ABSTRACT
Liposuction is the one of the most frequently performed cosmetic operations and usually has an easy recovery, with a reportedly low overall complication rate. Here, we report the case of a 60‐year‐old woman with type II diabetes mellitus and a previous burn injury of the abdomen who underwent abdominal liposuction and subsequently developed necrotising fasciitis. Following radical debridement, systemic antibiotic administration, negative pressure wound therapy and hyperbaric oxygen therapy, the wound healed completely. This case demonstrates the success of the combination treatment and highlights the need for clinicians to be aware of the risk of serious complications in selected patients.
Keywords: Burn scar, Hyperbaric oxygen, Liposuction, Necrotising fasciitis, Negative pressure wound therapy
Introduction
Liposuction is one of the most frequently performed cosmetic surgeries worldwide for reshaping the body contour. Although liposuction is minimally invasive, relatively safe and usually has an easy recovery, it carries the risk of major and minor complications 1, 2, 3.
The overall complication rate is reported to be in the range of 8·6–20%; the most common complication in abdominal liposuction is contour deformity, followed by hyperpigmentation, infection, abdominal wall injury, bowel herniation, bleeding, haematoma, seroma and lymphoedema. Major or lethal complications such as necrotising fasciitis (NF), abdominal or viscus perforation, deep vein thrombosis, pulmonary embolism and even mortality are reported in 0·02–0·25% of cases 1, 2, 3. We report the case of a 60‐year‐old woman with a history of abdominal burn injury presenting with NF of the abdomen secondary to liposuction; she was treated successfully with surgery, hyperbaric oxygen (HBO) and negative pressure wound therapy (NPWT).
Case report
A 60‐year‐old woman, body mass index of 28 kg/m2, presented with local fat deposition on her central abdomen and bilateral lateral thighs. She had a history of a second‐degree flame burn injury of the face, abdomen and four extremities, involving 45% of the total body surface area, with secondary healing 10 years prior. She also had type II diabetes mellitus, which was poorly controlled with oral hypoglycaemic agents. She underwent day surgery for aesthetic liposuction of the abdominal and bilateral thighs at a private cosmetic clinic. The procedure was apparently uneventful, and she was discharged the same day. A total of 72 hours later, she developed fever, increasing pain and tenderness at the operative site, with a small amount of purulent discharge and minor skin changes, such as erythema and swelling. She returned to the same clinic for medical attention, and she was advised to increase her intake of analgesics and oral antibiotics. Penrose drains were placed at the suction wound subcutaneously. There was no relief despite administration of a high dose of non‐steroidal anti‐inflammatory drugs.
She was sent to the emergency department on the sixth postoperative day with severe, diffuse and persistent abdominal pain. She was febrile, with a temperature of 38·7 degrees Celsius, appeared to have toxicity and was haemodynamically unstable, with a heart rate of 120 beats/minute and a blood pressure of 80/50 mmHg. Physical examination revealed swelling, erythema, blistering and subcutaneous crepitus of the left abdomen with a circle of skin necrosis (Figure 1). A large amount of purulent discharge was found in the suction wound. Initial laboratory analysis revealed leukocytosis (22 × 109/l) and an elevated C‐reactive protein level (35 mg/dl), decreased haemoglobin level (7 g/dl) and normal platelet count. Serum creatinine and blood urea nitrogen levels were also elevated. Serum creatine kinase level was slightly elevated (250 U/l). Computed tomography of the abdomen showed fatty stranding, free air, localised fluid accumulation in the subcutaneous layer and a skin defect of the left abdomen wall, suggestive of an infectious process (Figure 2).
Figure 1.
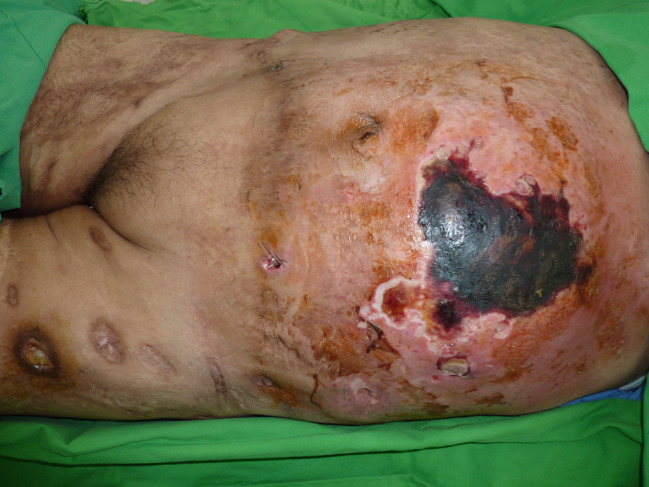
The patient presented with tenderness, purulent discharge and skin necrosis of the abdomen at admission.
Figure 2.
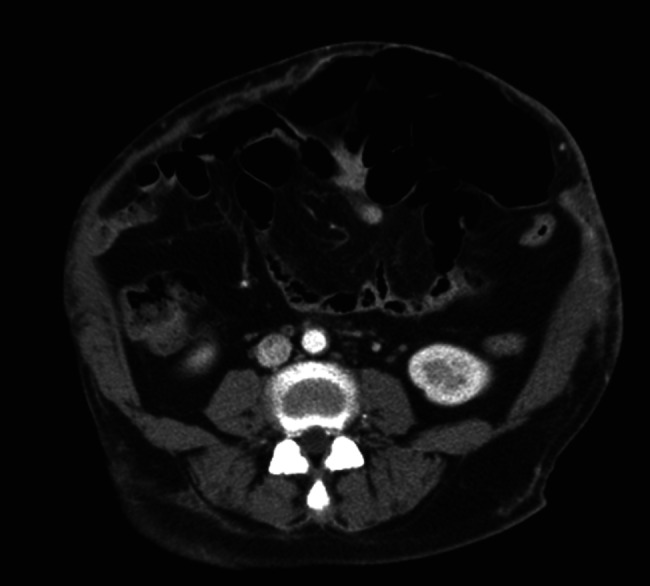
Computed tomography scan showing an infectious process and a thin abdominal wall.
The patient underwent emergency surgery under general anaesthesia for radical exploration and debridement of the lesion. Intraoperatively, greyish and oedematous subcutaneous tissue and superficial fascial necrosis and myonecrosis were observed (Figure 3). A diagnosis of NF was made. Microbiological culturing was performed from the tissue sample. Because of the burn scar and skin necrosis with a large defect of the abdominal wall, the wound was initially treated with NPWT dressing at 125 mmHg continuous topical negative pressure.
Figure 3.
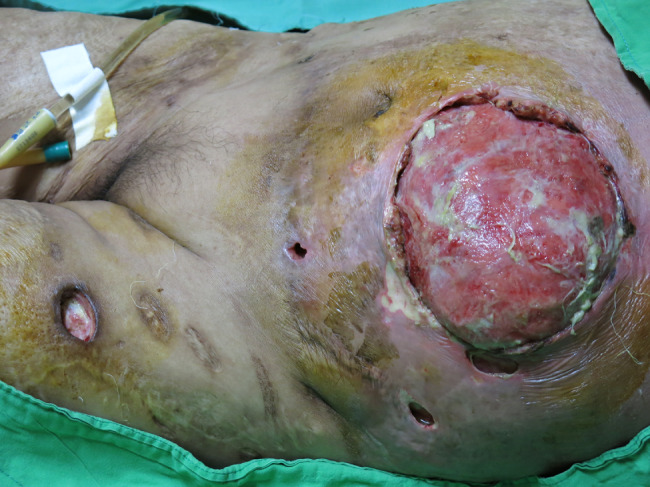
Intraoperative view with removal of necrotic skin.
Postoperatively, the patient was transferred to the intensive care unit and remained intubated for respiratory and inotropic support. The patient was administered empirical broad‐spectrum antimicrobial therapy, which was initiated soon after the diagnosis of NF. The pathology reports indicated acute necrotising inflammation of the fascia. Wound cultures revealed group A Streptococcus pyogenes. Targeted antibiotic coverage was started immediately following the report.
HBO was immediately started within 6 hours, and the patient received 90 minutes of 100% oxygen at 2·5 absolute atmospheres while inside the chamber. She used the chamber 5 days per week, once per day, with the exception of the day of operation. We repeated serial debridement and NPWT dressing twice per week for the next 3 weeks. After 21 days of NPWT, the infection was controlled, and the wound demonstrated marked improvements with fresh granulation tissue. Finally, the large wound was covered with a 2:1 meshed skin graft obtained from the back in a single stage. NPWT dressing was used for graft fixation for 5 days. The patient underwent a total of 20 sessions of HBO therapy, including five sessions after the skin graft operation, without any discomfort. The surgical wound completely healed (Figure 4), and the patient was discharged 35 days after hospital admission.
Figure 4.
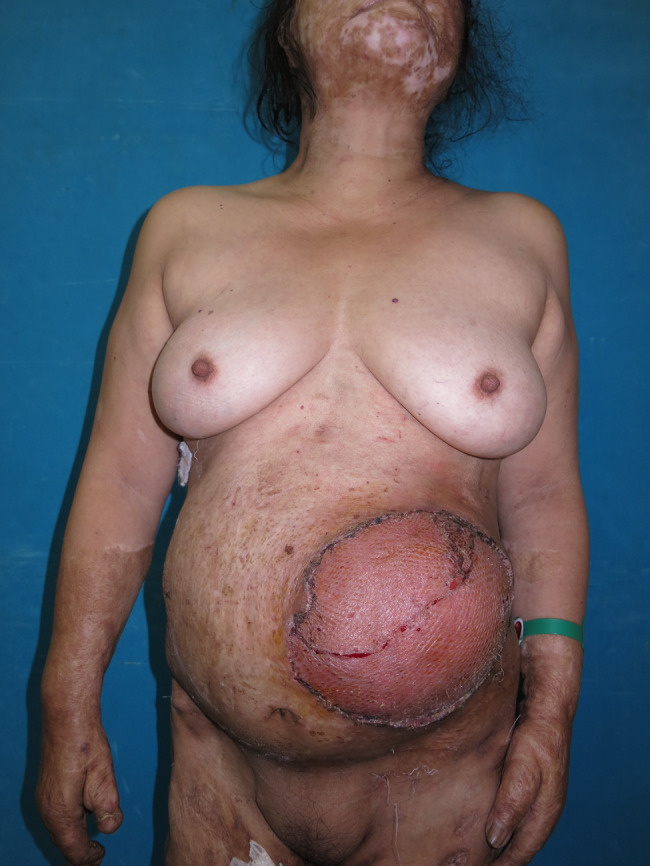
The patient was discharged after complete healing of the wound.
Discussion
NF is a rare, soft‐tissue infection with an incidence of 0–4 cases per 100 000. It is characterised by rapidly spreading necrosis of the soft tissue and fascia. The diagnosis of NF is primarily based on clinical findings. Without prompt recognition and immediate aggressive management, NF is often rapidly fatal 4, 5. NF of the central part of the body (trunk and perineal regions) has a higher mortality rate than that of the extremities because amputation is not feasible 5. Risk factors for NF include diabetes mellitus, trauma, wound infections, decubitus ulcers, alcoholism, carcinoma, peripheral vascular disease, smoking and intravenous drug abuse 6.
The key therapeutic intervention is early aggressive surgical debridement of all dead tissue, accompanied by prompt commencement of broad‐spectrum antibiotics and appropriate high‐dependency supportive care 7. Following radical debridement, closure of the remaining wound can pose significant reconstructive challenges. NPWT, also known as vacuum‐assisted closure, is a well‐known wound care system for the treatment of complex wounds. Accumulating evidence indicates that NPWT is useful for the management of infected wounds, including NF. The cyclical application of negative pressure can accelerate wound healing, where optimised blood flow increases local oxygenation and promotes angiogenesis, decreases local tissue oedema and accelerates removal of excessive fluid from the wound bed, which in turn reduces bacterial contamination 6, 8, 9.
Previous comparative studies showed that with the use of NPWT, patients had fewer dressing changes, less pain, fewer skipped meals, greater mobility and reduced length of hospital stay 6, 10, 11. In addition, NPWT has become a well‐established method for bolstering grafts to the recipient beds and improving graft outcomes by removal of exudate, reducing the risk of haematoma and seroma formation and increasing granulation tissue formation to improve revascularisation and attachment of the graft to the wound bed. This stabilises the graft and helps prevent shearing and removal 11.
Another important adjuvant treatment is the use of HBO, which has been used as an adjunct to surgery and antibiotics in the treatment of patients with NF with the aim of reducing morbidity and mortality compared to exclusive surgical debridement 8, 12. In a study of split‐thickness skin grafts, Perrins et al. showed a significantly higher graft survival percentage in patients treated with HBO 13.
HBO includes intermittent administration of 100% oxygen at pressures >1 atmosphere absolute in a pressure vessel. With intermittent HBO treatment, there are improvements in tissue oxygenation, phagocytosis and oedema and impairment of bacterial metabolism and exotoxin production. Furthermore, oxygen has a synergistic effect with antibiotics, enhancing angiogenesis and promoting wound healing. Hyperbaric chambers are safe and are used routinely for treating critically ill patients, with appropriate monitoring precautions and careful patient selection. Additionally, complications of HBO are rare and are usually self‐limiting 12, 14.
In our patient, a previous burn injury of the abdomen with scar bands along the soft tissue made liposuction difficult to manage. The dissected skin and subcutaneous layer can be vulnerable to microorganism infections and tissue swelling because of insufficient hygienic standards. Furthermore, the subcutaneous vascular system can be injured during liposuction, resulting in the worsening of abdominal circulation. Therefore, the vicious cycle of infection and ischaemia of the abdominal wall results in necrosis of the skin and NF.
There are limited published reports concerning the combined use of surgery with HBO and NPWT in the management of NF of the abdomen secondary to liposuction in a patient with a previous burn injury. Because of the efficacy of HBO and NPWT in infection control, wound healing and skin graft survival, our patient showed excellent results with combination treatment (surgery, antibiotics, HBO and NPWT).
Conclusion
Liposuction is one of the most frequently performed cosmetic surgeries worldwide. Particular care must be taken with patients who have a history of abdominal burn scars. Surgeons need to be aware of the clinical signs of NF and act promptly given the high mortality rate of the disease. We report an effective therapeutic approach with surgery, HBO and NPWT.
Author Contribution
IHC contributed to the literature search, data collection, data analysis, data interpretation, writing and figure collection and formatting. SCC contributed to the literature search, data collection and critical revision. CHW contributed to the study design, data analysis, data interpretation, writing and editing.
Acknowledgements
The authors thank the Civilian Administration Division of Tri‐Service General Hospital, National Defense Medical Center, Taipei, Taiwan. Conflicts of interest and sources of funding are not declared.
References
- 1. Grazer FM, de Jong RH. Fatal outcomes from liposuction: census survey of cosmetic surgeons. Plast Reconstr Surg 2000;105:436–46; discussion 447–8. [DOI] [PubMed] [Google Scholar]
- 2. Di Candia M, Malata CM. Aesthetic and functional abdominal wall reconstruction after multiple bowel perforations secondary to liposuction. Aesthetic Plast Surg 2011;35:274–7. [DOI] [PubMed] [Google Scholar]
- 3. You JS, Chung YE, Baek SE, Chung SP, Kim MJ. Imaging findings of liposuction with an emphasis on postsurgical complications. Korean J Radiol 2015;16:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine EG, Manders SM. Life‐threatening necrotizing fasciitis. Clin Dermatol 2005;23:144–7. [DOI] [PubMed] [Google Scholar]
- 5. Vayvada H, Demirdover C, Menderes A, Karaca C. Necrotising fasciitis in the central part of the body: diagnosis, management and review of the literature. Int Wound J 2013;10:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mizuguchi Y, Matsumoto S, Kan H, Koizumi M, Kuriyama S, Uchida E. Successful treatment of necrotizing fasciitis after rectal surgery with the application of a negative‐pressure wound therapy: a case study. J Nippon Med Sch 2015;82:290–4. [DOI] [PubMed] [Google Scholar]
- 7. Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg 2009;208:279–88. [DOI] [PubMed] [Google Scholar]
- 8. Pastore AL, Palleschi G, Ripoli A, Silvestri L, Leto A, Autieri D, Maggioni C, Moschese D, Petrozza V, Carbone A. A multistep approach to manage Fournier's gangrene in a patient with unknown type II diabetes: surgery, hyperbaric oxygen, and vacuum‐assisted closure therapy: a case report. J Med Case Rep 2013;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambert KV, Hayes P, McCarthy M. Vacuum assisted closure: a review of development and current applications. Eur J Vasc Endovasc Surg 2005;29:219–26. [DOI] [PubMed] [Google Scholar]
- 10. Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg 2009;197:660–5; discussion 665. [DOI] [PubMed] [Google Scholar]
- 11. Gupta S. Optimal use of negative pressure wound therapy for skin grafts. Int Wound J 2012;9(1 Suppl):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levett D, Bennett MH, Millar I. Adjunctive hyperbaric oxygen for necrotizing fasciitis. Cochrane Database Syst Rev 2015;1:CD007937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perrins DJ. Influence of hyperbaric oxygen on the survival of split skin grafts. Lancet 1967;1:868–71. [DOI] [PubMed] [Google Scholar]
- 14. Chiang IH, Chen SH, Huang KL, Chou YC, Dai NT, Peng CK. Adjunctive hyperbaric oxygen therapy in severe burns: experience in Taiwan Formosa Water Park dust explosion disaster. Burns 2016; pii: S0305-4179(16)30442-9. [DOI] [PubMed] [Google Scholar]


