Abstract
To identify risk factors for periprosthetic joint infection following primary total joint arthroplasty, a systematic search was performed in Pubmed, Embase and Cochrane library databases. Pooled odds ratios (ORs) or standardised mean differences (SMDs) with 95% confidence intervals (CIs) were calculated. Patient characteristics, surgical‐related factors and comorbidities, as potential risk factors, were investigated. The main factors associated with infection after total joint arthroplasty (TJA) were male gender (OR, 1·48; 95% CI, 1.19–1.85), age (SMD, −0·10; 95% CI, −0.17–−0.03), obesity (OR, 1·54; 95% CI, 1·25–1·90), alcohol abuse (OR, 1·88; 95% CI, 1·32–2·68), American Society of Anesthesiologists (ASA) scale > 2 (OR, 2·06; 95% CI, 1·77–2·39), operative time (SMD, 0·49; 95% CI, 0·19–0·78), drain usage (OR, 0·36; 95% CI, 0·18–0·74), diabetes mellitus (OR, 1·58; 95% CI, 1·37–1·81), urinary tract infection (OR, 1·53; 95% CI, 1.09–2.16) and rheumatoid arthritis (OR, 1·57; 95% CI, 1·30–1·88). Among these risk factors, ASA score > 2 was a high risk factor, and drain usage was a protective factor. There was positive evidence for some factors that could be used to prevent the onset of infection after TJA.
Keywords: Meta‐analysis, Periprosthetic joint infection, Risk factor, Systematic review, Total joint arthroplasty
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) have been proven to be highly successful procedures for improving quality of life and reducing pain in patients with severe joint diseases. However, despite the widely reported success of the procedures, prosthetic joint infection (PJI), as an infrequent but well‐recognised complication, affects some patients after joint arthroplasty 1. As reported by previous studies, PJI is associated with extremely poor postoperative outcomes and high incidence of mortality 2, 3. Several investigators have shown that the management of PJI is extremely costly, and it has placed a large economic burden on the health care system 4, 5. Thus, identifying potential risk factors is of great importance.
A number of risk factors for PJI after total joint arthroplasty (TJA) have been described, including obesity 6, 7, rheumatoid arthritis 8, operating time 6, urinary tract infection 9, blood transfusion 10 and diabetes mellitus 7. However, the results of clinical trials were various and markedly disagree. Meta‐analysis, by the method of pooling the results of high‐quality studies, could increase the statistical power of the association analysis and obtain more precise estimates of effect. Thus, we collected all relevant studies and made a comprehensive review. The purpose of this meta‐analysis was to identify risk factors, including intrinsic patient characteristics, surgical‐related factors and comorbid conditions, and quantify the magnitude of the risk in the patients undergoing TJA surgery.
Materials and methods
Literature search
We performed this meta‐analysis in accordance with the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) statement 11. Retrieval online was conducted in the following databases: PubMed, Embase and Cochrane library databases. The data of search was from the earliest available records in 1966 to 25 December 2015. The following search terms and Boolean operators were used to identify potential studies: (‘knee’ or ‘hip’ or ‘joint’) and (‘infection’) and (‘risk’ or ‘predictor’ or ‘factor’). The search was restricted to human subjects and those written in English. We also retrieved the references of all publications to identify additional studies for potential inclusion.
Inclusion and exclusion criteria
Two authors conducted the literature screening independently according to inclusion and excluding criteria. The following inclusive selection criteria were used 1: the study design was an observational study, including both cohort and case–control studies 2; patients underwent primary THA or TKA 3; PJI was investigated 4; possible risk factors for infection were explored; and 5 sufficient data were present to estimate the odds ratios (ORs) or standardised mean differences (SMDs) with 95% confidence intervals (CIs).
According to the Centres for Disease Control criteria 12, surgical site infections were classified into superficial incisional infection, deep incisional infection and space infection. For the joint, it is difficult to make a distinction between deep incisional infection and space infection. Thus, both types of infection were deemed to be PJI. Besides, during follow‐up, patients who underwent revised surgery because of infection were also considered PJI.
To decrease the heterogeneity, studies were excluded if they included patients with superficial surgical site infection or involved revised joint arthroplasty.
Data extraction and outcome measures
The following general characteristics were extracted from included studies: first author, publication year, country, period of investigation, number of patients in case and control group, infection ratio and identified significant risk factors. When the same population was reported in several publications, we retained only the most informative article or complete study to avoid duplication of information. Data were extracted independently by two authors, and any disagreements concerning paper eligibility were resolved by discussion and consensus.
Briefly, we investigated risk factors involving three aspects: patient‐related factors, surgical‐related factors and comorbid conditions. There were a total of 16 risk factors, including male gender, age, obesity, smoking, alcohol abuse, steroid usage, American Society of Anesthesiologists (ASA) scale, operative time, bilateral surgery, transfusion, drain usage, cementation, diabetes mellitus, urinary tract infection, hypertension and rheumatoid arthritis. In this study, obesity was defined as the body mass index ≥ 30 kg/m 2; ASA score greater than three was interpreted as significant system disease in decompensated state, and we tried to figure out whether greater ASA > 2 was a risk factor.
The methodological quality of studies was assessed independently by two reviewers using the Newcastle–Ottawa Scale (NOS) for observational studies 13. The scores ranged from 0 to 9, and a study with an NOS score ≥ 6 was considered high quality.
Statistical analysis
We used Stata 11·0 (Stata Corporation, College Station, TX) to conduct all statistical analyses. From every study, we extracted the data to calculate ORs for dichotomous outcomes and SMDs for continuous outcomes. If the exact number of patients with risk factors were not available, the ORs with 95% CI from univariate analysis were used. The associations between every potential factor and the risk of PJI were assessed, with P < 0·05 indicating a significant difference. Heterogeneity amongst the studies was qualitatively evaluated by Q‐test statistics, with the significance set at P < 0·10, and quantitatively tested by I 2 statistics, with I 2 > 50% indicating large inconsistency. A random‐effects model was used in the case of significant heterogeneity (P < 0·10 or I 2 > 50%); otherwise, a fixed‐effects model was used. Besides, we used OR value as a criteria to better illustrate the associations. If a significant difference exists, it was considered a high risk factor if OR ≥ 2, a moderate risk factor if 1 < OR < 2 and a protective factor if OR < 1.
As both THA and TKA were investigated, subgroup analyses were performed according to the position of surgery. Publication bias was assessed by the Begg test when the number of involved studies ≥ 10.
Results
Search results and study characteristics
As shown in Figure 1, the literature search yielded 254 titles of potentially relevant articles. Of these, 89 articles were excluded because of duplication. After title and abstract review, 129 records were excluded, and 36 full articles retained for further assessment. After full text review, 12 articles were excluded. Finally, 24 unique studies were included in the meta‐analysis 9, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36.
Figure 1.
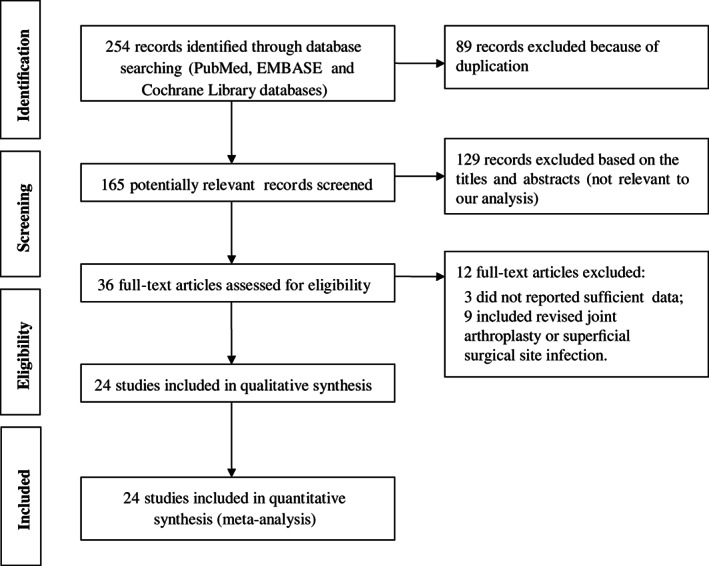
Flow diagram of literature search.
The basic characteristics of the included studies are summarised in Table 1. The publication year of the 24 studies ranged from 1998 to 2016. According to the NOS score, 9 studies scored 8 9, 17, 19, 22, 23, 25, 27, 32, 34, 11 studies scored 7 15, 16, 18, 24, 26, 28, 29, 30, 33, 35, 36 and 4 studies scored 6 14, 20, 21, 31. All studies were of high quality.
Table 1.
The basic characteristics of the 24 included studies
| First author | Publication year | Country | Period | Position | Case | Control | Infection ratio | NOS score | Identified significant risk factors |
|---|---|---|---|---|---|---|---|---|---|
| Berbari 36 | 1998 | United states | 1969–1991 | Hip, knee | 462 | 462 | – | 7 | Rheumatoid arthritis steroid therapy, diabetes mellitus |
| Dowsey 35 | 2008 | Australia | 1998–2005 | Hip | 22 | 1185 | 22/1207 | 7 | Obesity |
| Pulido 9 | 2008 | United states | 2001–2006 | Hip, knee | 63 | 9182 | 63/9245 | 8 | ASA score > 2, allogenic blood transfusion, urinary tract infection |
| Dowsey 34 | 2009 | Australia | 1998–2005 | Knee | 18 | 1196 | 18/1214 | 8 | Male gender, drain usage |
| Jamsen 33 | 2009 | Finland | 1997–2004 | Knee | – | – | ‐/40135 | 7 | Rheumatoid arthritis, male gender |
| Malinzak 32 | 2009 | United states | 1991–2004 | Hip, knee | 43 | 6065 | 43/6108 | 8 | Diabetes mellitus, obesity, younger age |
| Asensio 31 | 2010 | Spain | 2005–2006 | Knee | 44 | 106 | 50/5496 | 6 | Diabetes mellitus |
| Cordero‐Ampuero 30 | 2010 | Spain | 1997–2007 | Hip | 24 | 100 | – | 7 | Longer operative time, urinary tract infection |
| Pedersen 29 | 2010 | Denmark | 1995–2008 | Hip | 597 | 80159 | 597/80756 | 7 | Male gender |
| Peel 28 | 2011 | Australia | 2000–2007 | Hip, knee | 63 | 126 | – | 7 | Blood transfusion |
| Suzuki 27 | 2011 | Japan | 1995–2006 | Knee | 17 | 2005 | 17/2022 | 8 | Male gender, smoking |
| Bozic 26 | 2012 | United states | 1998–2007 | Knee | – | – | ‐/83011 | 7 | Diabetes mellitus, obesity, rheumatoid arthritis |
| Bozic 25 | 2012 | United states | 1998–2007 | Hip | 1371 | 39548 | 1371/40919 | 8 | Rheumatoid arthritis, obesity, diabetes mellitus |
| Jamsen 24 | 2012 | Finland | 2002–2008 | Hip, knee | 52 | 7129 | 52/7181 | 7 | Obesity, diabetes mellitus |
| Namba 23 | 2012 | United states | 2001–2009 | Hip | 155 | 30336 | 155/30491 | 8 | Diabetes mellitus, obesity, ASA score >2, bilateral surgery, longer operating time |
| Namba 22 | 2013 | United states | 2001–2009 | Knee | 404 | 55812 | 404/56216 | 8 | Male gender, bilateral surgery |
| Somayaji 21 | 2013 | Canada | 2000–2010 | Hip, knee | 5 | 254 | 5/259 | 6 | – |
| Bozic 20 | 2014 | United states | 1990–2011 | Hip | 89 | 499 | – | 6 | Obesity, female gender |
| Gomez‐Lesmes 19 | 2014 | Spain | 2007–2009 | Knee | 32 | 1299 | 32/1331 | 8 | Male gender, diabetes mellitus, ASA score >2, blood transfusion |
| Sousa 18 | 2014 | United Kingdom, Portugal, and Spain | 2010–2011 | Hip, knee | 43 | 2454 | 43/2497 | 7 | ASA score >2 |
| Wu 17 | 2014 | China | 2000–2012 | Hip, knee | 45 | 252 | – | 8 | Old age, obesity, alcohol abuse |
| Crowe 16 | 2015 | United states | 2009–2011 | Knee | 26 | 3393 | 26/3419 | 7 | Male gender, smoking |
| Gupta 15 | 2015 | United states | 2001–2006 | Hip, knee | 339 | 339 | – | 7 | Obesity, diabetes mellitus |
| Amin 14 | 2016 | United states | 2008–2012 | Knee | 16 | 1612 | 16/1628 | 6 | – |
ASA, American Society of Anesthesiologists; NOS, Newcastle–Ottawa Scale.
Main results of meta‐analysis
Of the 24 studies, 16 reported the incidence of PJI 9, 14, 16, 18, 19, 21, 22, 23, 24, 25, 27, 29, 31, 32, 34, 35. Based on the results of 16 studies, the infection rate ranged from 0·51% to 3·35%, and the cumulated infection rate was 1·17%. Significant heterogeneity was observed among the studies when evaluating the following potential risk factors: obesity, operative time, bilateral surgery, transfusion, cementation, diabetes mellitus, urinary tract infection, hypertension and rheumatoid arthritis. Based on the combined ORs or SMDs, we identified the following risk factors: male gender (OR, 1·48; 95% CI, 1·19–1·85), age (SMD, −0·10; 95% CI, −0.17–−0·03), obesity (OR, 1·54; 95% CI, 1·25–1.90), alcohol abuse (OR, 1·88; 95% CI, 1·32–2·68), ASA score > 2 (OR, 2·06; 95% CI, 1·77–2·39), operative time (SMD, 0·49; 95% CI, 0·19–0·78), drain usage (OR, 0·36; 95% CI, 0·18–0·74), diabetes mellitus (OR, 1·58; 95% CI, 1·37–1·81), urinary tract infection (OR, 1·53; 95% CI, 1·09–2·16) and rheumatoid arthritis (OR, 1·57; 95% CI, 1·30–1·88). Among the significant risk factors, ASA score > 2 was a high risk factor, and drain usage was a protective factor. Other factors were not identified as significant risk factors for infection following TJA (P > 0.05).
We further conducted subgroup analyses according to the position of surgery. In the THA subgroup, male gender was not a significant risk factor (OR, 1·21; 95% CI, 0·95–1·55); however, it was considered to be a significant risk factor in TKA subgroup (OR, 2·39; 95% CI, 1·69–3·39). Besides, in the THA subgroup, there was no significant difference in age between case and control groups (SMD, −0·04; 95% CI, −0·19–0·11), but the age was significantly younger in case group than control group in the TKA subgroup (SMD, −0·10; 95% CI, −0·19–−0·01). The main results of this meta‐analysis are listed in Table 2, and analysis for male gender, obesity, diabetes mellitus and rheumatoid arthritis as significant risk factors is presented by forest plots in Figure 2.
Table 2.
The main outcomes of meta‐analysis and subgroup analysis
| Risk factors | No. of studies | OR or SMD | LL 95%CI | UL 95%CI | P value | Q‐test (P) | I 2 (%) |
|---|---|---|---|---|---|---|---|
| Male gender | 15 | 1·48* | 1·19 | 1·85 | <0·01‡ | <0·01 | 74·2 |
| THA subgroup | 5 | 1·21* | 0·95 | 1·55 | 0·85‡ | <0·01 | 74·7 |
| TKA subgroup | 6 | 2·39* | 1·69 | 3·39 | <0·01‡ | <0·01 | 68·2 |
| Age | 8 | −0·10† | −0·17 | −0·03 | <0·01§ | 0·55 | 0·0 |
| THA subgroup | 2 | −0·04† | −0·19 | 0·11 | 0·63§ | 0·47 | 0·0 |
| TKA subgroup | 4 | −0·10† | −0·19 | −0·01 | 0·03§ | 0·91 | 0·0 |
| Obesity (BMI ≥ 30) | 12 | 1·54* | 1·25 | 1·90 | <0·01‡ | 0·01 | 58·8 |
| THA subgroup | 4 | 2·04* | 1·71 | 2·44 | <0·01§ | 0·97 | 0·0 |
| TKA subgroup | 4 | 1·39* | 1·21 | 1·60 | <0·01§ | 0·69 | 0·0 |
| Smoking | 5 | 1·48* | 0·83 | 2·64 | 0·18§ | 0·55 | 0·0 |
| THA subgroup | 1 | 0·32* | 0·04 | 2·37 | 0·26§ | – | – |
| TKA subgroup | 3 | 1·84* | 0·93 | 3·63 | 0·08§ | 0·85 | 0·0 |
| Alcohol abuse | 3 | 1·88* | 1·32 | 2·68 | <0·01§ | 0·42 | 0·0 |
| THA subgroup | 1 | 2·09* | 1·25 | 3·50 | <0·01§ | – | – |
| TKA subgroup | 1 | 1·45* | 0·83 | 2·53 | 0·19§ | – | – |
| Steroid usage | 2 | 0·85* | 0·34 | 2·11 | 0·73§ | 0·84 | 0·0 |
| THA subgroup | 2 | 0·85* | 0·34 | 2·11 | 0·73§ | 0·84 | 0·0 |
| TKA subgroup | 0 | – | – | – | – | – | – |
| ASA score >2 | 6 | 2·06* | 1·77 | 2·39 | <0·01§ | 0·62 | 0·0 |
| THA subgroup | 1 | 2·37* | 1·71 | 3·27 | <0·01§ | – | – |
| TKA subgroup | 3 | 1·89* | 1·57 | 2·28 | 0·01§ | 0·62 | 0·0 |
| Operative time | 4 | 0·49† | 0·19 | 0·78 | <0·01‡ | <0·01 | 87·5 |
| THA subgroup | 2 | 0·32† | 0·17 | 0·47 | <0·01‡ | <0·01 | 95·8 |
| TKA subgroup | 2 | 0·31† | 0·22 | 0·40 | <0·01§ | 0·78 | 0·0 |
| Bilateral surgery | 2 | 0·80* | 0·53 | 1·21 | 0·30‡ | <0·01 | 94·6 |
| THA subgroup | 1 | 3·80* | 1·67 | 8·66 | <0·01‡ | – | – |
| TKA subgroup | 1 | 0·47* | 0·29 | 0·76 | <0·01‡ | – | – |
| Transfusion | 8 | 1·44* | 0·87 | 2·39 | 0·16‡ | <0·01 | 76·9 |
| THA subgroup | 2 | 0·57* | 0·06 | 5·73 | 0·63‡ | <0·01 | 92·4 |
| TKA subgroup | 3 | 1·95* | 0·79 | 4·83 | 0·15‡ | 0·04 | 68·2 |
| Drain usage | 4 | 0·36* | 0·18 | 0·74 | <0·01§ | 0·18 | 38·0 |
| THA subgroup | 1 | 1·36* | 0·32 | 5·89 | 0·68§ | – | – |
| TKA subgroup | 1 | 0·24* | 0·06 | 0·96 | 0·04§ | – | – |
| Cementation | 2 | 0·95* | 0·34 | 2·65 | 0·91‡ | 0·14 | 55·1 |
| THA subgroup | 1 | 2·10* | 0·49 | 9·07 | 0·32‡ | – | – |
| TKA subgroup | 0 | – | – | – | – | – | – |
| Diabetes mellitus | 15 | 1·58* | 1·37 | 1·81 | <0·01‡ | 0·05 | 41·9 |
| THA subgroup | 5 | 1·51* | 1·33 | 1·71 | <0·01§ | 0·47 | 0·0 |
| TKA subgroup | 7 | 1·66* | 1·25 | 2·19 | <0·01‡ | 0·01 | 63·1 |
| Urinary tract infection | 5 | 1·53* | 1·09 | 2·16 | 0·01‡ | <0·01 | 81·7 |
| THA subgroup | 2 | 4·80* | 0·25 | 92·9 | 0·30‡ | <0·01 | 92·4 |
| TKA subgroup | 1 | 1·14* | 1·03 | 1·27 | 0·01‡ | – | – |
| Hypertension | 7 | 1·09* | 0·92 | 1·28 | 0·32‡ | 0·10 | 43·3 |
| THA subgroup | 2 | 1·07* | 0·68 | 1·69 | 0·78‡ | 0·14 | 54·0 |
| TKA subgroup | 3 | 1·07* | 0·98 | 1·17 | 0·15§ | 0·15 | 47·7 |
| Rheumatoid arthritis | 12 | 1·57* | 1·30 | 1·88 | 0·03‡ | 0·06 | 41·8 |
| THA subgroup | 4 | 1·75* | 1·49 | 2·06 | <0·01§ | 0·30 | 18·7 |
| TKA subgroup | 5 | 1·34* | 1·18 | 1·52 | <0·01§ | 0·17 | 37·9 |
ASA, American Society of Anesthesiologists; BMI, body mass index; LL, lower limit; OR, odds ratios; SMD, standardised mean differences; THA, total hip arthroplasty; TKA, total knee arthroplasty; UL, upper limit.
OR.
SMD.
Random‐effects model was performed.
Fixed‐effects model was performed.
Figure 2.
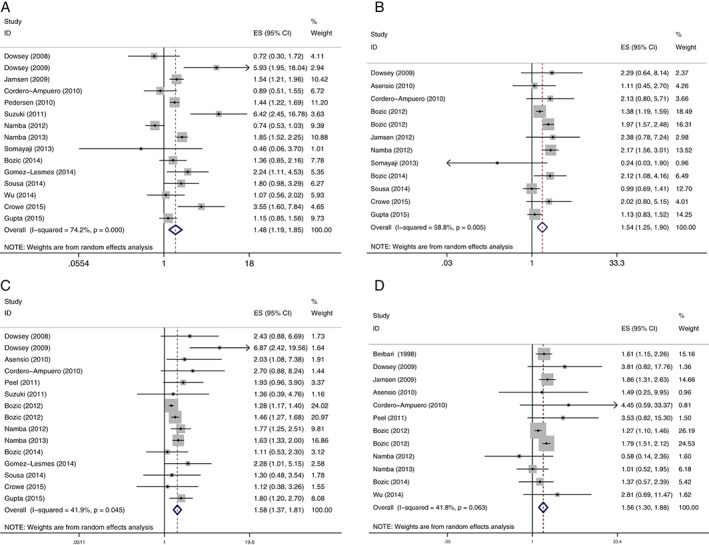
Forest plots of the meta‐analysis of (A) male gender, (B) obesity, (C) diabetes mellitus and (D) rheumatoid arthritis as significant risk factors for periprosthetic joint infection after total joint arthroplasty.
Publication bias
The Begg test showed that no significant publication bias was found among the studies investigating infection and risk factors for male gender (P = 0.49), obesity (P = 0.84), diabetes mellitus (P = 0.84) or rheumatoid arthritis (P = 0.73).
Discussion
Our meta‐analysis comprehensively and systematically reviewed the current available literature and found that (a) the accumulated incidence of PJI after TJA was 1·17%; (b) multiple risk factors were identified to be associated with PJI, including male gender, age, obesity, alcohol abuse, ASA score > 2, operative time, drain usage, diabetes mellitus, urinary tract infection and rheumatoid arthritis; (c) smoking, steroid use, bilateral surgery, transfusion, cementation or hypertension has not been proven to be risk factors and (d) younger age and male gender may have had an effect on infection after TKA but may not be associated with infection after THA.
In the clinical practice, obese patients are more likely to undergo TJA 37. Our results showed that the risk of PJI in obese patients is 1·9‐fold higher than non‐obese patients. Obese patients may be at an increased risk of PJI because of prolonged operative time or the presence of other medical comorbidities. Besides, this patient population is also at an increased risk of wound complications such as haematoma formation and wound dehiscence. ASA score was a physical status score of preoperative risk as documented in an anaesthetist's preoperative assessment chart. A score greater than three was interpreted as significant system disease in decompensated state. In our study, we found that ASA ≥ 2 was another patient‐related risk factor of infection after TJA, and this result is in accordance with several previous studies 6, 38.
PJI developed in THA and TKA had many similarities, but there are still some differences that deserve a discussion. In the subgroup analysis, younger age and male gender was proven to be associated with PJI after TKA, but the difference between groups was not seen in the THA subgroup. The underlying mechanism causing this difference has yet to be defined. We assumed that younger and male patients are generally more active than older and female patients and thus may potentially cycle their implant in greater numbers, leading to a higher chance of infection. It is possible that this effect is more obvious in knee joint prostheses than hip joint prostheses. However, we cannot draw a definite conclusion yet without further studies.
Operative time and drain usage were two surgical‐related factors that were proven to be associated with joint infection following TJA. A previous study conducted by Namba et al. observed an increased risk of infection per every additional 15 minutes of operative time 22. In the study conducted by Kurtz et al., the authors found that a TKA operative time of longer than 210 minutes, compared with less than 120 minutes, was associated with an increased risk of infection 39. Their conclusions were in accordance with our findings. Drain usage was found to have a protective effect against PJI. There is a chance that drain usage decreases the incidence of haematoma formation and subsequently decreased the risk of infection.
Among the comorbid conditions, diabetes mellitus and rheumatoid arthritis were proven to be associated with PJI after TJA. Several studies have suggested that diabetes increase the risk of postoperative infection in TJA patients 7, 24. Diabetic patients are known to be susceptible to infection because of their impaired defences against bacteria. What is more, diabetes mellitus could impair wound healing because microangiopathic changes could reduce the tissue concentrations of antibiotics and lead to local tissue ischaemia 40. Previous epidemiological studies have identified that rheumatoid arthritis predisposes patients to PJI because of immunosuppressive conditions. However, a challenge is to differentiate whether the increased risk is because of the underlying condition or the immunomodulatory therapy 41, 42, 43. Rheumatoid arthritis may also be associated with poor nutritional status, which could also lead to an increased risk of postoperative PJI.
This meta‐analysis was conducted in a strict and comprehensive process, but there are still some limitations that should be noticed. First, nearly all of the included studies were observational and retrospective. This could result in considerable bias and had potential impacts on our final results. Second, the original diseases, the race of patients and duration of follow‐up were varied among these studies, which definitely resulted in considerable heterogeneity and affected our results. Third, because of the limited numbers of studies, it was impossible to estimate the effects of every possible risk factor. Further studies should pay more attention to other factors.
In summary, the present analysis demonstrates that male gender, age, obesity, alcohol abuse, ASA score > 2, operative time, drain usage, diabetes mellitus, urinary tract infection and rheumatoid arthritis are significant risk factors for PJI after TJA surgery. Awareness of these risk factors will help surgeons optimise the patient's preoperative condition and surgical procedure and help decrease the incidence of postoperative infection. However, there was still a need for further high‐quality research to strengthen the evidence.
Author contribution
LK and JC conducted the literatures screening; WD and YS extracted data; and LK and YZ assessed the methodological quality of studies. LK and YS wrote the manuscript. JC and YS reviewed the manuscript. All persons designated as authors are qualified for authorship.
References
- 1. Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ 2009;338:b1773. [DOI] [PubMed] [Google Scholar]
- 2. Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one‐year mortality. J Bone Joint Surg Am 2013;95:2177–84. [DOI] [PubMed] [Google Scholar]
- 3. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic‐joint infections. N Engl J Med 2004;351:1645–54. [DOI] [PubMed] [Google Scholar]
- 4. Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin‐resistant infections. J Arthroplasty 2010;25:103–7. [DOI] [PubMed] [Google Scholar]
- 5. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;27:61–5. [DOI] [PubMed] [Google Scholar]
- 6. Maoz G, Phillips M, Bosco J, Slover J, Stachel A, Inneh I, Iorio R. The Otto Aufranc Award: Modifiable versus nonmodifiable risk factors for infection after hip arthroplasty. Clin Orthop Relat Res 2015;473:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Everhart JS, Altneu E, Calhoun JH. Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty. Clin Orthop Relat Res 2013;471:3112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, Fevang BT. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population‐based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res (Hoboken) 2010;62:473–9. [DOI] [PubMed] [Google Scholar]
- 9. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinitz D, Harvey EJ, Leighton RK, Petrie DP. Is homologous blood transfusion a risk factor for infection after hip replacement? Can J Surg 2001;44:355–8. [PMC free article] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 12. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–8. [PubMed] [Google Scholar]
- 13. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 14. Amin NH, Omiyi D, Kuczynski B, Cushner FD, Scuderi GR. The risk of a deep infection associated with intraarticular injections before a total knee arthroplasty. J Arthroplasty 2016;31:240–4. [DOI] [PubMed] [Google Scholar]
- 15. Gupta A, Osmon DR, Hanssen AD, Lightner DJ, Wilson WR, Steckelberg JM, Baddour LM, Harmsen WS, Mandrekar JN, Berbari EF. Genitourinary procedures as risk factors for prosthetic hip or knee infection: a hospital‐based prospective case–control study. Open Forum Infect Dis 2015;2:v97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowe B, Payne A, Evangelista PJ, Stachel A, Phillips MS, Slover JD, Inneh IA, Iorio R, Bosco JA. Risk factors for infection following total knee arthroplasty: a series of 3836 cases from one institution. J Arthroplasty 2015;30:2275–8. [DOI] [PubMed] [Google Scholar]
- 17. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One 2014;9:e95300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sousa R, Munoz‐Mahamud E, Quayle J, Dias DCL, Casals C, Scott P, Leite P, Vilanova P, Garcia S, Ramos MH, Dias J, Soriano A, Guyot A. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014;59:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomez‐Lesmes SP, Tornero E, Martinez‐Pastor JC, Pereira A, Marcos M, Soriano A. Length of storage of transfused red blood cells and risk of prosthetic joint infection after primary knee arthroplasty. J Arthroplasty 2014;29:2016–20. [DOI] [PubMed] [Google Scholar]
- 20. Bozic KJ, Ward DT, Lau EC, Chan V, Wetters NG, Naziri Q, Odum S, Fehring TK, Mont MA, Gioe TJ, Della VC. Risk factors for periprosthetic joint infection following primary total hip arthroplasty: a case control study. J Arthroplasty 2014;29:154–6. [DOI] [PubMed] [Google Scholar]
- 21. Somayaji R, Barnabe C, Martin L. Risk factors for infection following total joint arthroplasty in rheumatoid arthritis. Open Rheumatol J 2013;7:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am 2013;95:775–82. [DOI] [PubMed] [Google Scholar]
- 23. Namba RS, Inacio MC, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br 2012;94:1330–8. [DOI] [PubMed] [Google Scholar]
- 24. Jamsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single‐center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am 2012;94:e101. [DOI] [PubMed] [Google Scholar]
- 25. Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, Vail TP, Berry DJ. Patient‐related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am 2012;94:794–800. [DOI] [PubMed] [Google Scholar]
- 26. Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient‐related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res 2012;470:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki G, Saito S, Ishii T, Motojima S, Tokuhashi Y, Ryu J. Previous fracture surgery is a major risk factor of infection after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2011;19:2040–4. [DOI] [PubMed] [Google Scholar]
- 28. Peel TN, Dowsey MM, Daffy JR, Stanley PA, Choong PF, Buising KL. Risk factors for prosthetic hip and knee infections according to arthroplasty site. J Hosp Infect 2011;79:129–33. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen AB, Svendsson JE, Johnsen SP, Riis A, Overgaard S. Risk factors for revision due to infection after primary total hip arthroplasty. A population‐based study of 80,756 primary procedures in the Danish Hip Arthroplasty Registry. Acta Orthop 2010;81:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cordero‐Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res 2010;468:3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asensio A, Antolin FJ, Sanchez‐Garcia JM, Hidalgo O, Hernandez‐Navarrete MJ, Bishopberger C, Miguel LG, Gay‐Pobes A, Cabrera‐Quintero A, Asensio P, Sanz‐Sebastian C, Gonzalez‐Torga A, Ortiz‐Espada A, Perez‐Serrano L, Ramos A. Timing of DVT prophylaxis and risk of postoperative knee prosthesis infection. Orthopedics 2010;33:800. [DOI] [PubMed] [Google Scholar]
- 32. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty 2009;24:84–8. [DOI] [PubMed] [Google Scholar]
- 33. Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register‐based analysis of 43,149 cases. J Bone Joint Surg Am 2009;91:38–47. [DOI] [PubMed] [Google Scholar]
- 34. Dowsey MM, Choong PF. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res 2009;467:1577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dowsey MM, Choong PF. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res 2008;466:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis 1998;27:1247–54. [DOI] [PubMed] [Google Scholar]
- 37. Salih S, Sutton P. Obesity, knee osteoarthritis and knee arthroplasty: a review. BMC Sports Sci Med Rehabil 2013;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song KH, Kim ES, Kim YK, Jin HY, Jeong SY, Kwak YG, Cho YK, Sung J, Lee YS, Oh HB, Kim TK, Koo KH, Kim EC, Kim JM, Choi TY, Kim HY, Choi HJ, Kim HB. Differences in the risk factors for surgical site infection between total hip arthroplasty and total knee arthroplasty in the Korean Nosocomial Infections Surveillance System (KONIS). Infect Control Hosp Epidemiol 2012;33:1086–93. [DOI] [PubMed] [Google Scholar]
- 39. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the medicare population. Clin Orthop Relat Res 2010;468:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodson WR, Hung TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res 1977;22:221–7. [DOI] [PubMed] [Google Scholar]
- 41. White RH, McCurdy SA, Marder RA. Early morbidity after total hip replacement: rheumatoid arthritis versus osteoarthritis. J Gen Intern Med 1990;5:304–9. [DOI] [PubMed] [Google Scholar]
- 42. Giles JT, Bartlett SJ, Gelber AC, Nanda S, Fontaine K, Ruffing V, Bathon JM. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum 2006;55:333–7. [DOI] [PubMed] [Google Scholar]
- 43. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014;27:302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


