Abstract
Chronic wounds are a significant burden to global patient and health care infrastructures, and there is a need for better methods of early wound diagnosis and treatment. Traditional diagnosis of chronic wound infection by pathogenic bacteria, using clinical signs and symptoms, is based on visual inspection under white light and microbiological sampling (e.g. swabbing and/or biopsy) of the wound, which are subjective and suboptimal. Diagnosing microbial infection based on traditional clinical signs and symptoms in wounds of asymptomatic patients is especially challenging at the bedside. Bacteria are invisible to the unaided eye and wound sampling for diagnostic testing can cause unacceptable delays in diagnosis and treatment. To address this problem, we developed a new prototype handheld, portable fluorescence imaging device that enables non‐contact, real‐time, high‐resolution visualisation of pathogenic bacteria and tissues in wounds. Herein, we report the clinical use of this imaging device in detecting subsurface heavy bacterial load and subclinical local infection in an asymptomatic 50‐year‐old patient with a non‐healing diabetic foot ulcer.
Keywords: Bacteria, Diabetic wounds, Infection, Medical device, Optical imaging
Introduction
Subsurface bacterial burden can be missed during standard wound examination protocols and this can be influenced by the level of clinician experience 1, possibly contributing to wound chronicity, if left unmanaged. Wound management poses a large burden on health care systems worldwide 2. Wounds are classified as having an acute or chronic aetiology 2. Acute wounds heal within a predictable time frame and rarely require intervention, while the contrary is true for chronic wounds 2. Infections often occur in chronic wounds and contribute to the delayed healing process 3.
Of the many types of chronic wounds, diabetic foot ulcers are the leading cause of amputations worldwide 4. Diabetic foot ulcers are caused primarily by diabetic neuropathy and accelerated atherosclerosis, leading to ischaemia 5. Diabetic foot ulcers develop in approximately 15% of diabetic individuals, costing the Canadian health care system upwards of $150 million annually 6. Treatment for chronic diabetic wounds involves various surgical debridement techniques to remove superficial necrotic tissue and the topical or systemic use of antimicrobials and antibiotics to accelerate the healing process 7, 8.
The current diagnostic standard of wound care involves the non‐quantitative and subjective visual assessment of the wound under standard room lighting conditions for clinical signs and symptoms, such as pain, erythema, oedema, lymphadenitis and purulence 7. Microbiology swabs are inconsistently and sometimes inappropriately used in conjunction with clinical signs and symptoms as semi‐quantitative assessments of the presence of bacteria in wounds 7. Semi‐quantitative surface swabs are the most common sampling method because of minimised expense and pain 9, 10, but there is no conclusive guideline for the most effective swabbing technique 11. The Levine swabbing technique 12 is most commonly used, but is limited because it samples only the centre of the wound, potentiating missed collection and identification of treatment‐relevant bacteria at the wound periphery or other distant locations. Results from microbiology laboratories typically return after 3–5 days, and thus cannot be used in real time.
To address these limitations, we developed a handheld, non‐contact, non‐invasive, autofluorescence imaging device called PRODIGI (Portable Real‐time Optical Detection, Identification and Guidance for Intervention; Figure 1) for use in wound management at the point‐of‐care. The PRODIGI device captures autofluorescence (AF) signals, which are intrinsically produced by different tissue components and bacteria upon excitation by blue‐violet light from the device, without the need for exogenous contrast agents. A composite colour image of green AF, produced by endogenous connective tissues in skin, and red AF, produced by endogenous porphyrins in clinically relevant bacteria, is captured in real time by the imaging sensor for assessment by a physician on the liquid crystal display (LCD) touch screen 13. Porphyrin complexes are usually found in wound bacteria 14 and their biofilms, producing characteristic red fluorescence under violet light excitation. Whereas some bacteria, like Pseudomonas aeruginosa, produce green fluorescence under violet light excitation mainly because of the presence of siderophores.
Figure 1.
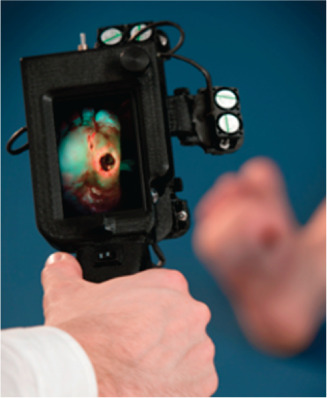
Photograph of PRODIGI device. The prototype handheld fluorescence imaging device aimed at a diabetic foot ulcer. Fluorescence images are displayed in real time on the colour liquid crystal display (LCD) touch screen. Dual broadband white light and violet light light emitting diode (LED) arrays provide illumination of the wound, while the autofluorescence signals from the wound and surrounding healthy tissues are detected by the device's complementary metal–oxide–semiconductor (CMOS) image sensor in real time.
PRODIGI has been extensively validated in preclinical wound models 13 as well as randomised clinical trials involving patients with chronic wounds (clinicaltrials.gov identifiers: NCT01378728, NCT01651845) 15. In these clinical studies, an accuracy comparison was performed between white light examination and AF imaging for detecting clinically significant bioburden in the wound bed, periphery and off‐site areas. In the wound bed, AF correctly detected 74·5% of wounds with clinically significant bioburden, compared with 52·5% for white light inspection (P = 0·003). In the wound periphery and off‐site areas, standard practice would not have assessed these areas, while PRODIGI accurately detected clinically significant bioburden 82·4% of the time in the wound periphery (P < 0·001) and 67·1% in other areas (P = 0·006). White light examination was correct 17·6% of the time in the peripheries and 32·9% in other areas (P < 0·001 and P = 0·006, respectively). Thus, by not assessing these areas at all, standard practice failed to detect 82·4% and 67·1% of clinically significant bacterial load in the periphery and off‐site areas, respectively.
Moreover, longitudinal FL imaging showed that 90% of patients would have been sent home at least once during the care cycle with a clinically significant bacterial load if assessed by white light examination (95% confidence interval: 81·0%, 96·0%). Overall, white light inspection had a positive predictive value of 94·5% versus 74·5% for AF imaging (P < 0·001), but the sensitivity of standard methods was only 14·2% (P < 0·001). The ratio of true positives from AF imaging to standard methods was 365/52 = 7·0. The overall accuracy of judging the presence of clinically significant bacterial load in chronic wounds for AF was 74·5% versus 35·5% (P < 0·001) for white light assessment, based on swab results, thus guiding the clinicians to consider the use of antimicrobial therapies following the detection of significant bacterial load with AF imaging.
Overall, these studies demonstrate that the technology can instantaneously detect the presence, location and extent of bacteria in and around the wound, which can guide wound sampling and debridement based on real‐time fluorescence imaging at the bed side 15. Furthermore, the technology was shown to be easy to use, non‐disruptive to clinical workflow and safe. Herein, we report a unique clinical case illustrating the use of AF imaging in detecting subclinical infection in an asymptomatic 50‐year‐old male patient with a non‐healing diabetic foot ulcer, where infection was occult to conventional clinical examination.
Case description
A 50‐year‐old male presented with a diabetic foot ulcer on the sole of his right foot. Comorbidities included obesity, hypertension and Charcot foot. In 1995, this patient experienced a motorcycle accident, resulting in a fractured right femur and severe nerve damage in his lower right extremity, which caused a drop foot. In 1997, he was diagnosed with type I diabetes, which has since been poorly maintained. In 1999, an ulcer on the mid‐plantar surface of his right foot was discovered; surgery was performed in 2002 to remove this ulcer. Post‐surgery, the patient injured himself in the midst of physiotherapy sessions, fracturing the tarsal, mid‐tarsal and calcaneus of his right foot. An orthopaedic surgeon recommended amputation of the right foot; however, after seeking a second opinion, he has been treated since 2007 at the Judy Dan Research and Treatment Centre (JDRTC; Toronto, Canada) with hyperbaric oxygen therapy (HBOT), various debridement techniques and antibacterial medications. Additional surgeries were performed to clip the protruding bones and to reconstruct the foot. This study wound was closed and re‐opened numerous times as a result of a combination of multiple surgeries and the nature of the healing process.
The patient presented at his weekly visit in April 2013 with hyperkeratosis (6 cm × 8 cm), which was examined under standard white light (Figure 2A). Based on the white light findings, the clinician was uncertain if debridement was necessary. A fluorescence image (Figure 2B) was then taken using PRODIGI, showing an area of bright red fluorescence at the wound site below the fifth toe, indicating the presence of bacteria in the wound. Based on the fluorescence image, the clinician decided to debride the wound with a scalpel and curette, exposing the underlying tissues. During the procedure, a second fluorescence image was taken with PRODIGI. A large area of red fluorescence was detected medial to the primary wound site on the right foot (Figure 2C). Based on the red fluorescence detected in the second image, debridement was continued and a secondary wound was observed, which was not visible under conventional clinical examination by white light. Microbiological culture testing of swabs obtained from the red fluorescent areas of the wound confirmed the presence of heavy growth of Staphyloccocus aureus in the wound before and after fluorescence image‐guided debridement – indicating an otherwise subclinical bacterial infection that was missed by conventional clinical examination. Post‐debridement images of this 0·6 cm × 0·2 cm wound were then taken using both white light and fluorescence imaging (Figures 2D, 2E). The wound was dressed with iodine and gauze and the patient was prescribed 500 mg of CIPRO® twice daily for 2 weeks, based on visual inspection and PRODIGI AF imaging of the open wound. Because of the severity of the wound infection found after opening up the wound, the attending physician determined that it was necessary to prescribe antibiotic therapy to suppress the infection (as the benefit of suppressing the infection outweighed the risk of the potential development of drug resistance in the future). In this case, AF imaging signalled the presence of a hidden bacterial infection in an otherwise asymptomatic patient that guided the decision to prescribe antibiotic and obtain a culture test for confirmation.
Figure 2.
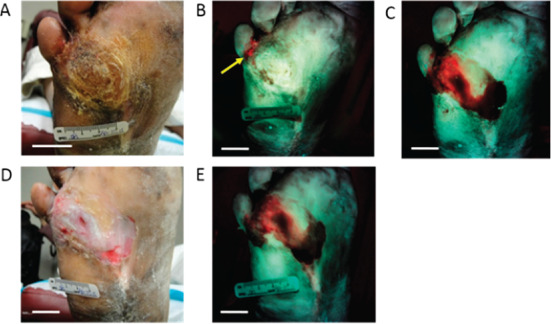
White light and corresponding fluorescence images of the study wound obtained using PRODIGI device. (A) White light image (plantar view) of the study patient's diabetic foot ulcer before debridement. (B) Corresponding fluorescence image of the ulcer before debridement shows area of bacterial red fluorescence (arrow) below small toe. (C) Fluorescence image of the ulcer during debridement showing larger extent of bioburden below the skin surface. The bacterial load before and after debridement was 0·9578 cm2 and 4·0985 cm2, respectively. (D) White light image of the ulcer after debridement. (E) Corresponding fluorescence image of the ulcer after debridement shows persistent area of bacterial load, which can be sampled in a targeted manner using fluorescence image guidance. (Scale bars: 2 cm).
Bacterial load was quantified (area in cm2) with custom fluorescence intensity threshold‐based software using MATLAB (Version 7.9.0; Figure 3) 13, 15. The bacterial load before and after debridement was 0·9578 cm2 and 4·0985 cm2, respectively. Comparison of bacterial load using fluorescence images obtained before and after debridement demonstrated the presence of heavy growth of S. aureus below the wound surface that was missed by conventional examination but shown by fluorescence‐guided debridement. Fluorescence detection of this underlying infection afforded useful and targeted sampling of the wound as well as timely therapeutic intervention that would have otherwise been a missed treatment opportunity.
Figure 3.
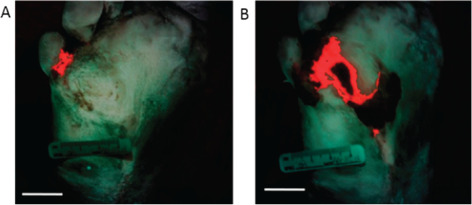
Fluorescence images enable quantification of bacterial load during wound assessment. Custom intensity threshold‐based image analysis software was used to determine the relative amount of bacteria. (A) prior to and (B) after wound debridement based on bacterial red fluorescence intensities. Image pixels containing bacterial red fluorescence are colour coded by the software to be bright red in this composite image, which also shows green fluorescence from connective tissues and skin for anatomical context of the location of the bacterial load. (Scale bars: 2 cm).
Discussion
This case report demonstrates the difficulty in detecting a subclinical surface bacterial infection and assigning timely treatment in asymptomatic chronic wound patients, based on best clinical practice guided by white light visual assessment of the wound site by the wound care clinician. Fluorescence imaging can be performed rapidly and safely before and during debridement to detect clinically relevant bacterial load at the wound site. In this case, imaging of the wound at presentation signalled a suspicious area (based on red bacterial fluorescence), which prompted the clinician to further investigate the wound by debridement. Indeed, fluorescence imaging enabled the discovery of a secondary infected wound below otherwise unremarkable skin and altered the clinician's decision‐making process by prompting the timely prescription of antibiotics, which benefited the patient. Importantly, fluorescence imaging was used by the clinician as an assistive tool during the conventional assessment of signs and symptoms of infection in the patient. In this assistive capacity, PRODIGI helped guide wound debridement at the bedside, aided ‘targeted’ sampling of the subsurface bioburden (based on bacterial fluorescence) for standard laboratory cultures and informed the decision for rapid administration of antibiotic treatment. PRODIGI can be used at the time of initial wound assessment and longitudinally to objectively monitor bacterial burden, including during the course of treatment 13, 15. In addition, fluorescence imaging can be used to objectively determine the response of a wound to treatment 13, 15. The current report illustrates the potential for missing the diagnosis of an infection using examination of standard clinical signs and symptoms in asymptomatic patients with chronic diabetic foot ulcers, possibly contributing to wound chronicity. Point‐of‐care fluorescence imaging may offer a new way to assist clinicians in mitigating this risk.
Acknowledgements
We acknowledge Ruth Baker and Julie Opoku for helping with patient recruitment at the JDRTC. Funding for this study was provided by Canadian Institutes of Health Research (DaCosta). RSD and his institution have a pending patent related to this work (‘Device and method for fluorescence‐based imaging and monitoring’ USPTO WO 2009140757 A1), which is being commercialised. RSD is an independent investigator with a Scientist appointment at the Princess Margaret Cancer Centre, University Health Network, where he has been the principal investigator on this project. He is also founder and a shareholder of a spin‐off company commercialising the technology. He has Board membership and receives compensation as Chief Scientific Officer. While LLT is a part‐time employee of the University Health Network (Clinical Associate), she also receives separate compensation as the clinical trials manager for the company, but her role in this project did not involve the company. At the time of this study, DS was a graduate student in RSD's laboratory, but is now a full‐time employee of the company. No other authors are associated with or receive compensation or own shares of the company. This work was funded, in part, by a Canadian Institutes of Health Research operating grant (CIHR MOP #93578), Ontario Centres of Excellence (MR60015‐08), Health Technologies Exchange, Biodiscovery Toronto (project # BDT04‐035‐S3A), Canadian Institute for Photonic Innovation, Cancer Care Ontario Research Chair in Cancer Imaging to RSD and by the Ontario Ministry of Health and Long Term Care (OMOHLTC). No funding for this project (including the experimental design, implementation of clinical trial, gathering, analysis, interpretation, publication or editing of any data obtained from it) or salary support for RSD or LLT or DS was provided by the company for any part of the project from which this (or other referenced publication herein) is based. RL has received compensation from the Ministry of Health for treating patients enrolled in this study.
References
- 1. Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA 2012;307:605–11. [DOI] [PubMed] [Google Scholar]
- 2. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Percival SL, Thomas JG, Williams DW. Biofilms and bacterial imbalances in chronic wounds:anti‐koch. Int Wound J 2010;7:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361:1545–51. [DOI] [PubMed] [Google Scholar]
- 5. Urbancic Rovan V. Causes of diabetic foot lesions. Lancet 2005;366:1675–6. [DOI] [PubMed] [Google Scholar]
- 6. Canadian Association of Wound Care . Statistics. Canadian Association of Wound Care, 2012. URL http://cawc.net/en/index.php/public/facts‐stats‐and‐tools/statistics/ [accessed on 23 July 2013]
- 7. McGuckin M, Goldman R, Bolton L, Salcido R. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care 2003;16:12–24. [DOI] [PubMed] [Google Scholar]
- 8. Howell‐Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomase DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother 2005;55:143–9. [DOI] [PubMed] [Google Scholar]
- 9. Spear M. When and how to culture a chronic wound: a culture is a valuable tool in wound care if used correctly. Wound Care Advisor 2014;3:23–5. [Google Scholar]
- 10. Angel DE, Lloyd P, Carville K, Santamaria N. The clinical efficacy of two semi‐quantitative wound‐swabbing techniques in identifying the causative organism(s) in infected cutaneous wounds. Int Wound J 2011;8:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Starr S, MacLeod T. Wound swabbing technique. Nurs Times 2003;99:57–9. [PubMed] [Google Scholar]
- 12. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu YC, Kulbatski I, Medeiros PJ, Maeda A, Bu J, Xu L, Chen Y, DaCosta RS. Autofluorescence imaging device for real‐time detection and tracking of pathogenic bacteria in a mouse skin wound model: preclinical feasibility studies. J Biomed Opt 2014;19:085002; doi: 10.1117/1.JBO.19.8.085002. [DOI] [PubMed] [Google Scholar]
- 14. Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, Nitzan Y. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol 1997;19:307–14. [DOI] [PubMed] [Google Scholar]
- 15. DaCosta RS, Kulbatski I, Lindvere‐Teene L, Starr D, Blackmore K, Silver JI, Opoku J, Wu YC, Medeiros PJ, Xu W, Xu L, Wilson BC, Rosen C, Linden R. Point‐of‐care autofluorescence imaging for real‐time sampling and treatment guidance of bioburden in chronic wounds: first‐in‐human results . PLoS One 2015;10:e0116623; doi: 10.1371/journal.pone.0116623. [DOI] [PMC free article] [PubMed] [Google Scholar]


