, such as ATP 80. Subsequent uptake of Ag+ into the cell cytoplasm, either via non‐specific or substrate‐specific transport systems, allows Ag+ to bind and interfere with the activity of essential intracellular enzymes and DNA 74, 78, 79.
In the presence of oxygen, Ag+ also promotes the generation of reactive oxygen species through the inhibition of respiratory enzymes, such as NADH dehydrogenase II 81, or by impairing superoxide‐radical‐scavenging enzymes, such as superoxide dismutase 82. Evidence also suggests that the antimicrobial activity of Ag+ may be a consequential result of its ability to bind to essential enzyme sulphydrals groups (thiols), thereby breaking these protein bonds 83. The binding of Ag+ to anionic groups, most notably disulfide, amino, imidazole, carbonyl and phosphate residues, results in intracellular and nuclear membrane (in eukaryotes) permeability changes as well as structural modifications of the cell wall 79, 84, 85.
Ag+ may also bind to DNA bases, causing condensation and degenerative changes of DNA strands, leading to inhibition of cell replication and cell death 72, 79. In general, the antimicrobial mechanism of action of Ag+ is described as a cascade of four steps 80:
The Ag+ binds to receptors (especially in protein residues including sulphydryl, amino, imidazole, phosphate and carboxyl groups) on the microbial cell membrane, resulting in membrane damage.
After entering the cytoplasm, Ag+ binds to other essential enzymes, restricting their activity and the production vital metabolites.
Binding to microbial DNA follows, thereby impairing cell replication.
With weakened membrane structure and inhibition of cellular processes, vital components leak out; the bacterial cell cannot maintain normal function, resulting in cell death.
Applications
Formulations containing silver are commonly used to treat a variety of Gram‐positive and Gram‐negative bacteria as well as common highly antibiotic‐resistant microorganisms such as P. aeruginosa 79, 86. Silver‐based pharmaceutical preparation, for example, silver sulfadiazine (Flamazine®, Smith and Nephew Healthcare Limited, Hull, UK), has been used for the treatment of burn wounds. It has been found that the antimicrobial activity of Ag+ depends not only on the amount of bioactive ions present but also on bioavailability, which is influenced by the physical and chemical form of silver, the affinity for moisture, rate of release and distribution 74, 79, 84, 87.
In the skin, silver may form a temporary reservoir by binding to proteins with an estimated half‐life of 10–12 hours 26. Being a highly reactive species, Ag+ can readily bind to components present in the wound bed, for example, negatively charged proteins, RNA, DNA and chloride ions 5, thereby limiting the bioavailability of the ions. This ‘quenching’ effect of the host tissues may lead to the need for the application of higher and potentially damaging doses of Ag+ for effective treatment.
Recent advances in controlled delivery systems, which incorporate antimicrobial agents such as Ag+ into delivery devices or dressings, may play a role in overcoming the potential problems caused by the increased exposure and concentration of active antimicrobial agents. Some examples of silver‐containing wound dressings available for current wound management applications include DuoDERM® (hydrocolloids, ConvaTec, Skillman, NJ, USA), Aquacel® Ag (hydrofibre dressings containing antimicrobial silver ions, ConvaTec, Skillman, NJ, USA), Tegaderm™ (films, 3M United Kingdom PLC, Bracknell, UK), Vaseline™ (gauze, Unilever, London, UK), Sorbsan® (alginates, Bertek Pharmaceuticals, Research Triangle Park, NC, USA), Lyofoam® C (foam dressing, ConvaTec, Skillman, NJ, USA) and Nu‐Gel™ (hydrogels, Johnson and Johnson Wound Management, Somerville, NJ, USA) 16, 17.
Wound‐healing benefits
As discussed above, Ag+ helps to promote wound healing by reducing the bacterial load at wound site. Ag+ can also enhance wound healing directly by modulating the inflammatory response 88. At wound sites, Ag+ is taken into epithelial cells responsible for the regulation of tissue metal homeostasis, heavy metal detoxification and wound healing 5. This uptake induces the synthesis of low molecular weight, cysteine‐rich, metal‐binding proteins called metallothioneins (metallothioneins I and II). This activity of metallothioneins, which protect the healthy cells from the toxic effects of metals, is induced by several other xenobiotic metals such as cadmium, gold and mercury 88. Metallothioneins also play an important role in promoting the uptake of key trace elements, such as zinc and copper, promoting RNA and DNA synthesis, cell proliferation, epithelialisation and tissue repair 5, 88. Rising zinc levels induced by the accumulation of Ag+ in wounds increased the activity of the zinc metalloenzymes, thus promoting cell proliferation and re‐epithelialisation in rats 88.
During wound repair and the inflammatory response, matrix metalloproteinases (MMPs) are present within the wound. MMPs function to cause breakdown of the extracellular matrix, autolytic debridement, dissolution of basement membranes, growth promotion of capillary beds, re‐epithelialisation and tissue remodelling 72. Hence, MMPs are essential in wound healing, but excessive levels degrade fibronectin and peptide growth factors needed for optimal re‐epithelialisation 5, 72. Ag+ may form stable complexes with MMPs, thus downregulating excessive localised inflammatory responses to promote wound healing 72, 88. Comparative studies revealed that patients had improved epithelialisation on the skin grafts when treated with nanocrystalline silver dressings in comparison to topical antibiotics 85. Results indicated that Acticoat™, a nanocrystalline silver‐containing product, was effective as a dressing that reduced the wound bio‐burden and altered the process of inflammation, thus facilitating the healing process 85, 89. The ability of Acticoat® to provide prolonged sustained release of silver onto the wound site helps to avoid the development of resistance microorganisms while reducing potentially toxic side effects 85, 89.
Resistance
While this multi‐locus action of silver makes development of resistant microorganisms less likely, such resistance has been observed 90, and with the increasing use of silver, it may be a cause for concern 68, 91. The occurrence of resistance genes has been reported and may be chromosomal‐ or plasmid‐born 92, 93. The identified silver resistance plasmid, pMG101, codes for periplasmic binding proteins (SilE and SilF) and a chemiosmotic efflux pump (SilCBA), which exports Ag + donated from an ATPase efflux pump (SilP) via SilF 90, 92, 93, 94.
The initial mechanism of resistance may be because of periplasmic Ag+‐binding proteins, SilE, each of which binds five Ag+ in the periplasmic space. Synthesis of SilE is stimulated by the presence of Ag+ during growth 90. Although SilE has high affinity for Ag+, the actual release mechanism of the bound Ag+ has yet to be determined. It is possible that the Ag+ may be released to the cell exterior via the SilCBA protein trimer. The SilCBA assembly functions as a transmembrane cation/proton antiporter, moving Ag+ from the cytoplasm directly to the cell exterior. It is classed as a member of the resistance, nodulation and cell division (RND) superfamily, sharing homologous sequences with similar resistance mechanisms in metal‐resistant Alcaligenes and multi‐drug resistance mechanisms in E. coli 90, 93, 94.
Silver resistance has been found in clinical isolates of E. coli, Enterobacter cloacae, Proteus mirabilis and Klebsiella pneumoniae in a burns unit. In addition, silver nitrate and SSD treatment has also been associated with resistance in Proteus spp., E. cloacae and miscellaneous Enterobacteriaceae 95. Similarly, resistance to multiple antibiotics and Ag+ has been shown in Salmonella species 96. Laboratory exposure of clinical strains of E. coli to stepwise increases of silver nitrate or SSD has been shown to induce cross‐resistance against both compounds 97.
A sil E gene has been detected in MRSA strains, isolated from dogs, and is ≥95% homologous with the sil E from plasmid, pMG101 98. However, these isolates were still sensitive to treatment with silver‐impregnated hydrofibres. The results from this study are compatible with those of Silver, 2003 and suggest that there is low prevalence of silver resistance in MRSA 90. The restricted occurrence of a single sil E gene encoding for resistance is not sufficient to induce significant resistance against silver 97.
Toxicity concerns
In common with many drug treatments, over exposure to the agents causes unwelcome side effects. Long‐term topical exposure to high concentrations of Ag+ leads to a build up of Ag0 in the dermis, causing an irreversible blue‐grey discolouration of the skin (argyria). This is particularly pronounced in areas exposed to sunlight, which accelerates the photoreduction and deposition of Ag0 27, 70. Some patients treated with silver‐containing dressings reported the occurrence of skin rashes, stinging and burning sensations when treated with silver‐impregnated dressings 26. Other more serious problems associated with topical application can include disturbances in electrolyte concentration, resulting in hyponatraemia or hypochloraemia 80.
Despite the beneficial properties of various silver‐based treatments, the potential toxicity and safety issues of silver use have to be carefully considered. With the increased availability of formulations, administration of silver can be tailored to the patient's condition, thus limiting the potential risk of side effects.
Conclusion
There has been increasing interest in the use of alternative, broad spectrum, pre‐antibiotic antimicrobial agents, such as essential oils and metal ions, to address issues relating to increased antibiotic‐resistant hospital infections. The versatility of alternative agents such as TTO and Ag+ against a wide range of different microorganisms because of their multiple target sites impedes the development of resistance and might be useful in improving the current wound treatment strategies. Despite the effectiveness of the agents, the potential development of side effects or toxicity to healthy host cells because of prolonged exposure at higher concentrations should be carefully monitored. Efforts to combine the use of these alternative antimicrobial agents with advances in targeted delivery techniques may help to address the issue of localised overloading and toxicity. Based on the current findings, which showed the efficacy and beneficial therapeutic properties of both Ag+ and TTO, the potential advantages of using these agents in wound treatment regimes should be explored further.
References
- 1. Glasser JS, Guymon CH, Mende K, Wolf SE, Hospenthal DR, Murray CK. Activity of topical antimicrobial agents against multidrug‐resistant bacteria recovered from burn patients. Burns 2010;36:1172–84. [DOI] [PubMed] [Google Scholar]
- 2. Toy LW, Macera L. Evidence‐based review of silver dressing use on chronic wounds. J Am Acad Nurse Pract 2011;23:183–92. [DOI] [PubMed] [Google Scholar]
- 3. Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 2010;10:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tacconelli E. Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis 2009;22:352–8. [DOI] [PubMed] [Google Scholar]
- 5. Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns 2007;33:139–48. [DOI] [PubMed] [Google Scholar]
- 6. Warnke PH, Becker ST, Podschun R, Sivananthan S, Springer IN, Russo PAJ, Wiltfang J, Fickenscher H, Sherry E. The battle against multi‐resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital‐acquired infections. J Craniomaxillofac Surg 2009;37:392–7. [DOI] [PubMed] [Google Scholar]
- 7. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karpanen TJ, Worthington T, Hendry ER, Conway BR, Lambert PA. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis . J Antimicrob Chemother 2008;62:1031–6. [DOI] [PubMed] [Google Scholar]
- 9. Low WL, Martin C, Hill DJ, Kenward MA. Antimicrobial efficacy of liposome encapsulated silver ions and tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans . Lett Appl Microbiol 2013;57:33–9. [DOI] [PubMed] [Google Scholar]
- 10. Martin C, Low WL, Amin MCIM, Radecka I, Raj P, Kenward MA. Current trends in the development of wound dressings, biomaterials and devices. Pharm Pat Anal 2013;2:341–59. [DOI] [PubMed] [Google Scholar]
- 11. Reddy PD, Swarnalatha D. Recent advances in novel drug delivery systems. Int J Res Ayurveda Pharm 2010;2:2025–7. [Google Scholar]
- 12. Martin C, Low WL, Gupta A, Amin MCIM, Radecka I, Britland ST, Raj P, Kenward MA. Strategies for antimicrobial drug delivery to the biofilm. Curr Pharm Des 2015;21:43–66. [DOI] [PubMed] [Google Scholar]
- 13. Vowden P, Cooper RA. An integrated approach to managing wound infection. European wound management association. Position document: management of wound infection. London: MEP Ltd., 2006: 2–6.
- 14. Robinson DA, Griffith RW, Shechtman D, Evans RB, Conzemius MG. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus . Acta Biomater 2010;6:1869–77. [DOI] [PubMed] [Google Scholar]
- 15. Mears A, White A, Cookson B, Devine M, Sedgwick J, Phillips E, Jenkinson H, Bardsley M. Healthcare‐associated infection in acute hospitals: which interventions are effective? J Hosp Infect 2009;71:307–13. [DOI] [PubMed] [Google Scholar]
- 16. Fonder MA, Lazarus GS, Cowan DA, Aronson‐Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206. [DOI] [PubMed] [Google Scholar]
- 17. Ovington LG. Advances in wound dressings. Clin Dermatol 2007;25:33–8. [DOI] [PubMed] [Google Scholar]
- 18. Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci 2009;122:3209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition 2010;26:862–6. [DOI] [PubMed] [Google Scholar]
- 20. Stojadinovic A, Carlson JW, Schultz GS, Davis TA, Elster EA. Topical advances in wound care. Gynecol Oncol 2008;111:S70–80. [DOI] [PubMed] [Google Scholar]
- 21. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1–28. [DOI] [PubMed] [Google Scholar]
- 22. Martin KW, Ernst E. Herbal medicines for treatment of bacterial infections: a review of controlled clinical trials. J Antimicrob Chemother 2003;51:241–6. [DOI] [PubMed] [Google Scholar]
- 23. Filoche SK, Soma K, Sissons CH. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol 2005;20:221–5. [DOI] [PubMed] [Google Scholar]
- 24. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12:564–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pandey RR, Dubey RC, Saini S. Phytochemical and antimicrobial studies on essential oils of some aromatic plants. Afr J Biotechnol 2010;9:4364–8. [Google Scholar]
- 26. Lansdown AB, Williams A. How safe is silver in wound care? J Wound Care 2004;13:131–6. [DOI] [PubMed] [Google Scholar]
- 27. Drake PL, Hazelwood KJ. Exposure‐related health effects of silver and silver compounds: a review. Ann Occup Hyg 2005;49:575–85. [DOI] [PubMed] [Google Scholar]
- 28. Castellano JJ, Shafii SM, Ko F, Donate G, Wright TE, Mannari RJ, Payne WG, Smith DJ, Robson MC. Comparative evaluation of silver‐containing antimicrobial dressings and drugs. Int Wound J 2007;4:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – a review. Food Chem Toxicol 2008;46:446–75. [DOI] [PubMed] [Google Scholar]
- 30. Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol 2007;3:408–14. [DOI] [PubMed] [Google Scholar]
- 31. Greay S, Ireland D, Kissick H, Levy A, Beilharz M, Riley T, Carson C. Induction of necrosis and cell cycle arrest in murine cancer cell lines by Melaleuca alternifolia (tea tree) oil and terpinen‐4‐ol. Cancer Chemother Pharmacol 2010;65:877–88. [DOI] [PubMed] [Google Scholar]
- 32. Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res 2007;21:308–23. [DOI] [PubMed] [Google Scholar]
- 33. Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 2006;19:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Low WL, Martin C, Hill DJ, Kenward MA. Antimicrobial efficacy of silver ions in combination with tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans . Int J Antimicrob Agents 2011;37:162–5. [DOI] [PubMed] [Google Scholar]
- 35. Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol 2000;88:170–5. [DOI] [PubMed] [Google Scholar]
- 36. Mann CM, Cox SD, Markham JL. The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil). Lett Appl Microbiol 2000;30:294–7. [DOI] [PubMed] [Google Scholar]
- 37. Cox S, Mann C, Markham J, Gustafson J, Warmington J, Wyllie S. Determining the antimicrobial actions of tea tree oil. Molecules 2001;6:87–91. [Google Scholar]
- 38. Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time‐kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 2002;46:1914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reichling J, Landvatter U, Wagner H, Kostka KH, Schaefer UF. In vitro studies on release and human skin permeation of Australian tea tree oil (TTO) from topical formulations. Eur J Pharm Biopharm 2006;64:222–8. [DOI] [PubMed] [Google Scholar]
- 40. Hammer KA, Carson CF, Riley TV. In‐vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother 1998;42:591–5. [DOI] [PubMed] [Google Scholar]
- 41. Messager S, Hammer KA, Carson CF, Riley TV. Effectiveness of hand‐cleansing formulations containing tea tree oil assessed ex vivo on human skin and in vivo with volunteers using European standard EN 1499. J Hosp Infect 2005;59:220–8. [DOI] [PubMed] [Google Scholar]
- 42. Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: a placebo‐controlled, double‐blind study. Trop Med Int Health 1996;1:505–9. [DOI] [PubMed] [Google Scholar]
- 43. Thornfeldt C. Cosmeceuticals containing herbs: fact, fiction, and future. Dermatol Surg 2005;31:873–81. [DOI] [PubMed] [Google Scholar]
- 44. Millar BC, Moore JE. Successful topical treatment of hand warts in a paediatric patient with tea tree oil (Melaleuca alternifolia). Complement Ther Clin Pract 2008;14:225–7. [DOI] [PubMed] [Google Scholar]
- 45. Caelli M, Porteous J, Carson CF, Heller R, Riley TV. Tea tree oil as an alternative topical decolonization agent for methicillin‐resistant Staphylococcus aureus . J Hosp Infect 2000;46:236–7. [DOI] [PubMed] [Google Scholar]
- 46. Dryden MS, Dailly S, Crouch M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. J Hosp Infect 2004;56:283–6. [DOI] [PubMed] [Google Scholar]
- 47. Cuttle L, Kempf M, Kravchuk O, George N, Liu PY, Chang HE, Mill J, Wang XQ, Kimble RM. The efficacy of Aloe vera, tea tree oil and saliva as first aid treatment for partial thickness burn injuries. Burns 2008;34:1176–82. [DOI] [PubMed] [Google Scholar]
- 48. D'Arrigo M, Ginestra G, Mandalari G, Furneri PM, Bisignano G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli . Phytomedicine 2010;17:317–22. [DOI] [PubMed] [Google Scholar]
- 49. Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay‐Jones JJ. Terpinen‐4‐ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res 2000;49:619–26. [DOI] [PubMed] [Google Scholar]
- 50. Koh KJ, Pearce AL, Marshman G, Finlay‐Jones JJ, Hart PH. Tea tree oil reduces histamine‐induced skin inflammation. Br J Dermatol 2002;147:1212–7. [DOI] [PubMed] [Google Scholar]
- 51. Warnke PH, Sherry E, Russo PAJ, Açil Y, Wiltfang J, Sivananthan S, Sprengel M, Roldàn JC, Schubert S, Bredee JP, Springer ING. Antibacterial essential oils in malodorous cancer patients: clinical observations in 30 patients. Phytomedicine 2006;13:463–7. [DOI] [PubMed] [Google Scholar]
- 52. Warnke PH, Terheyden H, Açil Y, Springer IN, Sherry E, Reynolds M, Russo PAJ, Bredee JP, Podschun R. Tumor smell reduction with antibacterial essential oils. Cancer 2004;100:879–80. [DOI] [PubMed] [Google Scholar]
- 53. Nelson RRS. Selection of resistance to the essential oil of Melaleuca alternifolia in Staphylococcus aureus . J Antimicrob Chemother 2000;45:549–50. [DOI] [PubMed] [Google Scholar]
- 54. Davies J. Microbes have the last word. A drastic re‐evaluation of antimicrobial treatment is needed to overcome the threat of antibiotic‐resistant bacteria. EMBO Rep 2007;8:616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hammer KA, Carson CF, Riley TV, Nielsen JB. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem Toxicol 2006;44:616–25. [DOI] [PubMed] [Google Scholar]
- 56. Mozelsio NB, Harris KE, McGrath KG, Grammer LC. Immediate systemic hypersensitivity reaction associated with topical application of Australian tea tree oil. Allergy Asthma Proc 2003;24:73–5. [PubMed] [Google Scholar]
- 57. Bischoff K, Guale F. Australian tea tree (Melaleuca alternifolia) oil poisoning in three purebred cats. J Vet Diagn Invest 1998;10:208–10. [DOI] [PubMed] [Google Scholar]
- 58. Nies DH. Microbial heavy‐metal resistance. Appl Microbiol Biotechnol 1999;51:730–50. [DOI] [PubMed] [Google Scholar]
- 59. Adarsh VK, Madhusmita M, Sanhita C, Sudarshan M, Thakur AR, Chaudhuri SR. Studies on metal microbe interaction of three bacterial isolates from East Calcutta Wetland. J Biol Sci 2007;7:80–8. [Google Scholar]
- 60. Ansari MA, Khan HM, Khan AA, Malik A, Sultan A, Shahid M, Shujatullah F, Azam A. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MSRA on isolates from skin infections. Biol Med 2011;3:141–6. [Google Scholar]
- 61. Gadd GM. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 2010;156:609–43. [DOI] [PubMed] [Google Scholar]
- 62. Karthikeyan S, Beveridge TJ. Pseudomonas aeruginosa biofilms react with and precipitate toxic soluble gold. Environ Microbiol 2002;4:667–75. [DOI] [PubMed] [Google Scholar]
- 63. Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 2005;99:703–15. [DOI] [PubMed] [Google Scholar]
- 64. Casey AL, Lambert PA, Elliott TSJ. Staphylococci. Int J Antimicrob Agents 2007;29:S23–32. [DOI] [PubMed] [Google Scholar]
- 65. Rusin P, Bright K, Gerba C. Rapid reduction of Legionella pneumophila on stainless steel with zeolite coatings containing silver and zinc ions. Lett Appl Microbiol 2003;36:69–72. [DOI] [PubMed] [Google Scholar]
- 66. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 2009;27:76–83. [DOI] [PubMed] [Google Scholar]
- 67. Edwards‐Jones V. The benefits of silver in hygiene, personal care and healthcare. Lett Appl Microbiol 2009;49:147–52. [DOI] [PubMed] [Google Scholar]
- 68. Chopra I. The increasing use of silver‐based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother 2007;59:587–90. [DOI] [PubMed] [Google Scholar]
- 69. Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomed Nanotech Biol Med 2007;3:95–101. [DOI] [PubMed] [Google Scholar]
- 70. Leak K, Johnson J. Silver therapy in practice: clinical considerations. Prim Health Care 2007;17:43–7. [Google Scholar]
- 71. Mooney EK, Lippitt C, Friedman J. Plastic Surgery Educational Foundation DC. Silver dressings. Plast Reconstr Surg 2006;117:666–9. [DOI] [PubMed] [Google Scholar]
- 72. Warriner R, Burrell R. Infection and the chronic wound: a focus on silver. Adv Skin Wound Care 2005;18:2–12. [DOI] [PubMed] [Google Scholar]
- 73. Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy‐filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol 2005;71:7589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maillard JY, Denyer SP. Demystifying silver. European wound management association. Position document: management of wound infection. London: MEP Ltd., 2006: 7–10.
- 75. Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus . J Biomed Mater Res 2000;52:662–8. [DOI] [PubMed] [Google Scholar]
- 76. Inoue Y, Hoshino M, Takahashi H, Noguchi T, Murata T, Kanzaki Y, Hamashima H, Sasatsu M. Bactericidal activity of Ag‐zeolite mediated by reactive oxygen species under aerated conditions. J Inorg Biochem 2002;92:37–42. [DOI] [PubMed] [Google Scholar]
- 77. Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver‐zeolite on oral bacteria under anaerobic conditions. Dent Mater 2000;16:452–5. [DOI] [PubMed] [Google Scholar]
- 78. Graham C. The role of silver in wound healing. Br J Nurs 2005;14:S22. [DOI] [PubMed] [Google Scholar]
- 79. Lansdown AB. Silver. I: its antibacterial properties and mechanism of action. J Wound Care 2002a;11:125–30. [DOI] [PubMed] [Google Scholar]
- 80. Hermans MH. Silver‐containing dressings and the need for evidence. Am J Nurs 2006;106:60–8. [DOI] [PubMed] [Google Scholar]
- 81. Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol 2003;69:4278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park HJ, Kim JY, Kim J, Lee JH, Hahn JS, Gu MB, Yoon J. Silver‐ion‐mediated reactive oxygen species generation affecting bactericidal activity. Water Res 2009;43:1027–32. [DOI] [PubMed] [Google Scholar]
- 83. Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli . Appl Environ Microbiol 2008;74:2171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol 2006;33:17–34. [DOI] [PubMed] [Google Scholar]
- 85. Leaper DJ. Silver dressings: their role in wound management. Int Wound J 2006;3:282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matsuura T, Abe Y, Sato Y, Okamoto K, Ueshige M, Akagawa Y. Prolonged antimicrobial effect of tissue conditioners containing silver‐zeolite. J Dent 1997;25:373–7. [DOI] [PubMed] [Google Scholar]
- 87. Burd A, Kwok CH, Hung SC, Chan HS, Gu H, Lam WK, Huang L. A comparative study of the cytotoxicity of silver‐based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen 2007;15:94–104. [DOI] [PubMed] [Google Scholar]
- 88. Lansdown AB. Silver. 2: toxicity in mammals and how its products aid wound repair. J Wound Care 2002b;11:173–7. [DOI] [PubMed] [Google Scholar]
- 89. Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine 2006;4:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 2003;27:341–53. [DOI] [PubMed] [Google Scholar]
- 91. Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett 2008;176:1–12. [DOI] [PubMed] [Google Scholar]
- 92. Cutting K, White R, Edmonds M. The safety and efficacy of dressings with silver – addressing clinical concerns. Int Wound J 2007;4:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Silver S, Phung le T, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol 2006;33:627–34. [DOI] [PubMed] [Google Scholar]
- 94. Gupta A, Matsui K, Lo JF, Silver S. Molecular basis for resistance to silver cations in Salmonella . Nat Med 1999;5:183–8. [DOI] [PubMed] [Google Scholar]
- 95. Landsdown AB, Williams A. Bacterial resistance to silver in wound care and medical devices. J Wound Care 2007;16:15–9. [DOI] [PubMed] [Google Scholar]
- 96. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005;60:1–7. [DOI] [PubMed] [Google Scholar]
- 97. Li XZ, Nikaido H, Williams KE. Silver‐resistant mutants of Escherichia coli display active efflux of Ag + and are deficient in porins. J Bacteriol 1997;179:6127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J 2009;6:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J Pharm Biomed Anal 2006;41:683–93. [DOI] [PubMed] [Google Scholar]
- 100. Slobodníková L, KoSt'álová D, Labudová D, Kotulová D, Kettmann V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother Res 2004;18:674–6. [DOI] [PubMed] [Google Scholar]
- 101. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–95. [DOI] [PubMed] [Google Scholar]
- 102. Okoro IO, Osagie A, Asibor EO. Antioxidant and antimicrobial activities of polyphenols from ethnomedicinal plants of Nigeria. Afr J Biotechnol 2010;9:2989–93. [Google Scholar]
- 103. Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of Aloe vera used for burn wound healing: a systematic review. Burns 2007;33:713–8. [DOI] [PubMed] [Google Scholar]
- 104. Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes . Lett Appl Microbiol 2009;50:30–5. [DOI] [PubMed] [Google Scholar]
- 105. Barcroft A, Myskja A. Aloe vera: nature's silent healer. London: BAAM, 2003:8–11. [Google Scholar]
- 106. Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract 1999;49:823–8. [PMC free article] [PubMed] [Google Scholar]
- 107. Grover JK, Yadav S, Vats V. Medicinal plants of India with anti‐diabetic potential. J Ethnopharmacol 2002;81:81–100. [DOI] [PubMed] [Google Scholar]
- 108. Wagner S, Merfort I. Skin penetration behaviour of sesquiterpene lactones from different Arnica preparations using a validated GC‐MSD method. J Pharm Biomed Anal 2007;43:32–8. [DOI] [PubMed] [Google Scholar]
- 109. Hussain AI, Anwar F, Hussain‐Sherazi ST, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 2008;108:986–95. [DOI] [PubMed] [Google Scholar]
- 110. Suppakul P, Sonneveld K, Bigger SW, Miltz J. Efficacy of polyethylene‐based antimicrobial films containing principal constituents of basil. LWT‐Food Sci Technol 2008;41:779–88. [Google Scholar]
- 111. Lauten JD, Boyd L, Hanson MB, Lillie D, Gullion C, Madden TE. A clinical study: Melaleuca, Manuka, Calendula and green tea mouth rinse. Phytother Res 2005;19:951–7. [DOI] [PubMed] [Google Scholar]
- 112. Okoh OO, Sadimenko AP, Asekun OT, Afolayan AJ. The effects of drying on the chemical components of essential oils of Calendula officinalis L. Afr J Biotechnol 2008;7:1500–2. [Google Scholar]
- 113. Roveroni‐Favaretto L, Lodi K, Almeida J. Topical Calendula officinalis L successfully treated exfoliative cheilitis: a case report. Cases J 2009;2:9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nuryastuti T, van der Mei HC, Busscher HJ, Iravati S, Aman AT, Krom BP. Effect of cinnamon oil on icaA expression and biofilm formation by Staphylococcus epidermidis . Appl Environ Microbiol 2009;75:6850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Trajano VN, Lima EO, Travassos AE, Souza EL. Inhibitory effect of the essential oil from Cinnamomum zeylanicum Blume leaves on some food‐related bacteria. Food Sci Technol 2010;30:771–5. [Google Scholar]
- 116. Angienda PO, Onyango DM, Hill DJ. Potential application of plant essential oils at sub‐lethal concentrations under extrinsic conditions that enhance their antimicrobial effectiveness against pathogenic bacteria. Afr J Microbiol Res 2010;4:1678–84. [Google Scholar]
- 117. Pinto E, Vale‐Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum (Eugenia caryophyllus) on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009;58:1454–62. [DOI] [PubMed] [Google Scholar]
- 118. Batish DR, Singh HP, Kohli RK, Kaur S. Eucalyptus essential oil as a natural pesticide. Forest Ecol Manag 2008;256:2166–74. [Google Scholar]
- 119. Karpanen T, Conway B, Worthington T, Hilton A, Elliott T, Lambert P. Enhanced chlorhexidine skin penetration with eucalyptus oil. BMC Infect Dis 2010;10:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gupta C, Garg AP, Uniyal RC, Kumari A. Antimicrobial activity of some herbal oils against common food‐borne pathogens. Afr J Microbiol Res 2008;2:258–61. [Google Scholar]
- 121. Souza EL, Lima EO, Freire KRL, Sousa CP. Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Braz Arch Biol Technol 2005;48:245–50. [Google Scholar]
- 122. O'Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori . Appl Environ Microbiol 2000;66:2269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sallam KI, Ishioroshi M, Samejima K. Antioxidant and antimicrobial effects of garlic in chicken sausage. LWT‐Food Sci Technol 2004;37:849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lee JH, Shim JS, Chung MS, Lim ST, Kim KH. In vitro anti‐adhesive activity of green tea extract against pathogen adhesion. Phytother Res 2009;23:460–6. [DOI] [PubMed] [Google Scholar]
- 125. Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea – a review. J Am Coll Nutr 2006;25:79–99. [DOI] [PubMed] [Google Scholar]
- 126. Yee YK, Koo MWL, Szeto ML. Chinese tea consumption and lower risk of Helicobacter infection. J Gastroenterol Hepatol 2002;17:552–5. [DOI] [PubMed] [Google Scholar]
- 127. Bilia AR, Giomi M, Innocenti M, Gallori S, Vincieri FF. HPLC‐DAD‐ESI‐MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J Pharm Biomed Anal 2008;46:463–70. [DOI] [PubMed] [Google Scholar]
- 128. Funes L, Fernández‐Arroyo S, Laporta O, Pons A, Roche E, Segura‐Carretero A, Fernández‐Gutiérrez A, Micol V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem 2009;117:589–98. [Google Scholar]
- 129. Ohno T, Kita M, Yamaoka Y, Imamura S, Yamamoto T, Mitsufuji S, Kodama T, Kashima K, Imanishi J. Antimicrobial activity of essential oils against Helicobacter pylori . Helicobacter 2003;8:207–15. [DOI] [PubMed] [Google Scholar]
- 130. Pereira CG, Meireles MAA. Evaluation of global yield, composition, antioxidant activity and cost of manufacturing of extracts from lemon verbena (Aloysia triphylla [L'herit.] Britton) and mango (Mangifera indica L.) leaves. J Food Process Eng 2007;30:150–73. [Google Scholar]
- 131. Burt S. Essential oils: their antibacterial properties and potential applications in foods – a review. Int J Food Microbiol 2004;94:223–53. [DOI] [PubMed] [Google Scholar]
- 132. Chouliara E, Karatapanis A, Savvaidis IN, Kontominas MG. Combined effect of oregano essential oil and modified atmosphere packaging on shelf‐life extension of fresh chicken breast meat, stored at 4°C. Food Microbiol 2007;24:607–17. [DOI] [PubMed] [Google Scholar]
- 133. Marnewick JL. Rooibos and honeybush: recent advances in chemistry, biological activity and pharmacognosy. In: African natural plant products: new discoveries and challenges in chemistry and quality. American Chemical Society, 2009:277–94. [Google Scholar]
- 134. Almajano MP, Carbó R, Jiménez JAL, Gordon MH. Antioxidant and antimicrobial activities of tea infusions. Food Chem 2008;108:55–63. [Google Scholar]
- 135. Nakano M, Nakashima H, Itoh Y. Anti‐human immunodeficiency virus activity of oligosaccharides from rooibos tea (Aspalathus linearis) extracts in vitro. Leukemia 1997;11:128–30. [PubMed] [Google Scholar]
- 136. Gutierrez J, Barry‐Ryan C, Bourke P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microbiol 2008;124:91–7. [DOI] [PubMed] [Google Scholar]
- 137. Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res 2007;21:989–94. [DOI] [PubMed] [Google Scholar]
- 138. Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res 2006;40:223–31. [DOI] [PubMed] [Google Scholar]
- 139. Prabhamanju M, Shankar GS, Babu K. Herbal vs. chemical actives as antidandruff ingredients – which are more effective in the management of dandruff? An overview. Ethnobot Leaflets 2009;13:1373–81. [Google Scholar]
- 140. Chopra I. Mechanism of plasmid‐mediated resistance tocadmium in Staphylococcus aureus . Antimicrob Agents Chemother 1975;7:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Noyce JO, Michels H, Keevil CW. Potential use of copper surfaces to reduce survival of epidemic meticillin‐resistant Staphylococcus aureus in the healthcare environment. J Hosp Infect 2006;63:289–97. [DOI] [PubMed] [Google Scholar]
- 142. Magnani D, Solioz M. How bacteria handle copper. In: Nies D, Silver S, editors. Molecular microbiology of heavy metals. Springer: Berlin/Heidelberg, 2007:259–85. [Google Scholar]
- 143. Bruggraber SFA, French G, Thompson RPH, Powell JJ. Selective and effective bactericidal activity of the cobalt (II) cation against Helicobacter pylori . Helicobacter 2004;9:422–8. [DOI] [PubMed] [Google Scholar]
- 144. Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol 2004;19:31–8. [DOI] [PubMed] [Google Scholar]
- 145. Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev 2011;2:CD001364. [DOI] [PubMed] [Google Scholar]
- 146. Hall TJ, Wren MWD, Jeanes A, Gant VA. A comparison of the antibacterial efficacy and cytotoxicity to cultured human skin cells of 7 commercial hand rubs and Xgel, a new copper‐based biocidal hand rub. Am J Infect Control 2009;37:322–6. [DOI] [PubMed] [Google Scholar]
- 147. Luna VA, Hall TJ, King DS, Cannons AC. Susceptibility of 169 USA300 methicillin‐resistant Staphylococcus aureus isolates to two copper‐based biocides, CuAL42 and CuWB50. J Antimicrob Chemother 2010;65:939–41. [DOI] [PubMed] [Google Scholar]
- 148. Faundez G, Troncoso M, Navarrete P, Figueroa G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni . BMC Microbiol 2004;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ibrahim SA, Yang H, Seo CW. Antimicrobial activity of lactic acid and copper on growth of Salmonella and Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Chem 2008;109:137–43. [DOI] [PubMed] [Google Scholar]
- 150. Coughlan A, Scanlon K, Mahon BP, Towler MR. Zinc and silver glass polyalkenoate cements: an evaluation of their antibacterial nature. Biomed Mater Eng 2010;20:99–106. [DOI] [PubMed] [Google Scholar]
- 151. Monteiro DR, Gorup LF, Takamiya AS, Ruvollo‐Filho AC, Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents 2009;34:103–10. [DOI] [PubMed] [Google Scholar]
- 152. Keegan G, Smart J, Ingram M, Barnes L, Rees G, Burnett G. An in vitro assessment of bioadhesive zinc/carbomer complexes for antimicrobial therapy within the oral cavity. Int J Pharm 2007;340:92–6. [DOI] [PubMed] [Google Scholar]
- 153. Dardenne M. Zinc and immune function. Eur J Clin Nutr 2002;56:S20–3. [DOI] [PubMed] [Google Scholar]


