Abstract
This observational case series reports the evaluation of a novel neuromuscular electrical stimulation device (geko™) that stimulates the common peroneal nerve at the fibular head as an adjunctive therapy in patients with non‐healing venous leg ulcers. The aim was to evaluate and determine if the geko™ device was effective in this population and should be added to the medical supply formulary. Patients whose wounds had failed to heal within 24 weeks of standard therapy were identified in two community settings in Ontario. A total of 11 patients consented to the evaluation with a combined 107‐year history of recalcitrant leg ulcers. Although the pre‐geko™ healing rate was unknown, all ulcers were considered non‐healing. With geko™, the average weekly percentage reduction in surface area for all patients was 4·5% and for the six adherent to geko™ and best practices 7·0%. By comparison, the average weekly percentage reduction for measurable wounds in the five non‐adherent patients was 1·8%. Requirements for success appear to include an arterial status adequate for healing, effective and prompt management of wound infections and adherence to the treatment schedule. The geko™ device has been added to the medical supply formulary in one centre and is pending in the other.
Keywords: Blood flow, geko, Neuromuscular electrical stimulation, Recalcitrant venous ulcers
Introduction
A total of 55 years after Dr. George Winter 1 identified moisture‐retentive dressings as an enhancement to wound healing, countless dressing choices now exist for local wound care, which constitute external treatment. However, a key first step in the wound bed preparation paradigm 2 is to ‘treat the cause’, optimising comorbidities that contribute to the initial wounding or delayed healing (e.g. compression therapy to decrease venous reflux). Venous leg ulcers (VLUs), which are the most frequent type of lower leg ulcerations, usually occur as a result of disease or dysfunction of the superficial, perforator or deep veins or of the calf muscle pump, collectively called chronic venous insufficiency. They can also have components of peripheral arterial disease, trauma, lymphoedema, diabetes, arthritis or malignancy 3.
VLUs can be a costly challenge to the health‐care system and to individuals. Affecting approximately 1% of the population in the Western world 4, some VLUs will heal within 12–24 weeks with appropriate wound care and compression of the leg 4, 5. Conversely, VLUs can be associated with poor healing even when the ankle brachial pressure index (ABPI) is >0·5, indicating arterial flow adequate for healing 6 and compression therapy with best practice wound care are utilised 7, 8, 9. Despite treatment with the ‘gold standard’, 10–20% of VLUs will remain unhealed after 2 years 10, increasing costs and affecting quality of life 11, 12. This has an adverse impact on the physical and psychological well‐being of the patient 13. Introducing more advanced therapies in combination with compression may reduce total treatment costs and shorten the length of time to closure 12.
Effective adjunctive therapies to improve the function of the calf and foot skeletal muscle pumps may include a structured physical therapy exercise programme for 6 months 14, requiring a referral to physiotherapy and a commitment to exercise. Intermittent pneumatic compression (IPC) is also recommended 15, which stimulates the calf muscle pump when at rest, consisting of a mechanical pump and inflatable sleeves that fit over the leg with variable cycle times and inflation pressures. The patient cannot ambulate during the therapy, which is up to 4 hours per day.
A new modality, the geko™ neuromuscular electrostimulation (NMES) medical device (FirstKind Ltd, High Wycombe, UK), is light weight (10 g), wrist watch‐sized, easy to use, self‐contained, self‐adhesive and battery operated (Figure 1) and may be of clinical importance in managing symptomatic venous disease 16, particularly in patients who are not physically active. When compared to IPC, in two studies, the geko™ device was superior in increasing both venous and arterial femoral blood volume flow in healthy volunteers by 101% and 75%, respectively 17, and microcirculatory flux by 400% (P ≤ 0·001) 18.
Figure 1.

OnPulse geko™ T‐2 and R‐2 devices.
The geko™ device is fitted to the skin of the inferolateral aspect of the knee joint at the fibular head, where it stimulates the common peroneal nerve prior to branching into the deep and superficial peroneal nerves. This activates the peroneus longus and peroneus brevis muscle groups, while the deeper peroneal nerve activates the tibialis anterior, extensor hallucis longus, extensor digitorum longus, peroneus tertius and extensor digitorum brevis muscle groups 19. They are all related to the reaction of the venous muscle pumps of the lower leg (Figure 2). The device is worn on both legs, and its stimulation level is set to the minimum setting that can achieve an upward and outward twitching of the foot when raised from the ground 20.
Figure 2.
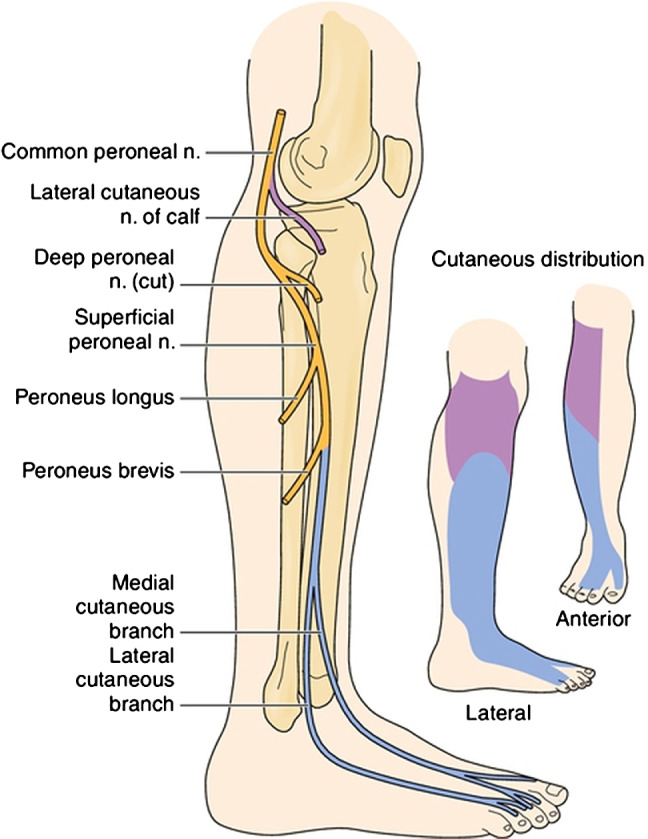
Common (blue) and superficial (purple) peroneal nerve branch cutaneous distributions and motor branches. (From Haymaker W, Woodhall B. Peripheral Nerve Injuries: Principles of Diagnosis. Philadelphia, WB Saunders, 1953.) Used with permission.
Initially developed to prevent postoperative deep vein thrombosis (DVT), it stimulates 50–70% of the blood flow generated by 10 consecutive full dorsiflexions (considered to equivalent that achieved by walking) measured by photophlesmography (PPG) (P = 0·0004) in the dorsal veins of the foot and by strain gauge phlesmography (SPG) in changes to mid‐calf circumference measured (P < 0·001) 20. The geko™ device increases venous, arterial and microcirculatory blood flow in the lower limb in people with chronic venous insufficiency 21, 22 and intermittent claudication 23, reduces chronic leg oedema in some individuals 21, 22, 24, acts as a calf‐muscle pump 25 and maintains TCpO2 20, promoting conditions suitable for wound healing.
It is licenced by Health Canada for clinical indications that include increasing blood flow, treating oedema and promoting wound healing.
This paper describes the evaluation of the geko™ device by the Erie‐St. Clair Community Care Access Centre (ESC CCAC) and Hamilton Niagara Haldiman Brant (HNHB) CCAC in Ontario, Canada. The objective was to determine the wound‐healing response to the geko™ device as an adjunct to standard of care for patients with non‐healing VLUs and if the technology should be made available for routine care in the community setting.
Materials and methods
The geko™ devices were provided free of charge by Perfuse Medtec Inc. The evaluation started with the geko™ T‐1 and T‐2 devices and then moved to R‐2 devices for all patients (see Box 1), set at a level that achieved discernible movement of the specified muscles of the calf and foot or the highest setting if no discernible movement was seen. Leg straps were provided as an additional optional method of securing the device.
BOX 1. geko™ SPECIFICATIONS.
T‐1: 27 mA constant current output with 7 pulse width settings of 70–560 µs
T‐2: 27 mA constant current output with 7 pulse width settings of 50–400 µs; lighter weight with longer strap
R‐2: 54 mA constant current output with 8 pulse width settings of 50–560 µs, also lighter, longer and with a higher current output
Education and training in the use of the geko™ device and skin care was provided to the nurses, the patient and/or family member. There is an increased risk of developing dermatitis in response to known sensitisers, including adhesives for people with CVI 26. When used for DVT prophylaxis, it is worn 24 hours per day continuously. Because the geko™ device adheres to the skin with a proprietary adhesive/ conductive gel, we wished to minimise the risk of rash. In consultation with First Kind, a titrating plan was developed in which the device would be worn for 2 hours per day ×7 days, then increased to 4, 6 and 8 hours per day based on client tolerance and skin integrity. This was to reduce the time that the skin would be in contact and tried to align with the number of hours per day when patients would be more likely to be sitting, where the device produces a more noticeable muscle response. The use of the geko™ device was to be combined with appropriate compression therapy of the affected lower leg as an adjunct to, not in place of, compression. Standard‐of‐care treatments would be modified as needed in response to wound characteristics.
Reassessment at 2, 4, 6 and 12 weeks would include wound measurements and photographs, determining patient tolerance and recording the geko™ setting. If there was over 50% improvement at 12 weeks, the device could be continued, with ongoing evaluation. If patients were deemed to be non‐adherent to the use of the device, they would be removed from the evaluation.
Participants and procedures
Patients whose wounds had failed to heal within 24 weeks of standard therapy were identified and consented to treatment. The group decided that although several of the following are considered to be warnings in the geko™ Instructions for Use, patients would be excluded in any of the following situations: <18 years of age, a recent history of DVT or development of DVT during evaluation, patients with cardiac demand pacemakers or implanted/external medical devices or patients presenting with a history of contact dermatitis and patients with a history of poor adherence to a prescribed plan of care (nursing/ physician/CCAC opinion). Standard of care for VLUs should include compression therapy following lower leg assessment and ankle brachial pressure index (ABPI) and moist wound healing. An ABPI of >0·6 was chosen to ensure that patients had arterial circulation adequate for healing.
Ethical considerations
Each patient consented to participate and to share their anonymised results for evaluation and publication. Patients could withdraw for any reason, without risk to their ongoing standard of care. Approval to publish the results was obtained from the Regional Centre for Excellence in Ethics, Homewood Health Centre, 150 Delhi Street, Guelph, Ontario, N1E 6 K9.
Statistics
Percent change in ulcer surface area (SA) is calculated from the initial ulcer SA (cm2) to the final ulcer SA (cm2) attained per the formula in Box 2 27. The weekly ulcer healing rate is determined by dividing the results from the formula by the number of weeks between the initial ulcer and final ulcer SA measurement. The ulcers were followed to complete reepithelialisation (healing), patient discharge or the point of evaluation. Cumulative proportion of ulcers healed were determined for patients adhering and not adhering to geko™ therapy through 50 weeks.
BOX 2. PERCENTAGE CHANGE IN SURFACE AREA (cm2).
*SA = Surface area calculated as longest length × perpendicular widest width
Results
A total of 11 patients with 30 wounds and a combined 107‐year history of VLUs started treatment with the geko™ devices between April and September 2014. All wounds were recalcitrant to current treatment and were considered non‐healing and followed up until June/July 2015. Table 1 summarises the demographics.
Table 1.
Patient demographics
| Demographic | Erie‐St. Clair CCAC and HNHB CCAC |
|---|---|
| Number of patients | 11 |
| Average age | 69·9 years (range: 49–90 years) |
| Gender | 54·5% were male and 45·5% female |
| ABPI values | >0·8:6 Not known:5 |
| Status of leg ulcer | 100% non‐healing |
| Average duration of leg ulcer(s) | 10·6 years (range: 0·54–53 years). Total of 107 years with ulcers |
| Average size of leg ulcer | 21 cm2 (range of 0·12–247 cm2) |
| Use of compression therapy | 64% wearing compression of some type |
| Use of advanced wound products | 100% were using advanced wound care products |
Six patients started on the geko™ T‐1, two on the T‐2 (both 27 mA) and three on the new higher‐current geko™ R‐2 (54 mA). Those requiring a higher current had a lack of visual calf muscle contraction and outward foot movement with geko™ stimulation because of oedema, obesity and/or woody fibrosis. Regardless of the type of device, some patients could decrease to a lower setting within a few weeks.
The average weekly % change in SA for the 28 measured wounds was a 4·5% reduction (range −3%–40%). Two circumferential leg wounds in one patient were never measured. Six patients (54%) with 16 wounds were adherent to geko™, and best practice wound care had a 7% reduction in SA per week. In retrospect, one patient who was adherent to care was likely not healable, having been offered amputation prior to the evaluation. By removing her data, the average weekly percentage change for adherent, healable patients is 7·6% (reduction). By comparison, the average weekly percentage change for wounds in the five (46%) patients non‐adherent with the wound care plan and/or geko™ with 12 measurable wounds was 1·82% (reduction). Four of the five were withdrawn from the evaluation and returned to previous standard of care. Figure 3 shows the cumulative proportion of wounds healed; Figure 4 shows the proportion of wounds healed, improving or deteriorating for adherent patients, while Figure 5 shows the same analysis for non‐adherent patients.
Figure 3.
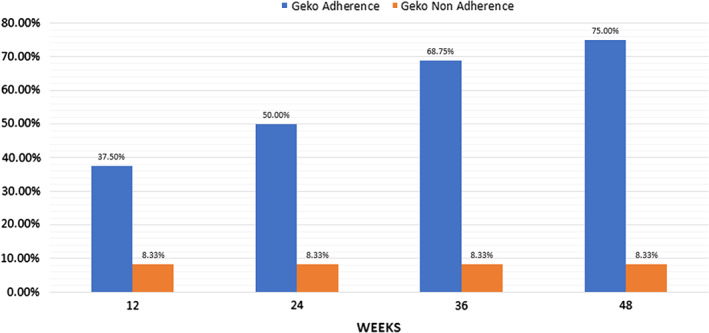
Cumulative proportion healed with geko™ and Best Practices.
Figure 4.
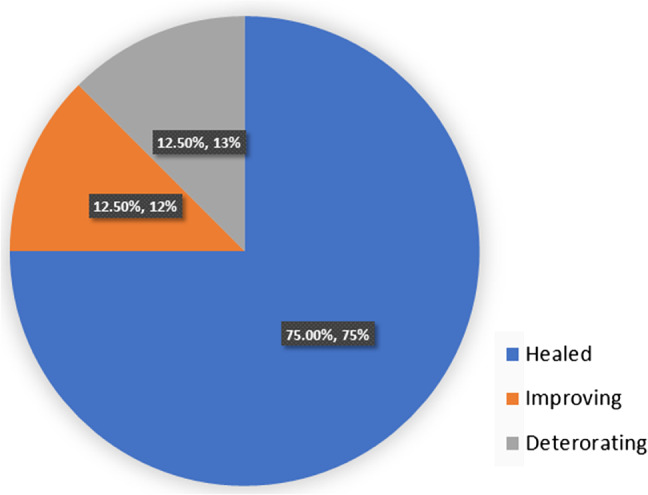
Proportion of wounds healed, improving or deteriorating for patients adherent to geko™ and/or Best Practices
Figure 5.
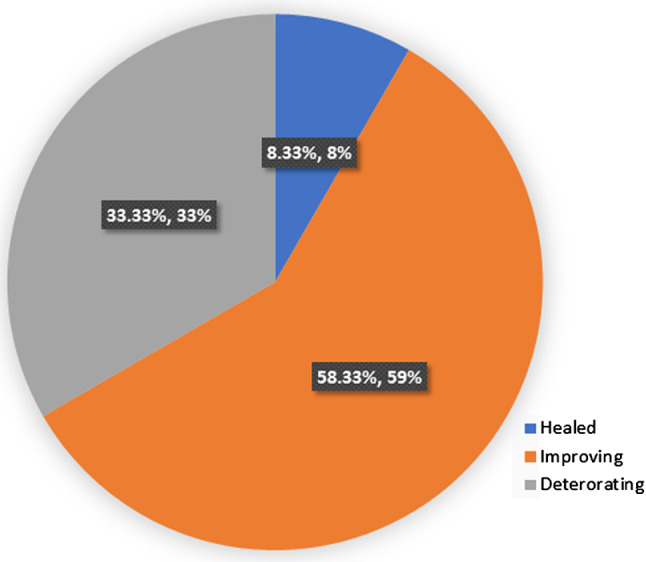
Proportion of wounds healed, improving or deteriorating for patients non‐adherent to geko™ and/or Best Practices.
The following case studies are an example of the cases seen. Patient 1 was male, aged 75, with a 10‐year history of ulcerations, closures and recurrences. His ABPIs were: Rt. 1·07, Lt. 1·09, with previous bilateral knee replacements with peripheral oedema, two ulcers on left leg: SAs: 8·74 and 4·5 cm2 (Figures 5A and B) and a history of several pseudomonas aeruginosa infections. His pain went from 8 of 10 at baseline to 5 of 10. He could not tolerate compression initially, but with geko™, he was able to start with low and move to moderate compression. He achieved 100% closure of both wounds at 32 weeks (Figure 6). He found the device very comfortable and often forgot that he was wearing it. He believed that he had increased sensation in his legs after starting to wear the geko™.
Figure 6.
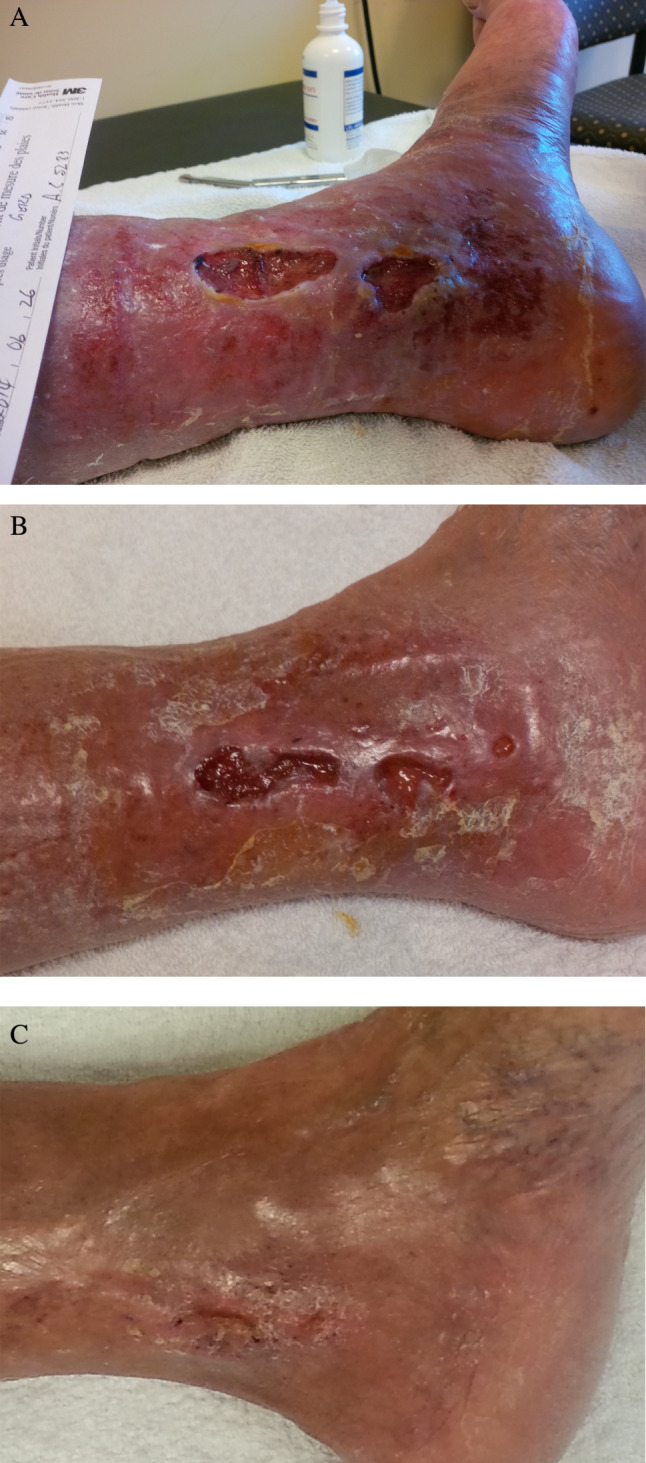
Patient 1 Left medial ulcers at baseline, (B) 26 weeks, (C) closed at 32 weeks.
Patient 2 was male who was 62 years of age with a 9‐year history of open VLUs bilaterally, with seven wounds, all 0·2–0·5 cm deep: two on left medial aspect with an SA of 0·63 and 5·28 cm2, respectively, one x‐shaped wound on the medial right leg with a SA of 52·5 cm2, four clustered on the lateral right leg with total SA of 6·93 cm2, a right posterior ulcer that occurred at 27 weeks SA 15 cm2 and a left posterior ulcer that occurred at 40 weeks with SA of 3·78 cm2. His ABPIs were adequate for high‐compression bandages. He had many wound infections prior to starting geko™. He was not initially adherent with wearing the geko™ and then wore it for longer than advised. When he developed rashes at the application sites, he tried to solve the problem on his own by modifying the device backing, making it worse. He missed wound clinic and nursing appointments, with dressings staying on longer than intended, developing infections that resulted in wound deterioration at 27 weeks. Once he was given long‐term antibiotic treatment, became more adherent to keeping his appointments and used the geko ™ as advised, the new right posterior wound closed 12 weeks after it was first documented, the left medial wound had a remarkable reduction of 90%, the right lateral had a reduction of 61%, and the right medial decreased by 77% by 50 weeks (Figure 7). He continued to wear the geko™ device past the evaluation date.
Figure 7.
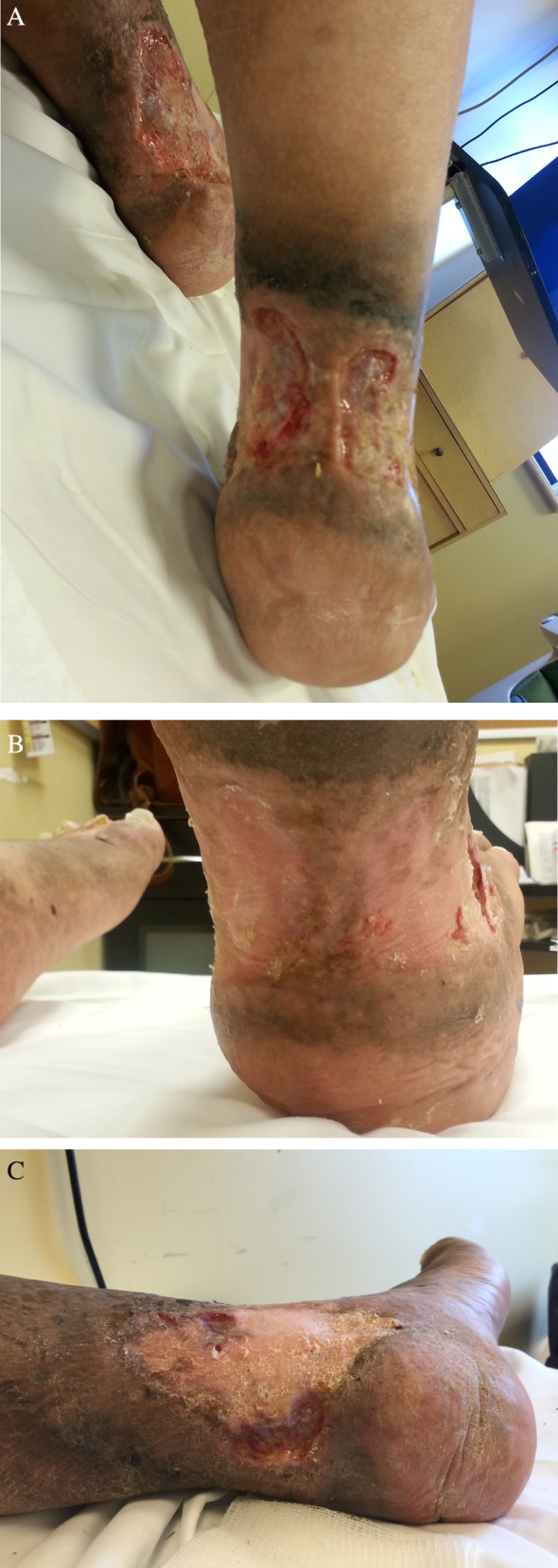
(A) Right posterior and left medial ulcers at 27 weeks with infection. (B) New right posterior ankle healed in 12 weeks. (C) Left medial at 50 weeks (23 weeks from largest size).
Discussion
The term ‘adherence to treatment’ describes the willingness and follow‐through required for patients to follow a therapeutic regimen 28. Five patients were considered to be non‐adherent because of underuse, overuse and tampering with the device. Barriers to adherence include the complexity of the regimen and failure of the patient to understand the importance of adherence, possibly because of poor communication 29. Post‐evaluation interviews could have helped to determine why the geko™ devices were overused or underused or not used as instructed. This is a new technology, and all care providers may not have provided consistent instructions on wear times and instructions for use. One patient preferred to wear the geko™ devices while standing at work for the entire shift. When standing, with 0° of knee flexion, compared with sitting with knee flexed to 90°, the common peroneal nerve moves away from the fibular head by an average of 17 mm, 30 and visual response of the muscles to the geko™ device decrease. When Jawad et al. tested the geko™ response compared to IPC in blood flow volume and velocity, they used a ‘threshold setting’, the minimum setting to elicit a minor muscular contraction in the calf, compared to the ‘normal clinical setting’ wherein the upward and outward twitching of the foot is seen 18. In the threshold setting, there was a 14% increase in venous blood flow, while in the normal clinical setting, there was a 33% increase. It appears reasonable to assume that a patient who is upright and is not able to be seated any of the time while wearing the geko™ will have a lower rate of healing.
An adverse event consisting of a skin rash appeared at the device application site involving 45·1% of patients occurring at 45 days on average. It is unclear whether the rash was related to folliculitis, sensitivity or other factors. Rashes were addressed by alternating the fitting site with two other locations, local skin care advised by nurses/physicians and, if required, taking a break from wearing the device to allow the rash to dissipate. Only two patients had to discontinue the product because of skin irritation, but both were non‐adherent to the advised plan of care. The development of skin reactions with the geko™ device in this CVI population are acknowledged, and at the time of writing, further research and development has resulted in a new product that should result in the decreased incidence of skin rash.
Although the hours of wear were initially titrated to avoid rash, it proved difficult to follow for patients and nurses. After review, it was decided that a standard of 6 hours per day, 5 days per week should be the protocol of use with 2 days off, specifically to give the skin a rest from the adhesive. The group decided on 2 hours per day, 3 days per week for 1 month after wound closure to give extra circulatory support while the new skin became more durably healed. This was based on the Canadian Physical Activity Guidelines for Older Adults, (http://www.csep.ca/CMFiles/Guidelines/CSEP_PAGuidelines_older-adults_en.pdf).
Unexpected but very positive results included pain reduction, so two patients who could not tolerate compression prior to the evaluation started compression therapy. One described having more sensation in his legs over the time of wearing the geko™ yet was comfortable and able to go from no compression to a moderate 2‐layer system, while a third increased their level of compression. Compression therapy is key in treating CVI, but it is not clear which interventions improve adherence to compression therapy 30. If the geko™ device can help to achieve optimal compression, it will be a valuable adjunct to best practice care.
Limitations
Although the intention was NOT to include patients who did not have circulation adequate for healing, we failed to insist on knowing the ABPI before patients were started in the evaluation. Even so, with ABPIs adequate for healing, success with the product was not guaranteed; non‐adherence to the geko™ use guidelines or failing to keep physician or nursing appointments appears to be a big factor in the failure to improve. We also were unsuccessful in predicting which patients would adhere to the treatment plan and may have created selection bias in trying to do so.
This was an evaluation involving several nursing agencies in two geographical areas, and patient information was not always available or recorded consistently. Much of it was collected retrospectively, which has disadvantages vis‐a‐vis prospective studies. Future evaluations or studies would benefit from one consistent, shareable method of documentation. It may be prudent to limit evaluations to a set period such as 8–10 weeks' maximum and, beyond measuring wound healing, consider changes in wound characteristics such as increased granulation, decreased exudate, decreased pain and oedema as markers of improvement. There should be clear endpoints of therapy when it is shown that patients are not following the treatment plan despite repeated learning opportunities.
Conclusions
This evaluation of the geko™ device was to determine if there would be a positive impact on wound healing, in patients with recalcitrant VLUs. To achieve a mean weekly 4·5% reduction in wound size in a group of 11 patients who had a combined 107‐year history (average of 10 years) is extremely exciting. The results suggest that the use of the novel geko™ device can play a positive role as an adjunct therapy for chronic VLUs. Requirements for success appear to include an arterial status adequate for healing, prompt management of wound infections and adherence to the treatment plan. Importantly, adherent patients or family members quickly mastered the application and removal of the device and care for the skin under the geko™ site, showing the ease of use of the device. At the time of writing, Erie St. Clair CCAC has added this to their formulary, and it is being added or is pending in four others in the Province of Ontario. Further research is required to fully understand the benefits provided by the device, the optimal dosage or wear time and the patient population most likely to respond. Many of these patients described feeling new hope when they started the evaluation, and for many, this hope appears to have been justified.
Acknowledgements
The authors acknowledge Erie St. Clair CCA; Hamilton Niagara Haldimand Brant CCAC; CarePartners; Bayshore Home Health Nursing; Saint Elizabeth Health Nursing; Victorian Order of Nurses; First Kind and Perfuse Medtec Inc.; Dr. Craig Pearce; Geoff Fournie, Dorace Ramage and Shannon McKie of Perfuse Medtec Inc.; and Ron Shannon, Global Health Economic Projects, LLC, whose support made this study possible. CH was the Clinical Nurse Specialist for Wounds at CarePartners at the time of these evaluations but has been contracted as a Consultant with Perfuse Medtec, Inc. since 1 April 2016, and with Hamilton Niagara Haldiman Brant CCAC since 11 May 2016.
No direct financial support was received for this study; however, educational support was provided by Perfuse Medtec Inc (London, Canada) and no‐charge geko devices by FirstKind Ltd. (High Wycombe, UK).
Perfuse Medtec, Inc. London, ON Canada and Firstkind Ltd. High Wycombe, UK provided in‐kind support in the way of geko™ devices, training and support as outlined in this paper.
[The copyright line for this article was changed on 26 October 2017 after original online publication]
References
- 1. Winter GD. Formation of the scab and rate of epithelialization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- 2. Schultz GS, Sibbald RG, Falanga V, Ayello E, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, VanScheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:1–28. [DOI] [PubMed] [Google Scholar]
- 3. Harding K, Dowsett C, Fias L, Jelnes R, Mosti G, Öien R,Partsch H, Reeder S, Senet P, Soriano JV, Vanscheidt W. Simplifying venous leg ulcer management. Consensus recommendations. Wounds International 2015. URL www.woundsinternational.com [accessed on 30 March 2017]. [Google Scholar]
- 4. O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11:CD000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantor J, Margolis DJ. A multicenter study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 6. Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg 2012;55:122–8. [DOI] [PubMed] [Google Scholar]
- 7. Lambourne LA, Moffatt CJ, Jones AC, Dorman MC, Franks PJ. Clinical audit and effective change in leg ulcer services. J Wound Care 1996;5:348–51. [PubMed] [Google Scholar]
- 8. Harrison MB, Légare F, Graham ID, Fervers B. Adapting clinical practice guidelines to local context and assessing barriers to their use. CMAJ 2010;182:E78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison MB, VanDenKerkhof EG, Hopman WM, Graham ID, Carley ME, Nelson EA; the Canadian Bandaging Trial Group. The Canadian Bandaging Trial: evidence‐informed leg ulcer care and the effectiveness of two compression technologies. BMC Nurs 2011;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson B, Hooper P, Powell R. Four‐layer bandaging and healing rates of venous leg ulcers. J Wound Care 1996;5:213–6. [DOI] [PubMed] [Google Scholar]
- 11. Pfeffer F, von Dobschuetz E, Riediger H, Moosmann C, Hopt UT. The nonhealing wound. MMW Fortsch Med 2004;146:45–8. [PubMed] [Google Scholar]
- 12. Tennvall GR, Hjelmgren J, Öien R. The cost of treating hard‐to‐heal venous leg ulcers: results from a Swedish survey. URL http://www.worldwidewounds.com/2006/november/Tennvall/Cost-of-treating-hard-to-heal-venous-leg-ulcers.html [accessed on 30 March 2017]
- 13. Persoon A, Heinen MM, Van Der Vleuten CJM, De Rooij MJ, Van De Kerkhof PCM, Van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004;13:341–54. [DOI] [PubMed] [Google Scholar]
- 14. Padberg FT, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. J Vasc Surg 2004;39:79–87. [DOI] [PubMed] [Google Scholar]
- 15. Registered Nurses Association of Ontario . Assessment and Management of Venous Leg Ulcers Guideline Supplement. Toronto, Canada: Registered Nurses Association of Ontario. 2007. URL http://rnao.ca/bpg/guidelines/assessment-and-management-venous-leg-ulcers [accessed 30 March 2017].
- 16. Williams KJ, Ayekoloye O, Moore HM, Davies AH. The calf muscle pump revisited. J Vasc Surg 2014;2:329–34. [DOI] [PubMed] [Google Scholar]
- 17. Williams KJ, Moore HM, Ellis M and Davies AH. Haemodynamic changes with the use of a neuromuscular stimulation device compared to intermittent pneumatic compression. Phlebology. 2015;30(5):365–72. URL http://phl.sagepub.com/content/early/2014/04/10/0268355514531255 [accessed 10 April 2014]. [DOI] [PubMed] [Google Scholar]
- 18. Jawad H, Bain DS, Dawson H, Crawford K, Johnston A, Tucker AT. The effectiveness of a novel neuromuscular electrostimulation method versus intermittent pneumatic compression in enhancing lower limb blood flow. J Vasc Surg 2014;2:160–5. [DOI] [PubMed] [Google Scholar]
- 19. Korthuis RJ. Regulation of vascular tone in skeletal muscle. In: Skeletal Muscle Circulation. San Rafael, CA: Morgan & Claypool Life Sciences, 2011. URL www.ncbi.nlm.nih.gov/books/NBK57142/ [accessed 3 July 2015]. [PubMed] [Google Scholar]
- 20. Tucker AT, Maass A, Bain DS, Chen L‐H, Azzam M, Dawson H, Johnston A. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol 2010;19:e31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams KJ, Babber A., Ravikumar R, Ellis M, Davies AH. Pilot Trial of neuromuscular stimulation in the management of chronic venous disease. 2 Posters from VEINS Conference, UK. 2014.
- 22. Williams KJ, Davies AH. Pilot trial of neuromuscular stimulation in the management of chronic venous disease. Br J Surg 2015;102:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnes R, Shahin Y, Tucker AT, Chetter IC. Haemodynamic efficacy of the geko™ electrical neuromuscular stimulation device in claudicants. Oral Presentation at Society of Academic & Research Surgery, 2014. Annual Meeting, 8 and 9 January 2014, Cambridge University, England. http://www.surgicalresearch.org.uk/wp-content/uploads/2013/10/1A_Vascular_Surgery_1.pdf
- 24. Ingves MV, Power AH. Two cases of transcutaneous electrical nerve stimulation of the common peroneal nerve successfully treating refractory, multifactorial leg edema. J Investig Med High Impact Case Rep 2014:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Q, Styf J, Ekström L, Holm AK. Effects of electrical nerve stimulation on force generation, oxygenation and blood volume in muscles of the immobilized human leg. Scand J Clin Lab Invest 2014;74:369–77. [DOI] [PubMed] [Google Scholar]
- 26. Rietschel RL, Fowler JF. Fischer's Contact Dermatitis, 5th edn. Philidelphia: Lippincott Williams & Wilkins, 2001:39. [Google Scholar]
- 27. Sussman C. Wound measurements and prediction of healing. In: Wound Care: A Collaborative Practice Manual. Philadelphia: Lippincott Williams & Wilkins, 2007:134. [Google Scholar]
- 28. Hildebrand G, Tompkin M, Macalena J. Fibular head as a landmark for identification of the common peroneal nerve: a cadaveric study. Arthroscopy 2015;31:99–103. [DOI] [PubMed] [Google Scholar]
- 29. Aronson JK. Editors' view compliance, concordance, adherence. Br J Pharmacol 2007;63:383–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weller CD, Buchbinder R, Johnston RV. Interventions for helping people adhere to compression treatments for venous leg ulceration. Cochrane Database Syst Rev 2016;3:CD008378. [DOI] [PMC free article] [PubMed] [Google Scholar]


