Abstract
The aims of this study were to observe the effects of vacuum sealing drainage (VSD) with three different negative pressures on the wound healing rate, macrophage count and the expression of hyaluronic acid (HA) as well as its receptor CD44 in seawater‐immersed blast‐injury wounds (SIBIW) and to determine the optimal negative pressure value. In a minipig SIBIW model, different suction pressures and routine dressing were applied. Histological and immunohistochemical comparisons as well as molecular biology methods were performed to compare the wound healing conditions, macrophage count and the levels of HA and CD44. The wound healing rate of the VSD group was significantly higher than that of the control group, with the −120 mmHg group exhibiting the best effects. The macrophage count of the VSD group was higher than that of the control group. The HA level fluctuation was higher in the VSD group, with the −120 mmHg and the −180 mmHg groups showing the most significant fluctuation (P < 0·05). CD44 was expressed in the full‐thickness wound‐limbic tissues and was higher in the treatment group than that in the control group, with the −120 mmHg group having the most obvious expression. VSD significantly improved the healing ability and increased the macrophage count and the HA content. It also promoted CD44 expression. −120 mmHg is the optimal negative pressure value.
Keywords: Blast injury, Macrophage, Seawater immersion, Vacuum sealing drainage, Wound healing rate
Introduction
In recent years, with the development of modern technology and the gradual increase in human exploration and exploitation of marine resources, marine activities have become very common. Marine operations, dangerous marine life attacks, naval wars and other accidents lead to the wounded falling into the sea and developing seawater‐immersed blast‐injury wounds (SIBIW). Moreover, the high temperature and pressure generated by the explosion interact with the low temperature, high bacterial content, high osmotic pressure and high alkalinity of the sea water, thus resulting in a wound with infection in the surrounding tissues and other conditions including microcirculation disorders. It also causes local oedema, haemorrhage, degeneration and other symptoms, with the severe cases exhibiting tissue necrosis and systemic complications 1, 2, 3.
Vacuum sealing drainage (VSD) is suitable for the treatment of refractory wounds of various causes including acute and chronic skin defects, infections and skin grafts and has a wide range of applications 4, 5. However, there are no reports on the effects of VSD on the wound healing rate of SIBIW, the macrophage count and the expression of hyaluronic acid (HA) and its receptor CD44 in the wound‐limbic tissues. In this study, the minipig model was used for the dynamic observation of the effects of VSD with three different negative pressures on the wound healing rate, macrophage count and the expression levels of HA and its receptor CD44 in SIBIW. We aimed to summarise the application guidelines of VSD treatment in SIBIW and to determine the ideal pressure value and the corresponding treatment programme for such wounds, which would be applicable in the clinical setting.
Materials and methods
Animals and grouping
Five pure‐bred minipigs (Chinese No. 1), with an average weight of 25 kg and no gender restrictions, were provided by the Kexing experimental animal breeding centre (Liulihe, Beijing). The animals were domesticated with the standard pig diet for 1 week before the experiment for improving adaptability. Each pig was kept individually, and normal eating and drinking was maintained. One SIBIW was inflicted bilaterally on the shoulders and hips of each pig. The animals were then randomly divided into the control group (routine dressing) and experiment group (VSD treatment with −120, −180 and −240 mmHg pressure). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the 309th Hospital of PLA.
Preparation of the animal model
Seawater (pH 7·9) was obtained from near Bohai Bay and was stored at 4°C. The temperature was maintained at 10°C during the experiment, and the laboratory temperature was 25°C. After 12 hour of fasting and water deprivation, the skin on the pigs' bilateral shoulders and hips was prepared, and a small incision, about 3 cm long and 1 cm deep, was made in the centre of the prepared skin area. An electric detonator (3·6 × 12 type, developed by the Xi'an 213 Institute, Xi'an, China) was implanted into the skin and fixed with scotch tape; the detonator was then triggered with direct current. During the preliminary experiment, three minipigs died in the morning and afternoon hours of the day after the explosion. The autopsy showed that the causes of death were haemorrhagic shock and pulmonary embolism; thus, it was presumed that the explosion was too strong and had caused massive blood loss, and the prolonged seawater immersion had caused hypothermia. Therefore, the explosive power was weakened, and the seawater immersion time was shortened.
The required minipigs were re‐purchased for the experiment; the detonator was placed 1·5 cm away from the skin and the wound immersion time was shortened to 1 hour. The rest of the procedure was as described above. After the immersion, a large amount of saline was used to perform complete debridement (including iron, wood, dust, other foreign matter and residual seawater). The pig received an intramuscular penicillin 800 000 U/day injection to prevent infection. According to the size and shape of the wound, the VSD dressing was trimmed (provided by the 4th Military Medical University). A transparent surgical film was then used to cover the wound completely. The VSD device (Yuyue 7A‐23D adjustable electric aspirator, Jiangsu Yuyue Medical Equipment Co., Ltd., Nanjing, China) was connected, the drainage pipe was connected with the tee junctor and VSD device and the surrounding wound was sealed with a medical adhesive plaster to prevent leakage. The pressure values were adjusted to −120, −180 and −240 mmHg. The foaming sponge‐like collapse and effusion were drained. The experimental animals were then sent back to the cage and were allowed water access 6 hour later and diet access 12 hour later. To maintain continuous vacuum drainage, the leakage and dressing blockage were kept under check; if these occur, the film or VSD dressing should be replaced in a timely fashion.
Gross morphological observation of wounds
The wounds were dressed on the 0, 3rd, 7th, 16th and 30th days of the experiment, and the gross morphology was photographed at the same time for collection of image data. Dynamic observation and comparisons were performed among the groups to assess the wound size, degree of swelling as well as the conditions of granulation growth and epithelial coverage on the wound edge and base. After 9 days, the VSD was removed and changed to a regular dressing, after which the wound was kept under observation.
Evaluation of wound healing
The healing conditions were observed and recorded at the designated postoperative time points. Total epithelialisation of the wound, no oozing, scab formation and falling of the scab were set as the criteria for wound healing. Transparent film plethysmography was used to record the wound area and wound healing rates 3, 5, 7, 9, 16, and 23 days after the explosion. The procedure was performed based on the Nagelschmidt method 6, and the specific steps were as follows: Following debridement, a sterile transparent plastic film was used to cover the wound, and the wound edges were carefully marked. The marked figures were then pictured out on a cardboard with a uniform density, which was then cut and weighed. The formula used was: wound healing rate = original wound area − wound area on the measuring day/original wound area × 100%.
where r is the wound healing rate; S 0 is the original wound area; S 1 is the unhealed area; M 0 and M 1 represent the quality of the cardboard at the beginning of the experiment and on the measuring day, respectively; and ρ and h represent the density and thickness of the cardboard, respectively.
Quantitative analysis of macrophages
A tissue piece, about 3 × 5 mm2, was cut from the edge of the wound in the experiment group and control group pigs on the first, third, fifth, seventh and ninth post‐wound days. The specimens were then fixed overnight in 10% neutral formalin, embedded in paraffin, cut into 5 µm‐thick slices and stained with haematoxylin and eosin. Then, the slices were observed with an optical microscope, and under five randomly chosen vision fields at 400‐fold magnification, the number of macrophages were observed in each field, and the mean value of these five vision fields was used as the final count.
Enzyme‐linked immunosorbent assay
The dressing was removed on the 0, 1st, 3rd, 5th, 7th, 9th, 16th and 23rd day post‐VSD, and fresh granulation tissue weighing about 1 g was taken from each wound. Then, a specific amount of phosphate buffer solution (PBS, pH 7·4) was added. Following this, the above mixture was rapidly frozen with liquid nitrogen and stored. After thawing, the specimen still remained at 2–8°C. After adding PBS (pH 7·4), the specimens were sufficiently homogenised, followed by centrifugation for 20 minutes (626–1049 g). The supernatant was carefully collected and packaged, with one part used for detection and the remaining frozen for future use. The double‐antibody sandwich enzyme‐linked immunosorbent assay (ELISA) was performed to detect the HA levels; the absorbance (OD) at 450 nm wavelength was then determined by the ELISA method, and the standard curve was used to calculate the HA content within the sample. The ELISA kit was produced by Abcam Company (Cambridge, MA), the primary antibody CD44 (rabbit anti‐pig) by Beijing Biosynthesis Co., Ltd. (Beijing, China) and the secondary antibody (goat anti‐rabbit) by the Beijing Zhongshan Golden Bridge Co. (Beijing, China).
Immunohistochemical staining
The skin of the minipigs was selected as the experimental sample, and paraffin embedding and dewaxing until water and antigen retrieval were performed; the endogenous catalase was then removed, and the normal goat serum blocking solution was added dropwise onto the slice. The primary antibody was then added dropwise onto the slice and incubated overnight at 4°C. After washing with PBS, one drop of biotinylated secondary antibody was added dropwise onto the slice (IgG) and incubated at 37°C for 20 minutes; the slice was then washed with PBS, which was followed by addition of one drop of horseradish peroxidase–labelled streptavidin and incubation at 37°C for 20 minutes. We then performed diaminobenzidine staining, re‐staining and mounting. The tissues of interest were selected at this time, and image analysis was performed.
Statistical processing
The SPSS version 20.0 software (SPSS Inc., Chicago, IL) was used for statistical processing; the experimental data were expressed as mean ± standard deviation () and the repeated measurements analysis of variance was used.
Results
Gross morphological observation
The wounds were almost similar, with an irregular shape, and a long diameter of about 8 cm, a short diameter of about 6 cm and a depth of about 1 cm. The wound exhibited a small amount of bleeding. The necrotic skin was cut.
On the third post‐VSD day, the surface of the wound in the control group was dirty, the muscular tissues exhibited obvious oedema and the bottom of the wound was uneven with more exudate. The skin around the wound was red, and the partial skin was necrotic. The surface of the wound in the treatment group was clean, dry and ruddy. It bled easily when touched, and the muscle exhibited slight oedema. The bottom of the wound was uneven and without exudate and odour; the skin around the wound was mildly red without necrosis, and there was a vigorous growth of granulation tissue (Figure 1A–D).
Figure 1.
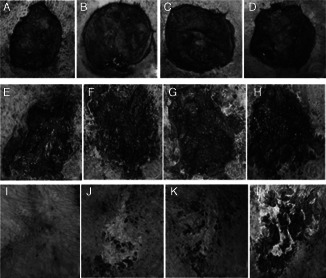
VSD results. (A–D): the −120 mmHg group, −180 mmHg group, −240 mmHg group and control group on the third day; (E–H): the −120 mmHg group, −180 mmHg group, −240 mmHg group and control group on the 16th day; (I–L): the −120 mmHg group, −180 mmHg group, −240 mmHg group and control group on the 30th day.
On the seventh post‐VSD day, the wound in the control group still had the blood scab attached, with a small amount of new epithelial tissue formation at the wound edge and a small amount of granulation tissue formed within the wound; the oozing, however, had increased. The wound of the treatment group was clean, with much more newly generated epithelium at the wound edge. A large amount of granulation tissue had formed within the wound, the oozing had decreased and the wound base was shallow.
On the 16th post‐VSD day, the wound formed a scab, which was followed by healing under the scab with no infection. Partial regeneration of a skin island on the wound surface of the control group was seen. The wound edge showed contraction, and the wound size decreased, but the wound surface continued to have blood crust adhesions. The wound of the treatment group was much cleaner than that of the control group, with an obvious contracture of the wound edge. The wound was significantly reduced in size; wound healing in the treatment group was significantly faster than that in the control group (Figure 1E–H).
On the 30th post‐VSD day, the wound in the treatment group was completely healed, while that in the control group still exhibited scabs (Figure 1I–L).
Evaluation of wound healing
The wound healing rate of each group was calculated by the aforementioned formula, shown in Table 1. The wound healing rate of the experiment group was significantly higher than that of the control group; epithelial migration took the least time in the −120 mmHg negative pressure group, and the increased wound healing rate was the most significant (P < 0·05).
Table 1.
Wound healing rate (%, )
| Time (days) | −120 mmHg group | −180 mmHg group | −240 mmHg group | 0 mmHg control group |
|---|---|---|---|---|
| 3 | 3·67 ± 1·29 | 4·68 ± 1·55 | 6·12 ± 4·22 | 7·35 ± 5·41 |
| 5 | 15·46 ± 7·46 | 10·33 ± 3·62 | 11·19 ± 7·60 | 12·67 ± 6·31 |
| 7 | 22·75 ± 7·34 | 18·49 ± 6·75 | 15·65 ± 6·46 | 18·89 ± 5·89 |
| 9 | 35·81 ± 13·48* , † , ‡ | 25·43 ± 5·73 | 24·44 ± 8·76 | 26·93 ± 7·08 |
| 16 | 49·47 ± 9·26* , † , ‡ | 37·88 ± 6·56 | 40·21 ± 12·04 | 37·37 ± 9·08 |
| 23 | 70·12 ± 6·64* , † , ‡ | 61·01 ± 6·63* | 55·18 ± 6·63* | 45·20 ± 8·19 |
Versus control, P < 0·05.
Versus −180, P < 0·05.
Versus −240, P < 0·05.
Quantitative analysis of macrophages
Results of the staining and counting of macrophages are shown in Figure 2 and Table 2. The macrophage count of the wounds in the experiment group was significantly higher than that of the wounds in the control group, and the count on the fifth day was the most significant (P < 0·05). After it reached a peak, the count of both groups exhibited a declining trend, and the negative pressure was positively related with the macrophage count of the wound.
Figure 2.
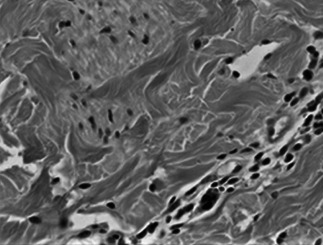
Macrophage staining and counting.
Table 2.
Wound macrophage countings ()
| Group | First day | Third day | Fifth day | Seventh day | Ninth day | |
|---|---|---|---|---|---|---|
| VSD | −120 mmHg | 1·8 ± 0·84 | 2·2 ± 0·45* | 2·8 ± 1·30 | 1·8 ± 1·10 | 1·0 ± 0·70 |
| −180 mmHg | 2·2 ± 0·84 | 2·4 ± 1·52* | 3·4 ± 1·14 | 2·2 ± 1·64 | 1·4 ± 0·89 | |
| −240 mmHg | 3·4 ± 1·14 | 3·6 ± 1·83* | 4·6 ± 1·14 | 3·4 ± 1·14 | 1·6 ± 0·55 | |
| Control | 0 mmHg | 0·6 ± 1·34 | 0·8 ± 1·30* | 2·2 ± 2·59 | 1·6 ± 0·55 | 0·4 ± 0·55 |
VSD, vacuum sealing drainage.
Versus 9 days, P < 0·05.
Enzyme‐linked immunosorbent assay
Preparation of the standard curve: The standard curve was drawn according to the HA standard OD (Figure 3). Linear regression equation: Y = 11·23·X2 + 107·29·X − 1·9411; R 2 = 0·9995. The HA content trends of both groups at different time points are as follows: after the VSD treatment, the HA levels of the −120 mmHg group and the −180 mmHg group were increased most significantly (P < 0·05); these reached a peak on the fifth day and showed a fluctuating decline after that. The HA content exhibited significant differences on the fifth and ninth post‐VSD days (P < 0·01), while the HA content at the remaining time points exhibited no statistical significance (Figure 3).
Figure 3.
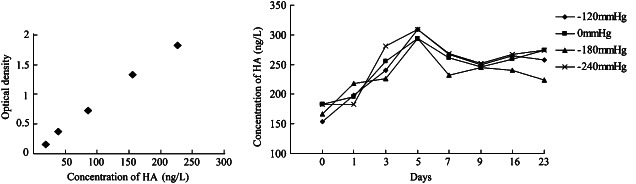
Absorbance (OD) value and hyaluronic acid (HA) concentration.
Immunohistochemical staining
CD44 was almost not expressed in the epidermis. After the trauma, the CD44 expression was significantly enhanced (Figure 4).
Figure 4.
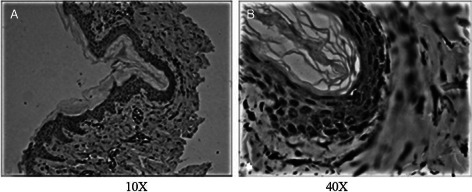
CD44 expression levels around the wound edge on the explosion day. (A): 10×, (B): 40×.
In the early stage of the experiment, the CD44 expression levels within the wounds of the experiment group were significantly higher than those in the control group wounds. The CD44 expression levels of both groups reached a peak on the third day, and the positive expression in the experiment group was still much more significant than that in the control group, as shown in Figure 5.
Figure 5.
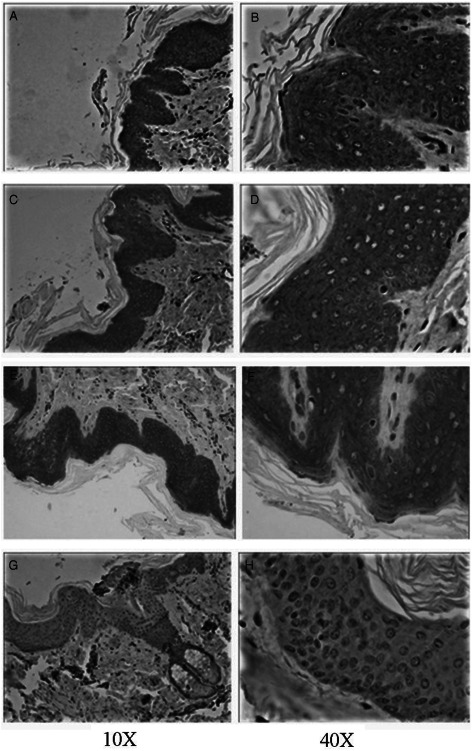
CD44 expression levels of wound tissues 3 days after vacuum sealing drainage (VSD) (A–B, C–D, E–F, G–H are the CD44 expression levels of the −120, −180, −240 mmHg group and the control group, respectively).
On the ninth day, the CD44 expression in the wounds of both groups showed a downward trend, while the positive expression of CD44 in the experiment group was still stronger than that in the control group.
On the 58th day, the positive CD44 expression levels of both groups were very weak, and there existed only a small amount of diffused distribution in the top layer of epidermis and dermis of the −120 mmHg group (Figure 6).
Figure 6.
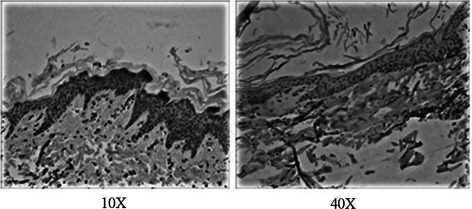
The CD44 expression levels within the wound tissues 58 days after vacuum sealing drainage (VSD). Left: the −120 mmHg group, right: the control group.
Discussion
VSD promotes cellular proliferation, inhibits apoptosis and accelerates epithelial migration, thereby accelerating wound healing.
The mechanism of VSD is as follows: It increases the blood flow to the wound, promotes angiogenesis, reduces the surface area of the wound, adjusts and inhibits the fluid flow in the wound, induces cell proliferation, reduces oedema and increases bacterial clearance 7, 8. Recently, VSD application has been proven to increase the survival rate of wound transplantation, with the rate and speed of transplantation survival being important indicators of the epithelial migration speed of the wound transplantation 9. de Laat 10 performed a controlled trial of VSD therapy and traditional sodium hypochlorite dressing therapy with 50% reduction of the wound size as the target. The study found that VSD therapy required 2 weeks to reduce the wound size by 50%, while traditional sodium hypochlorite dressing therapy accomplished the same in 3·5 weeks. The healing rate of topical VSD was almost two times that of conventional dressing therapy, and it was also safe and effective in the treatment of refractory wounds.
In this experiment, the wound healing rates of both groups were significantly increased from the beginning of treatment, among which the −120 mmHg group exhibited the most significant increase; the average healing rate on the 23rd day was 70·12 ± 6·64% while that in the control group was 45·20 ± 8·19%. The repeated measurement analysis of variance showed that there existed interactions between time and VSD (P = 0·006 < 0·01), and indicated that the difference in the healing rates at different negative pressures between the 3rd and the 23rd days was statistically significant (P < 0·01), whereas the difference in the healing rates between the 16th and 23rd days was not statistically significant (P > 0·05). Thus, on the ninth day of VSD treatment, the −120 mmHg treatment exhibited the fastest speed and the best effects on the healing of SIBIW, indicating that −120 mmHg might be the optimal negative pressure for early treatment of SIBIW.
VSD activates the macrophages, reduces the secretion of inflammatory mediators and prevents the excessive inflammatory response.
The number of macrophages in normal skin tissues was ∼1–2/mm2; however, it can increase by as much as five times during the wound healing period. The macrophages are the ‘general commanders’ during the processes of wound healing and tissue repair, exhibiting an irreplaceable guiding role in the entire wound healing process 11. They are an important medium for wound healing, tissue remodelling and cytokine secretion. The active inflammation defence is mediated by M1 macrophage, and the wound healing and tissue repair are regulated by M2/M2‐like macrophages 12.
Normally, the macrophages increase in number beginning the first day of injury, and the numbers increase with time, not only reaching a peak 2–4 days after the injury, but also reaching 80% of total cells in the granulation tissues on the seventh post‐injury day, thus becoming the inflammatory cells that persist for the longest period and are the largest in number 13. The possible mechanisms that lead to this phenomenon include the continuous negative pressure environment stimulating the chemotaxis of macrophages towards the wound, thus increasing the number of macrophages and inhibiting the increase of bacteria, so that the inflammation response is localised and reduced. Application of VSD by Phillips showed that the moist environment under the sealed wound provided favourable conditions for the host to play the role in cell phagocytosis, and compared with the dry wound, it would be much more favourable for migration and infiltration of immune cells, thus preventing the occurrence of wound infection 14, 15.
In this experiment, microscopic observation showed that the macrophage count of the wounds in both groups was increased from the first day, and the count of the experiment group was significantly higher than that of the control group. The higher the negative pressure, the greater the macrophage cell count per unit area. The macrophage count of the two groups reached a peak on the fifth day and then declined, but the macrophage count of the experiment group was still higher than that of the control group. The repeated measurements analysis of variance showed P = 0·184 > 0·05, indicating that there was no virtual correlation among the data of repeated measurements. So, these were consistent with the Huynh–Feldt condition, in which there was a statistical significance between the time and the number of macrophages (P < 0·05). The data could therefore be processed by one‐way ANOVA. The interaction of time and negative pressure showed no statistical significance with the number of macrophages (P > 0·05). The analysis of variance showed that there was a statistically significant difference in the macrophage count between the two groups on the fifth and ninth days (P < 0·05).
VSD adjusts HA metabolism, thereby promoting wound healing.
HA is an indispensable component of extracellular matrix (ECM) during wound healing process. ECM is composed of biological macromolecules such as glycoproteins, proteoglycans and glycosaminoglycans; among these, the glycosaminoglycans are the main component and HA exhibits the highest content within the glycosaminoglycans. Therefore, HA is an important indicator that could impact wound healing.
After the trauma, the HA content inside the skin was significantly increased 16. A very high or very low HA content would not be conducive for wound healing. Our previous study showed that VSD could bi‐directionally regulate HA metabolism, restoring its content to the level that was conducive for healing. Cigna 17 found that when a posterior tibial injury wound was treated with HA and continuous negative pressure (−125 mmHg), the patient could walk independently 6 months later, and when supplemented with the appropriate physical therapy, the patient could have an early return to daily activities. Morykwas 18 confirmed that granulation tissues grew fastest under the −125 mmHg pressure. The main component of early granulation tissues is HA, which promotes the activity and division of cells within ECM.
The line chart (Figure 3) showed that prior to the VSD treatment (day 0), the HA concentrations in both groups were at a low level. After the treatment, the HA concentrations exhibited a fluctuating upward trend with passage of time; on the fifth VSD treatment day, the HA levels of the two groups reached a peak and that of the −120 mmHg group was the highest. With time and the bi‐adjustment of VSD towards HA, the HA concentrations of the two groups showed dynamic fluctuations. After the data were processed, it showed that the HA concentrations exhibited a statistically significant difference between the fifth and ninth days between the two groups, and the −120 mmHg and −180 mmHg groups showed adjustment of the HA concentrations, thus promoting wound healing.
VSD promotes the expression of HA and its receptor CD44 inside the wound tissues.
On observation, the skin had the highest HA content, and its content within the dermis was about five times that within the epidermis; among the intercellular substances, the HA content within the epidermis was eight times that within the dermis. Currently, there is no uniform standard for the detection of HA expression in the skin, and this is an area of disagreement among many scholars. In Laugier's study 19, immunohistochemistry of the skin showed that HA was expressed only in the basal cells and basal upper layers of the epidermis and not inside the granular layer and stratum corneum. Lindqvist 20 found that HA was mainly distributed within the papillary layer's intercellular regions of normal skin, which exhibited strong and non‐uniform staining distribution inside the reticular layer of dermis. In addition, the epidermal basement membrane area was the region that had the most intense staining. The epidermal HA was mainly located within the intercellular spaces of keratinocytes of the spinous layer, and it was also distributed within the granular layer. Both HA and its receptor CD44 were expressed in the epidermis and dermis, with only a small part of CD44 expressed in the basal layer, which was mainly distributed in the top layer of epidermis. There was a small amount of diffused distribution of CD44 within the dermal blood vessels. A possible reason for the differences in immunohistochemical staining might be the fact that the HA distributed inside the stratum corneum was of low molecular weight, and the binding between the high molecular weight HA and biotin‐binding protein was weak. Moreover, this might also be combined with the coverage of HA‐binding sites by the other ingredients of stratum corneum 21.
This study showed that CD44 was expressed in the epidermis (within the cell membrane and cytoplasm) and dermis in SIBIW. In normal skin tissues, CD44 was only rarely expressed in the dermal layer. In the early stage of this experiment, the CD44 expression in the experiment group was stronger than that in the control group, and the −120 mmHg group exhibited the strongest expression. The CD44 expression reached a peak on the third post‐injury day, and exhibited a decreasing trend from the ninth day. On the 58th day, the CD44 expression of both groups was weak, and only a small amount was diffusely distributed in the top layer of epidermis and dermis. In addition, we also observed that the CD44 expression is higher within the regions that have more epidermal cells (the matrix was relatively less) than in the regions with a small number of epidermal cells (the matrix was relatively more). This also confirmed that HA was the main component of ECM.
We confirmed that the HA concentration was positively correlated with the CD44 content; a large amount of HA stimulates the body to produce more CD44, thereby enhancing the enzymatic metabolism of HA, and CD44 could transport more HA, resulting in the reduction of local HA concentration. In addition, the synthesis and degradation of HA were closely linked with the HA‐stimulating factor (HASF) and HA‐binding protein (HABP), which could affect the HA content, thereby affecting the expression of CD44.
References
- 1. Rodrigues CS, Souza SS, Rezende RP, Silva A, Andrioli JL, Costa H, Fontana R, Dias JC. Application of denaturing gradient gel electrophoresis for detection of bacterial and yeast communities along a salinity gradient in the estuary of the Cachoeira River in Brazil. Genet Mol Res 2013;12:1752–60. [DOI] [PubMed] [Google Scholar]
- 2. Kirkman E, Watts S. Haemodynamic changes in trauma. Br J Anaesth 2014;113:266–75. [DOI] [PubMed] [Google Scholar]
- 3. Ning J, Mo L, Zhao H, Lu K, Wang L, Lai X, Yang B, Zhao H, Sanders RD, Ma D. Transient regional hypothermia applied to a traumatic limb attenuates distant lung injury following blast limb trauma. Crit Care Med 2014;42:e68–e78. [DOI] [PubMed] [Google Scholar]
- 4. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002;137:930–3. [DOI] [PubMed] [Google Scholar]
- 5. Zhang C, Liu D, Liang Z, Liu F, Lin H, Guo Z. Repair of refractory wounds through grafting of artificial dermis and autologous epidermis aided by vacuum‐assisted closure. Aesthetic Plast Surg 2014;38:727–32. [DOI] [PubMed] [Google Scholar]
- 6. Nagelschmidt M, Becker D, Bönninghoff N, Engelhardt GH. Effect of fibronectin therapy and fibronectin deficiency on wound healing: a study in rats. J Trauma 1987;27:1267–71. [DOI] [PubMed] [Google Scholar]
- 7. Mouës CM, Heule F, Hovius SE. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg 2011;201:544–56. [DOI] [PubMed] [Google Scholar]
- 8. Li RG, Ren GH, Tan XJ, Yu B, Hu JJ. Free flap transplantation combined with skin grafting and vacuum sealing drainage for repair of circumferential or sub‐circumferential soft‐tissue wounds of the lower leg. Med Sci Monit 2013;19:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloemen MC, van der Wal MB, Verhaegen PD, Nieuwenhuis MK, van Baar ME, van Zuijlen PP, Middelkoop E. Clinical effectiveness of dermal substitution in burns by topical negative pressure: a multicenter randomized controlled trial. Wound Repair Regen 2012;20:797–805. [DOI] [PubMed] [Google Scholar]
- 10. de Laat EH, van den Boogaard MH, Spauwen PH, van Kuppevelt DH, van Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult‐to‐heal wounds: a prospective randomized controlled trial. Ann Plast Surg 2011;67:626–31. [DOI] [PubMed] [Google Scholar]
- 11. Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology 2011;216:753–62. [DOI] [PubMed] [Google Scholar]
- 12. Rodeo MP, Legrand JM, Bou‐Garios G, Khosrotehrani K. Wound‐associated macrophages control collagen 1α2 transcription during the early stages of skin wound healing. Exp Dermatol 2013;22:143–5. [DOI] [PubMed] [Google Scholar]
- 13. Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil‐depleted mice. J Leukoc Biol 2003;73:448–55. [DOI] [PubMed] [Google Scholar]
- 14. Phillips PG, Birnby LM, Narendran A. Hypoxia induces capillary network for mation in eultured bovine pulmonary microvessel endothelial cells. Am J Physiol 1995;268:789–800. [DOI] [PubMed] [Google Scholar]
- 15. Chen S, Li J, Li XY, Xu LS. Effects of vacuum‐assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28:213–7. [DOI] [PubMed] [Google Scholar]
- 16. Baymurat AC, Ozturk AM, Yetkin H, Ergun MA, Helvacıoglu F, Ozkızılcık A, Tuzlakoğlu K, Sener EE, Erdogan D. Bio‐engineered synovial membrane to prevent tendon adhesions in rabbit flexor tendon model. J Biomed Mater Res A 2015;103:84–90. [DOI] [PubMed] [Google Scholar]
- 17. Cigna E, Maruccia M, Sorvillo V, Parisi P, Palumbo F, Onesti MG. The use of negative pressure therapy and hyaluronic acid for the management of post‐traumatic lower limb injury. Int Wound J 2013;10:534–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 19. Laugier JP, Shuster S, Rosdy M, Csóka AB, Stern R, Maibach HI. Topical hyaluronidase decreases hyaluronic acid and CD44 in human skin and in reconstituted human epidermis: evidence that hyaluronidase can permeate the stratum corneum. Br J Dermatol 2000;142:226–33. [DOI] [PubMed] [Google Scholar]
- 20. Lindqvist U, Pihl‐Lundin I, Engström‐Laurent A. Dermal distribution of hyaluronan in psoriatic arthritis; coexistence of CD44, MMP3 and MMP9. Acta Derm Venereol 2012;92:372–7. [DOI] [PubMed] [Google Scholar]
- 21. Sakai S, Yasuda R, Sayo T, Ishikawa O, Inoue S. Hyaluronan exists in the normal stratum corneum. J Invest Dermatol 2000;114:1184–7. [DOI] [PubMed] [Google Scholar]


