ABSTRACT
The aim of this study was to assess the relationship between length of surgery (LOS) and pressure ulcer (PU) risk in cardiovascular surgery patients. PubMed and Web of Science were systematically searched. We compared LOS difference between PU (+) group and PU (‐) group. We also examined the dose–response effect of this relationship. The mean LOS in the PU(+) groups ranged from 252·5 to 335·7 minutes, compared with 233·0 to 298·3 minutes in PU(−) groups. The LOS was higher in PU(+) groups compared with PU(−) groups [weighted mean difference (WMD) = 36·081 minutes; 95% CI: 21·640–50·522 minutes; Z = 4·90, P = 0·000]. The funnel plot showed no publication bias. A significant dose–response association was also found between the LOS and the risk of surgery‐related pressure ulcers (SRPU, model χ 2 = 9·29, P = 0·000). In the linear model, the PU OR was 1·296 (95% CI 1·097–1·531) for a 60‐minute increase in the LOS intervals and 13·344 (95% CI 2·521–70·636) for a 600‐minute increase. In a spline model, the OR of PU increased almost linearly along with the LOS. Our meta‐analysis indicated that LOS was an important risk factor for pressure ulcers in cardiovascular surgical patients.
Keywords: Cardiovascular surgery, Dose–response relationship, Length of surgery, Meta‐analysis, Pressure ulcers
Introduction
Cardiovascular surgery patients are among those most at risk of developing surgery‐related pressure ulcers (SRPU), with a reported incidence as high as 29·5% 1. Pressure ulcers not only make a significant independent contribution to the excess length of hospitalisation 2 but also increase significant additional costs for pressure ulcer treatment 3. Most importantly, pressure ulcers are associated with a higher risk of mortality 4. Many studies have examined factors associated with SRPU development in cardiovascular surgery patients. Commonly cited risk factors include temperature manipulation, vasoactive drugs, hypotensive periods and reduced haemoglobin and haematocrit levels 5.
A pressure ulcer is a localised injury as a result of pressure or pressure in combination with shear and/or friction. Cardiovascular surgery is always related with prolonged surgery, which increases the pressure on local tissue and increases the risk of SRPU. There have been some studies investigating the relationship between length of surgery (LOS) and the SRPU risk in cardiovascular surgery patients. Some
studies found that LOS was an important risk factor for SRPU 6, but other studies found there was no significant difference of LOS between SRPUs developed and no SRPUs developed in cardiovascular surgery patients (P‐value was 0·34 in Stordeur's study 7 and 0·43 in Jesurum's study 8). We found that there is still no systematic review summarising the relationship between LOS and SRPU risk in cardiovascular surgery patients.
The aim of this study was to assess this relationship by meta‐analysis and find the dose–response effect of this relationship.
Methods
Databases and literature search
We searched MEDLINE and Web of Science up to 20 September 2016. The search terms included ‘pressure ulcer’, ‘cardiac surgery’, ‘cardiovascular surgery’ and ‘cardiothoracic surgery’. The search detail in MEDLINE was ‘pressure ulcer’[MeSH Terms] AND [cardiac (Title/abstract) OR cardiovascular (Title/abstract) OR cardiothoracic (Title/abstract)]. In Web of Science, the search detail used was as follows: (Ts = pressure ulcer*) AND (Ts = cardiac OR Ts = cardiovascular OR Ts = cardiothoracic). We supplemented our searches by manually reviewing the references of all relevant studies.
Eligibility criteria
The following eligibility criteria had to be fulfilled: (i) observational studies (cross‐sectional, case–control or cohort study) that permitted assessment of LOS and SRPU risk; (ii) outcome was the incidence of SRPU during the cardiovascular perioperative period; (iii) study reported the sample size, mean and standard deviation of LOS both in the SRPU group and the no SRPU group; and (iv) study divided LOS into three or more quantitative categorised levels and reported the number of cases and controls for each LOS category. The eligibility criteria was described as: (i) AND (ii) AND ((iii) OR (iv)).
Data extraction and critical appraisal
The following data were abstracted onto standardised forms: first author; publication year; country; study design; enrolment year; sample size of participants; age and gender of participants; SRPU definition (I–IV or II–IV); SRPU incidence; sample size, mean and standard deviation of LOS both in the SRPU group and the no SRPU group; LOS category; and number of patients in the case or control group in each LOS category.
The quality of the case–control and cohort studies was assessed according to the Newcastle–Ottawa Scale (NOS) 9. The quality of the cross‐sectional study was also assessed by the NOS. The NOS contains eight items, categorised into three dimensions, including selection, comparability and exposure. The NOS ranges between zero and nine.
Statistical analysis
First, we compared LOS between the SRPU(+) group and SRPU(−) group of the included studies. The meta‐analysis model was chosen by the heterogeneity between included studies. Statistical heterogeneity was explored by χ 2 and inconsistency (I 2) statistics; P < 0·05 for χ 2 or I 2 value of 25% or more represented heterogeneity. In the absence of significant heterogeneity, the LOS of the included studies were pooled for weighted mean difference (WMD) using a fixed‐effect model. If heterogeneity was observed, a random‐effects model was used. Overall effects were determined using the Z test. Meta‐regression was conducted for the source of heterogeneity by SRPU incidence. Publication bias was evaluated using a funnel graph, Egger's regression test and Begg's adjusted rank correlation test.
Second, we examined the dose–response relationship of LOS and pressure ulcer risk. The dose–response meta‐analysis was carried out using the method proposed by Greenland and Longnecker 10 and Orsini et al. 11, 12. Non‐linear dose–response relationship was tested by using restricted cubic splines with three knots.
All statistical analyses were performed with Stata software, version 13·0 (Stata Corp, College Station, TX). Two‐sided P < 0·050 was considered statistically significant.
Results
Characteristics of included studies
We identified eight studies 6, 7, 8, 13, 14, 15, 16, 17 that met our inclusion criteria for meta‐analysis. The detailed steps of our literature search are shown in Figure 1. The studies included three prospective cohort studies, three retrospective cohorts and two cross‐sectional surveys. Of these studies, three were conducted in USA, three in China, two in Japan, and Netherlands and Belgium contributed one study each. The quality rating of the included studies ranged from seven to eight on a scale of nine. Table 1 showed the characteristics of the eight identified studies.
Figure 1.
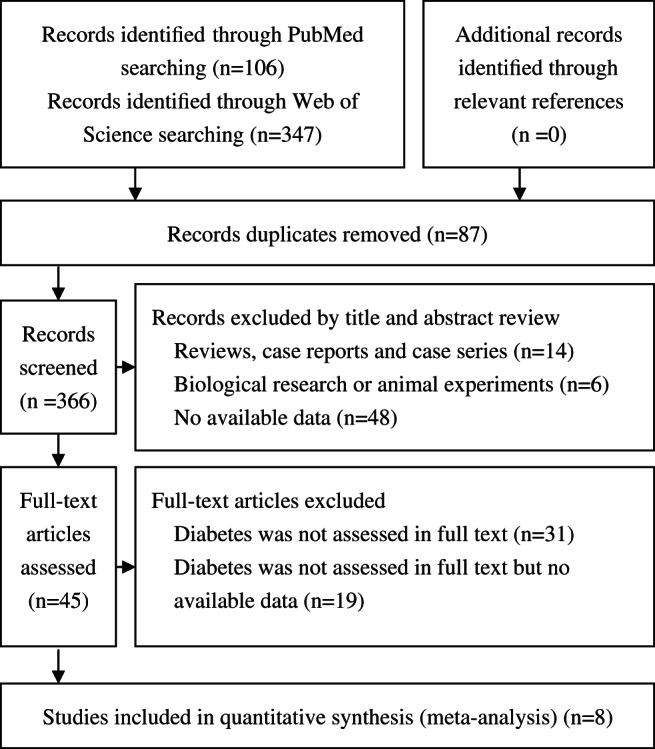
Flow diagram showing selection of studies.
Table 1.
Characteristics of the included studies accessing length of surgery and pressure ulcer risk in cardiovascular surgical patients
| First author, year | Country | Study design | Enrolment year | Sample size | Age (year) | Gender (M/F) | SRPU definition (stage) | SRPU incidence | SRPU(+) group | SRPU(‐) group | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | LOS (min) | N | LOS (min) | ||||||||||
| Lu, 2016 13 | China | Prospective cohort | 2015 | 149 | 49·8 ± 17·7 | 79/70 | I–IV | 24·8% | 37 | 263·6 ± 93·0 | 112 | 221·7 ± 85·8 | 8 |
| Chen, 201514 | China | Retrospective cohort | 2012 | 286 | 46·9 ± 22·1 | 160/126 | I–IV | 16·4% | 45 | 259·7 ± 108·9 | 230 | 182·6 ± 98·8 | 8 |
| Schuurman, 2009 15 | Netherlands | Prospective cohort | 2000 | 204 | 68·1 ± 9·6 | 129/75 | I–IV | 57·4% | 117 | 252·5 ± 89·6 | 87 | 233·0 ± 81·2 | 7 |
| Stordeur, 1998 7 | Belgium | Cross‐sectional | 1995 | 163 | 64·5 ± 11·3 | NR | II–IV | 29·4% | 48 | 283·8 ± 122·1 | 115 | 265·1 ± 91·8 | 7 |
| Jesurum, 1996 8 | USA | Prospective cohort | 1995‐ 1996 | 36 | 63 ± 10·7 | 26/10 | I–IV | 16·7% | 6 | 335 ± 167·4 | 30 | 280 ± 81·2 | 7 |
| Papantonio, 1994 16 | USA | Cross‐sectional | 1994 | 136 | 61·9 ± 13·4 | 90/46 | I–IV | 27·2% | 36 | 335·7 ± 120·9 | 97 | 298·3 ± 85·2 | 7 |
LOS, length of surgery; SRPU, surgery‐related pressure ulcer
LOS difference between the SRPU group and the no SRPU group
Six studies 7, 8, 13, 14, 15, 16 with 976 patients were included in this comparison. The mean LOS in the SRPU (+) groups ranged from 252·5 to 335·7 minutes, compared with 233·0–298·3 minutes in the SRPU (−) groups. There was moderate heterogeneity between the included studies (χ 2 = 8·38, P = 0·137; I 2 = 40·3%) 5. The pooled analysis using a random‐effects model showed that LOS was higher in SRPU (+) groups compared with SRPU (−) groups (WMD = 36·081 minutes; 95% CI: 21·640–50·522 minutes; Z = 4·90, P = 0·000). Table 1 and Figure 2 showed the comparison of LOS between the two SRPU groups of the eight identified studies. The funnel plot showed no asymmetry, which indicated no evidence of publication bias (Figure 3). Begg's test (z = 0·38, P = 707) and Egger's test (t = 0·60, P = 0·583) also suggested no publication bias. Meta‐regression analysis showed that the heterogeneity did not come from SRPU incidence (t = −0·59, P = 0·587) (Figure 4).
Figure 2.
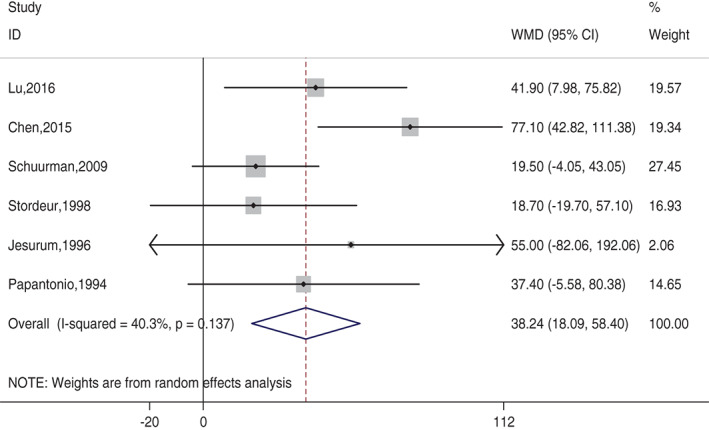
Comparison of length of surgery between SRPU (+) group and SRPU (−) group of the eight identified studies. Forest plot showed that length of surgery was higher in SRPU (+) groups.
Figure 3.
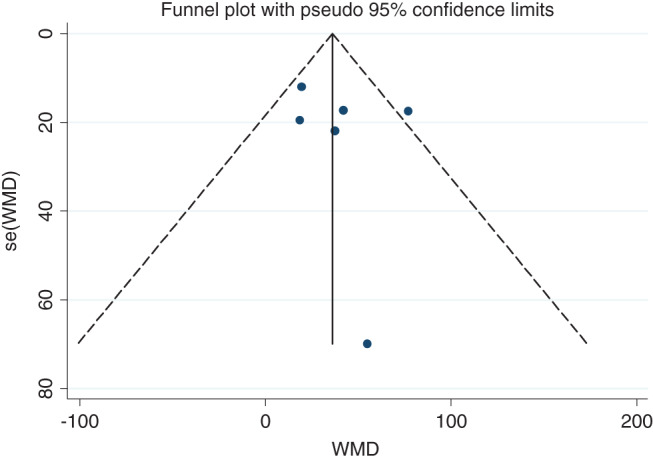
Publication bias analysis for length of surgery comparison between two groups. Funnel plot was asymmetry, which indicated no evidence of publication bias
Figure 4.
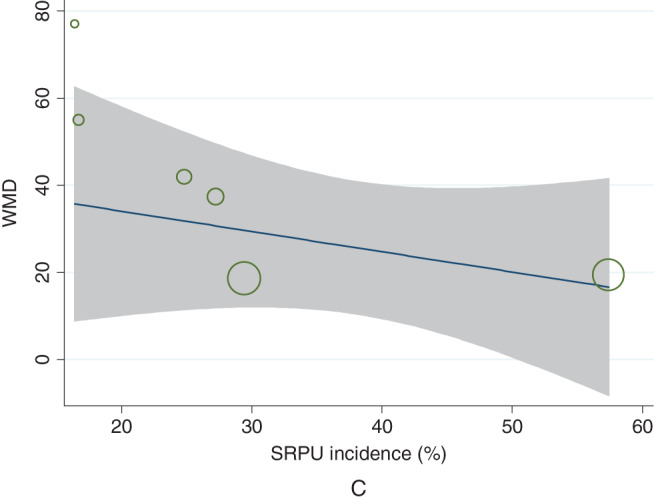
Meta‐regression that showed heterogeneity was not obtained from SRPU incidence (t = −0·59, P = 0·587).
Dose–response association between LOS and SRPU risk
Two studies 6, 17 with 1254 patients were included in the dose–response meta‐analysis (Table 2). A significant dose–response association was also found between LOS and risk of SRPU (model χ 2 = 9·29, P = 0·000). In the linear model, the SRPU OR was 1·296 (95% CI 1·097–1·531) for a 60‐minute increase in LOS intervals, 2·176 (95% CI 1·320–3·587) for a 180‐minute increase, for a 300‐minute increase and 13·344 (95% CI 2·521–70·636) for a 600‐minute increase. In the spline model, the OR of SRPU increased almost linearly along with the LOS. Figure 5 shows the dose–response association between LOS and SRPU risk.
Table 2.
Characteristics of the included studies for dose–response meta‐analysis for length of surgery and pressure ulcer risk
| First author, year, country, study design, NOS SRPU definition (stage), SRPU total incidence | LOS category (min) | SRPU (+) | SRPU (−) | SRPU incidence | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| Shen, 2015 6 | <120 | 2 | 74 | 2·6% | 1·0[ref.] |
| China | 120‐180 | 5 | 27 | 15·6% | 6·851(1·025–74·392) |
| Retrospective cohort | 180‐240 | 13 | 65 | 16·7% | 7·4(1·568–69·209) |
| 8 | 240‐300 | 12 | 42 | 22·2% | 10·571(2·16–99·986) |
| I–IV | 300‐360 | 10 | 13 | 43·5% | 28·462(4·98–280·784) |
| 16·4%· | 360‐600 | 1 | 8 | 11·1% | 4·625(0·07–95·572) |
| >600 | 2 | 1 | 66·7% | 74(2·277–4411·217) | |
| Aronovitch, 1999 17 | <180 | 26 | 423 | 5·8% | 1·0[ref.] |
| USA | 180‐240 | 27 | 276 | 8·9% | 1·592(0·873–2·903) |
| Retrospective survey | 240‐300 | 20 | 182 | 9·9% | 1·788(0·92–3·422) |
| 7 | 300‐360 | 6 | 75 | 7·4% | 1·302(0·423–3·377) |
| I–IV | 360‐420 | 3 | 34 | 8·1% | 1·356(0·265–5·058) |
| 8·1% | 420‐480 | 2 | 19 | 9·5% | 1·713(0·184–7·743) |
| >480 | 8 | 15 | 34·8% | 8·677(2·883–24·059) |
Figure 5.
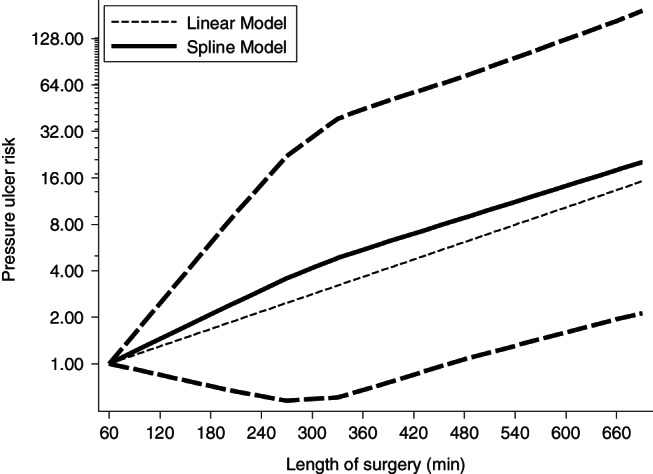
Dose–response relationships between length of surgery and pressure ulcers risk in cardiovascular surgical patients.
Discussion
Our meta‐analysis indicated that SRPU risk increased almost linearly along with the LOS in cardiovascular surgical patients. The point estimate of LOS at 300 and 600 minutes increased the SRPU risk of 3·653 (95% CI 1·588–8·405) and 13·344 (95% CI 2·521–70·636), respectively, compared with the reference (60 minutes). In a pig model, Daniel found that pressure exceeding 35 mm Hg for 2 hours would cause ischemia resulting in a pressure ulcer 18. In rat models, Linder et al. found that even relatively short exposures (15 minutes to 1 hour) to pressures greater than 240 mm Hg would cause cell death; for exposures of 2 hours or greater, the magnitude of pressure is the important factor for causing cell death, and for the intermediate exposures (over 1 and 2 hours), the magnitude of cell death‐causing pressure strongly depends on the time of exposure 19. In cardiac procedures, the LOS often exceeds 2 hours. The results of these animal experiments well explained why prolonged surgery leads to pressure ulcer and why LOS has a dose–response relationship with SRPU risk.
However, SRPUs in cardiovascular surgical patients not only result in prolonged pressure from operating table during cardiopulmonary bypass, hypothermia is often required for the purpose of organ protection, vasoactive drugs, hypotensive periods and reduced haemoglobin and haematocrit levels 5. This status mainly related to the cardiopulmonary bypass. Some studies also assessed the length of cardiopulmonary bypass and the risk of SRPUs in cardiovascular surgical patients. Lewicki reported that the mean extracorporeal circulation time for patients who developed pressure ulcers was 1·82 hours (SD = 0·84 hours) compared with 1·75 hours (SD = 0·67 hours) for patients who did not develop pressure ulcers 20. While Shen et al. reported that the length of cardiopulmonary bypass was not found to be statistically significantly different between the two groups [37 minutes (15–144 minutes) versus 44 minutes (16–107 minutes), P = 0·830] 6. However, we did not assess the relationship between length of cardiopulmonary bypass and the risk of SRPU by meta‐analysis because the existed studies did not provide sufficient data for this meta‐analysis.
Some strategies have been used for the prevention SRPUs in cardiovascular surgery. A meta‐analysis showed that patients who were provided a support surface had a significantly decreased incidence of surgery‐related PUs (OR 0·31 [95% Cl 0·17–0·59]) compared to patients using a standard mattress 21. Pham also reported that support surfaces for intraoperative prevention of pressure ulcers in patients undergoing surgery is cost‐effective 22.Our meta‐analysis found that LOS has dose–response relationship with SRPU risk in cardiovascular surgery patients. However, we still do not know what are the best prevention strategies stratified by LOS. It appears that well‐designed prospective cohorts are needed.
There are some limitations in this meta‐analysis. First, some of the included studies were retrospective case–control studies, which were more prone to bias. Second, there was moderate heterogeneity between the included studies. Meta‐regression analysis of heterogeneity was not obtained from SRPU incidence. Heterogeneity may come from different study designs, ethnicity and/or SRPU stage. These two limitations need to be considered when evaluating the conclusion.
In conclusion, our meta‐analysis indicated that LOS was an important risk factor for pressure ulcers in cardiovascular surgical patients. Some pressure ulcer prevention strategies should be carried out for the prolonged surgery for cardiovascular patients.
Acknowledgements
The authors have no competing interests.
References
- 1. Rao AD, Preston AM, Strauss R, Stamm R, Zalman DC. Risk factors associated with pressure ulcer formation in critically ill cardiac surgery patients: a systematic review. J Wound Ostomy Continence 2016;43:242–7. [DOI] [PubMed] [Google Scholar]
- 2. Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Control Hosp Epidemiol 2005;26:293–7. [DOI] [PubMed] [Google Scholar]
- 3. Demarre L, Van Lancker A, Van Hecke A, Verhaeghe S, Grypdonck M, Lemey J, Annemans L, Beeckman D. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud 2015;52:1754–74. [DOI] [PubMed] [Google Scholar]
- 4. Moore Z. US medicare data show incidence of hospital‐acquired pressure ulcers is 4.5%, and they are associated with longer hospital stay and higher risk of death. Evid Based Nurs 2013;16:118–9. [DOI] [PubMed] [Google Scholar]
- 5. Feuchtinger J, Halfens RJG, Dassen T. Pressure ulcer risk factors in cardiac surgery: a review of the research literature. Heart Lung 2005;34:375–85. [DOI] [PubMed] [Google Scholar]
- 6. Shen WQ, Chen HL, Xu YH, Zhang Q, Wu J. The relationship between length of surgery and the incidence of pressure ulcers in cardiovascular surgical patients: a retrospective study. Adv Skin Wound Care 2015;28:444–50. [DOI] [PubMed] [Google Scholar]
- 7. Stordeur S, Laurent S, D'Hoore W. The importance of repeated risk assessment for pressure sores in cardiovascular surgery. J Cardiovasc Surg (Torino) 1998;39:343–9. [PubMed] [Google Scholar]
- 8. Jesurum J, Joseph K, Davis JM, Suki R. Balloons, beds, and breakdown. Effects of low‐air loss therapy on the development of pressure ulcers in cardiovascular surgical patients with intra‐aortic balloon pump support. Crit Care Nurs Clin North Am 1996;8:423–40. [PubMed] [Google Scholar]
- 9. Wells GA O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa scale (NOS) for assessing the quality of non‐randomized studies in meta‐analysis. URL http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed on 20 Sep 2016].
- 10. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 11. Orsini N. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6:40–57. [Google Scholar]
- 12. Orsini N, Li RF, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu CX, Chen HL, Shen WQ, Feng LP. A new nomogram score for predicting surgery‐related pressure ulcers in cardiovascular surgical patients. Int Wound J. 2017;14(1):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen HL, Shen WQ, Xu YH, Zhang Q, Wu J. Perioperative corticosteroids administration as a risk factor for pressure ulcers in cardiovascular surgical patients: a retrospective study. Int Wound J 2015;12:581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuurman JP, Schoonhoven L, Keller BPJ, Van Ramshorst B. Do pressure ulcers influence length of hospital stay in surgical cardiothoracic patients? A prospective evaluation. J Clin Nurs 2009;18:2456–63. [DOI] [PubMed] [Google Scholar]
- 16. Papantonio CT, Wallop JM, Kolodner KB. Sacral ulcers following cardiac surgery: incidence and risks. Adv Wound Care 1994;7:24–36. [PubMed] [Google Scholar]
- 17. Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. J Wound Ostomy Continence Nurs 1999;26:130–6. [DOI] [PubMed] [Google Scholar]
- 18. Daniel RK, Priest DL, Wheatley DC. Etiologic factors in pressure sores: an experimental model. Arch Phys Med Rehabil 1981;62:492–8. [PubMed] [Google Scholar]
- 19. Linder‐Ganz E, Engelberg S, Scheinowitz M, Gefen A. Pressure‐time cell death threshold for albino rat skeletal muscles as related to pressure sore biomechanics. J Biomech 2006;39:2725–32. [DOI] [PubMed] [Google Scholar]
- 20. Lewicki LJ, Mion L, Splane KG, Samstag D, Secic M. Patient risk factors for pressure ulcers during cardiac surgery. AORN J 1997;65:933–42. [DOI] [PubMed] [Google Scholar]
- 21. Huang HY, Chen HL, Xu XJ. Pressure‐redistribution surfaces for prevention of surgery‐related pressure ulcers: a meta‐analysis. Ostomy Wound Manag 2013;59:36–48. [PubMed] [Google Scholar]
- 22. Pham B, Teague L, Mahoney J, Goodman L, Paulden M, Poss J, Li JL, Sikich NJ, Lourenco R, Ieraci L, Carcone S, Krahn M. Support surfaces for intraoperative prevention of pressure ulcers in patients undergoing surgery: a cost‐effectiveness analysis. Surgery 2011;150:122–32. [DOI] [PubMed] [Google Scholar]


