ABSTRACT
This study confirms that botulinum neurotoxin type A (BoNT‐A) decreases capsular contracture and elucidates a possible mechanism. Silicone blocks were implanted subcutaneously in 20 mice. The experimental groups received BoNT‐A (1, 2·5 or 5 U) instilled into the subcutaneous pocket. After 30 days, periprosthetic capsules were harvested and evaluated. The effect of BoNT‐A on the differentiation of human dermal fibroblasts to myofibroblasts in culture was examined by Western blot analysis. Changes in transforming growth factor‐beta1 (TGF‐β1) expression in cultured fibroblasts were determined by enzyme‐linked immunosorbent assay (ELISA). In in vivo study, the thickness of capsules (P < 0·05) and the number of alpha‐smooth muscle actin (α‐SMA)+ cells in capsules (P < 0·05) were significantly decreased in the experimental groups. TGF‐β1 was significantly underexpressed in the experimental groups (P < 0·05). In in vitro study, BoNT‐A did not significantly affect fibroblast viability. Western blot analysis showed that α‐SMA protein levels were significantly decreased in the experimental groups (P < 0·05). Based on ELISA, the amount of TGF‐β1 was significantly decreased in the experimental groups (P < 0·05), especially cells treated with a high dose of BoNT‐A (P < 0·001). This study confirms that BoNT‐A prevents capsular formation around silicone implants, possibly by blocking TGF‐β1 signalling and interrupting the differentiation of fibroblasts to myofibroblasts.
Keywords: Botulinum neurotoxin type A, Capsule, Myofibroblast, TGF‐β1
Introduction
Silicone breast implants are generally accepted for use in breast reconstruction and augmentation mammoplasty. Capsular contracture remains one of the most common complications of both aesthetic and reconstructive breast surgery. Many possible causes of capsular contracture have been studied in breast reconstruction and augmentation mammoplasty 1, 2, 3, 4. Potential explanations include hypertrophic scar hypothesis, myofibroblasts, silicone gel bleed, hematoma theory, age and the infectious theory 5. Research concerning the cause and prevention of capsular contracture has been inconclusive 5, 6, 7, 8, 9, 10.
In the early 1980s, botulinum neurotoxin type A (BoNT‐A) was first introduced for medical indications such as strabismusand blepharospasm 11, 12. BoNT induceschemodenervation through its action on presynaptic neurons, preventing the release of acetylcholine and leading to functional denervation of striated muscle for 2–6 months after injection. This chemodenervation results in muscle fibre atrophy and subsequent clinical flaccid paralysis 13. Thus, its cosmetic use in treating wrinkles induced by muscle hyperactivity is widespread 14, 15.
Many off‐label cosmetic applications of BoNT are currently under evaluation, and some physicians have observed improvement in the cosmetic appearance of scars 16, 17, 18. The development of a capsule around an implanted device is a normal response of the body to a foreign object, and a mature capsule is typically constructed of cells similar to those found in scar tissue 19, 20. BoNT‐A has been shown to reduce the incidence of capsular contracture in breast augmentation with silicone implants 21.
To further demonstrate that BoNT reduces capsule formation around silicone implants, we studied the effects of BoNT in vivo in a mouse model for silicone‐induced capsules and in vitro in human fibroblasts. Additionally, these experiments aimed to elucidate a possible mechanism for the BoNT‐related effects on capsular contracture.
Materials and methods
In vivo analysis
Twenty female mice (Orient Bio, Inc., Seoul, Korea) were used in the animal experiments according to the Guidelines for the Care and Use of Laboratory Animals and as approved by the Chungnam National University's Institutional Animal Care and Use Committee (CNUCOM‐2007‐017). Solid, smooth‐surfaced, hemisphere silicone blocks (1·0 cm diameter) were crafted for the experiment and were sterilised using ethylene oxide gas. Anaesthesia was induced in 7‐week‐old mice, and a 1·0‐cm incision was made through the skin and panniculus carnosus 0·5 cm from the medial dorsal line on each side of the proximal dorsal region of the mouse. A pocket measuring 1·5 cm in diameter was created, the implants were introduced and the skin incision was closed. The animals were distributed into four groups (n = 5 per group). The control group received saline infiltrated into the subcutaneous pocket, and the three experimental groups, groups 1, 2 and 3, were infiltrated with 1, 2·5 and 5 U BoNT‐A (Botox®; Allergan, Irvine, CA), respectively.
After the surgical procedure, the mice were followed for 30 days. At the end of this period, the animals were sacrificed, and the silicone implant, wound and surrounding soft tissue were harvested en bloc. After separation from the silicone implant, the wound and surrounding soft tissue sample from the right side of each mouse were fixed in 10% formaldehyde overnight, embedded in paraffin and cut into 5‐µm‐thick sections. The wound and surrounding soft tissue sample from the left side were placed in RNAlater solution (Applied Biosystems, Foster City, CA) and stored at −80°C.
Histological analysis
For histological analysis, capsules around the silicone disc were fixed in 4% paraformaldehyde for at least 24 hours, incubated in a graded alcohol series, embedded in paraffin and cut into 4‐µm‐thick sections. The sections were stained with haematoxylin and eosin and examined under a light microscope. The thickness of the capsule was measured in three different areas of each section and the mean thickness was calculated.
Immunofluorescence microscopy of α‐SMA
To examine the myofibroblast content in the capsule, immunofluorescence microscopy was performed. For immunofluorescence staining, the capsules were thoroughly washed in 0·01 M phosphate‐buffered saline (PBS), placed into sodium citrate for antigen retrieval, boiled and then cooled for 5 minutes. After three washes with PBS, the sections were placed in PBS supplemented with 10% goat serum and 0·1% Triton X‐100 for 30 minutes. Samples were incubated with antibody (1:200 dilution) against human alpha‐smooth muscle actin (α‐SMA) (R&D Systems, Minneapolis, MN) overnight at 4°C, washed again with PBS and incubated with Cy3‐conjugated anti‐rabbit secondary antibody (1:500; Amersham, Little Chalfont, UK) for 2 hours at room temperature. The nuclei were stained with DAPI (40‐60‐diamidino‐2 phenylindole; Sigma, St. Louis, MO). The sections were examined using a Leica digital DMI 6000 fluorescence microscope (Leica Microsystems, Heidelberg, Germany) with a DAPI filter (365 nm excitation). α‐SMA fluorescence was visualised with a PE filter (546 nm excitation) under 400× magnification, and the images were captured with a photometric camera (Cool Snap HQ, Tucson, AZ). To quantify the myofibroblasts, sectioned tissues were immunostained with anti‐α‐SMA antibody and the α‐SMA+ cells were counted in four randomly selected fields.
Reverse transcription PCR analysis of cytokine expression
To examine the changes in growth factor expression associated with capsule formation, we analysed the messenger RNA (mRNA) expression of transforming growth factor‐beta1 (TGF‐β1), tumor necrosis factor‐alhpa (TNF‐α) and vascular endothelial growth factor (VEGF) receptor. Total RNA from the capsule around the silicone implant was extracted using Trizol reagent. Approximately 2 µg of total RNA from each sample was used to generate cDNA using 200 U of SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo(dT) in a 40‐µl reaction. Subsequently, 2 µl of each cDNA sample was amplified by polymerase chain reaction (PCR) using DNA polymerase with the following specific primer sets: TGF‐β1 (BC013738.1), 5′‐CTT CAG CTC CAC AGA GAA GAA‐3′ and 5′‐TCA TGT TGG ACA ACT GCT CC‐3′; TNF‐α (NM 013693.2), 5′‐ATG AGC ACA GAA AGC ATG ATC‐3′ and 5′‐CCA AAG TAG ACC TGC CCG GAC TC‐3′; VEGF (NM 001025250.3), 5′‐TGA ACT TTC TGC TCT CTT GG‐3′ and 5′‐AAC AAA TGC TTT CTC CGC TC‐3′; and β‐actin, 5′‐GTC ACC AAC TGG GAC GAC ATG‐3′ and 5′‐GCC GTC AGG CAG CTC GTA GC‐3′. The PCR program was 33 cycles of denaturation for 40 seconds at 95°C, annealing for 30 seconds at 52°C and extension for 30 seconds at 72°C, with a final extension at 72°C for 10 minutes. The PCR products were quantified by 2% agarose gel electrophoresis with normalisation to β‐actin mRNA expression.
In vitro analysis
Primary fibroblast culture and treatment with BoNT‐A
Normal human skin samples were obtained from circumcisions, in accordance with the Ethics Committee approval process of Chungnam National University Hospital. Specimens were briefly sterilised in 70% ethanol, minced and incubated in Dulbecco's Modified Eagle's Medium supplemented with 10% foetal bovine serum and antibiotics. Dermal fibroblasts developed from the explants after 5–7 days. At confluence, cells were harvested, seeded at 1 × 105/ml and cultured in serum for 24 hours. Cells were treated with 0, 1, 2·5 or 5 U of BoNT‐A in serum‐free conditions.
MTT assay
Human dermal fibroblasts (1 × 105/ml) were seeded in 96‐well plates, incubated for 24 hours and treated with 0, 1, 2·5 or 5 U of BoNT‐A for 6 hours. Then, 20 µl of MTT (5 mg/ml) was added to each well for 4 hours at 37°C, followed by removal of the culture medium and addition of 150 µl of dimethyl sulfoxide (Sigma). The absorbance was measured at 570 nm, with 655 nm as the reference wavelength. All experiments were performed in triplicate.
Western blot analysis of α‐SMA
Cultured fibroblasts were collected by scraping, and the pellet was solubilised in lysis buffer using PRO‐PREP reagent (Intron Biotechnology, Sungnam, Korea) with a protease inhibitor cocktail. After vigorous pipetting, extracts were clarified by centrifugation for 15 minutes at 12 700 g. Total protein was measured using a Bradford protein assay kit (Bio‐Rad Laboratories, Hercules, CA). Following normalisation of the protein content in each sample, 30 mg of total cellular protein from each sample was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transblotted onto a nitrocellulose membrane. The blot was probed with anti‐α‐SMA (Santa Cruz Biotechnology, Santa Cruz, CA) and anti‐actin (Santa Cruz Biotechnology) antibodies in blocking solution and then incubated with peroxidase‐conjugated secondary antibodies for 1 hour at room temperature. Immunoreactive proteins were detected by enhanced chemiluminescence (Amersham Biosciences, Uppsala, Sweden). Densitometry was performed using ImageJ software (NIH, Bethesda, MD).
Determination of TGF‐β1 by ELISA
To determine the total TGF‐β1 levels in tissues, wound homogenates were incubated with 1 N HCl (5:1) for 10 minutes, and the acidified sample was neutralised by the addition of 1·2 N NaOH/0·5 M HEPES. TGF‐β1 levels in culture supernatants of serum‐free‐cultured cells treated with various doses of BoNT‐A were evaluated using an enzyme‐linked immunosorbent assay (ELISA) kit (DY240; R&D Systems, Minneapolis, MN). TGF‐β1 levels are expressed as picograms of TGF‐β1/µg of total protein.
Statistical analysis
Statistical analysis was performed using SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL). The t‐test and parametric analysis of variance (ANOVA) with the Scheffe post hoc test were used for between‐group comparisons. Values of P < 0·05 indicated statistical significance. Data are presented as means ± SD.
Results
In vivo analysis
Histological analysis of the periprosthetic capsules showed a significant difference in capsule thickness between the postoperative control and experimental groups. Capsules in mice treated with BoNT‐A were significantly thinner than those in the control group at the 30‐day time point (P < 0·05), indicating that capsule formation was significantly affected by BoNT‐A. In addition, the decrease in capsular thickness was dose‐dependent (Figure 1). Capsules in mice treated with 1 U of BoNT‐A (experimental group 1) were significantly thicker than those in mice treated with higher doses (2·5 and 5 U) (P < 0·05), but capsule thickness did not differ significantly (P > 0·05) between experimental groups 2 (2·5 U) and 3 (5 U).
Figure 1.
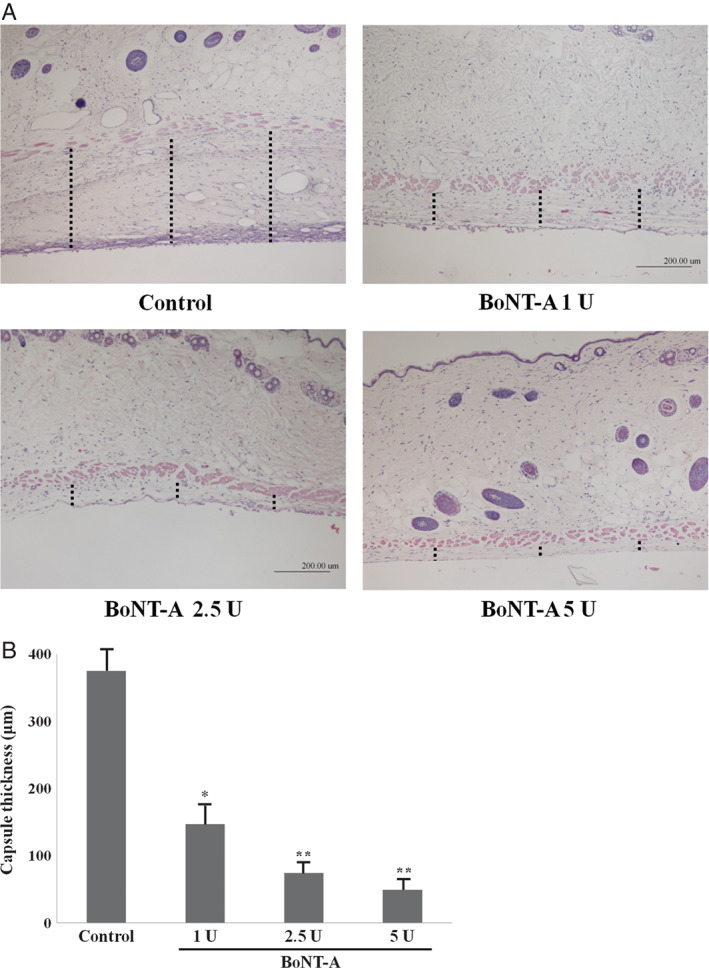
Representative histological sections of capsular tissues in experimental groups or control group at the 30‐day time point (original magnification, ×40). Sections were stained with haematoxylin and eosin. Length of dot line is capsular thickness. The capsules of experimental groups were significantly thinner than those of control group (P < 0·05). The capsules of experimental group 1 were significantly thicker than those of experimental groups 2 and 3 (P < 0·05). *P < 0·05 versus control; **P < 0·01 versus control.
To examine the myofibroblast content in the capsules, α‐SMA expression on capsules from control and experimental mice was detected by immunofluorescence microscopy. The number of α‐SMA+ cells in the capsules was lower in the experimental groups than in the control group (P < 0·05), indicating that BoNT‐A significantly affected the differentiation of fibroblasts to myofibroblasts. In addition, the decrease in the number of α‐SMA+ cells was dose‐dependent, although the trend was not significant (P > 0·05) (Figure 2).
Figure 2.
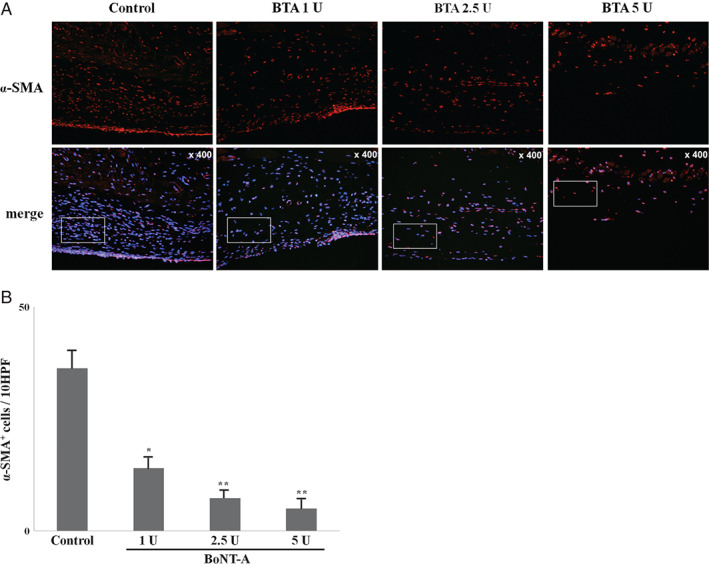
Immunofluorescence microscopic sections of capsular tissues in experimental groups and control group at the 30‐day time point (original magnification, × 400). To quantify the myofibroblasts, sectioned tissues were immunostained with anti‐alpha‐smooth muscle actin (α‐SMA) antibody and the α‐SMA + cells were counted in four randomly selected fields. The number of α‐SMA + cells in capsules was decreased in experimental groups compared with control group (P < 0·05).*P < 0·05 versus control; **P < 0·01 versus control.
To examine the changes in growth factor expression associated with capsule formation, the mRNA expression of TGF‐β1, TNF‐α and VEGF receptor in the capsules was analysed. TNF‐α and VEGF receptor expression did not differ significantly among any of the four groups. In contrast, TGF‐β1 was significantly underexpressed in the experimental groups compared with the control group (P < 0·05) (Figure 3), suggesting a relationship between capsule formation and TGF‐β1.
Figure 3.
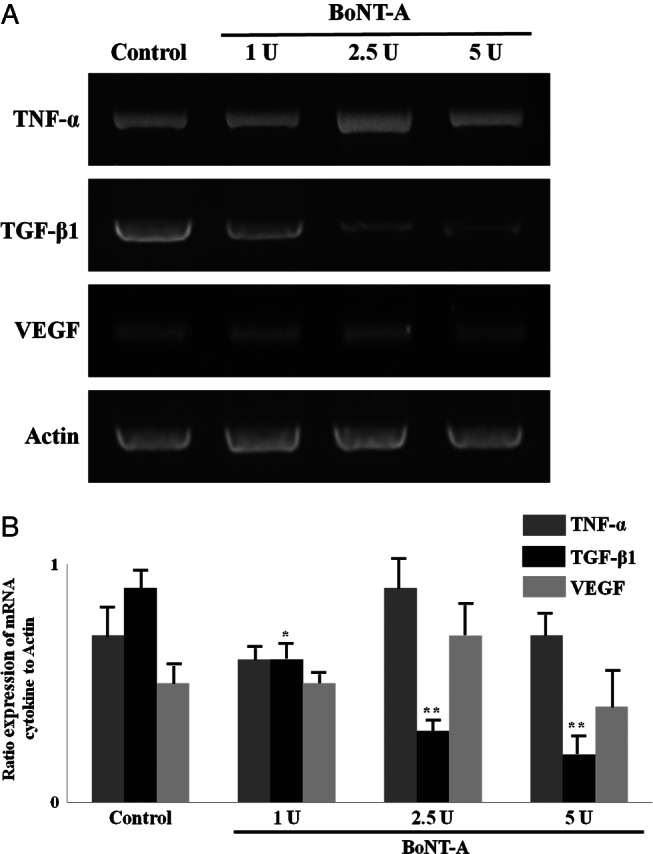
Reverse transcription PCR analysis of TGF‐β1, TNF‐α and VEGF gene expression at harvested capsules from mice. Expression of TNF‐α and VEGF in all samples showed no significant differences (P > 0·05). TGF‐β1 was significantly decreased in experimental groups (P < 0·05). *P < 0·05 versus control; **P < 0·01 versus control.
In vitro analysis
Fibroblasts were cultured in the absence of BoNT‐A or in the presence of 1, 2·5 or 5 U of BoNT‐A per 1 × 105 cells, and after 1–2 days, cell viability was assayed using MTT. Fibroblasts treated with BoNT‐A showed greater proliferation than untreated fibroblasts (P > 0·05), indicating that cell viability was not significantly decreased by BoNT‐A (Figure 4).
Figure 4.
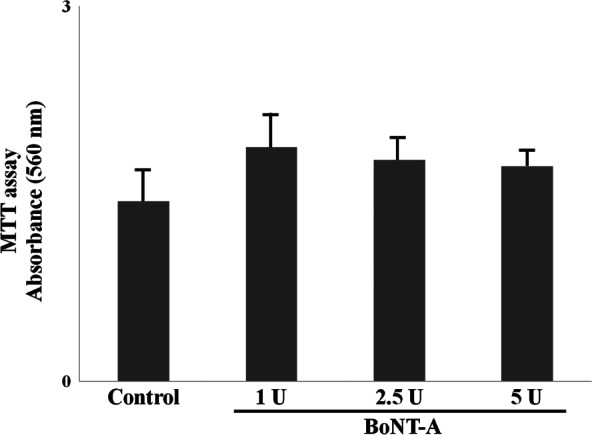
In MTT assay, fibroblasts with the various doses of botulinum neurotoxin type A (BoNT‐A) had a higher vaibility than those without BoNT‐A. In addition, this effect was dose‐dependent. But they did not show an apparent difference (P > 0·05). *P < 0·05 versus control; **P < 0·01 versus control.
The α‐SMA protein levels expressed by untreated and BoNT‐A‐treated dermal fibroblasts were analysed on Western blots. α‐SMA protein expression was significantly decreased in fibroblasts treated with BoNT‐A compared with expression in untreated fibroblasts (P < 0·05), and the effect was dose‐dependent (Figure 5). These results indicate that BoNT‐A inhibits α‐SMA protein expression. When TGF‐β1 protein expression in untreated and BoNT‐A‐treated cultured fibroblasts was analysed by ELISA, the results indicated that TGF‐β1 expression was significantly decreased at the highest dose of BoNT‐A (5 U per 1 × 105 cells) (Figure 6). The decrease in α‐SMA protein expression might have resulted from the downregulation of TGF‐β1 expression in cultured fibroblasts treated with BoNT‐A, which would be consistent with our in vivo results.
Figure 5.
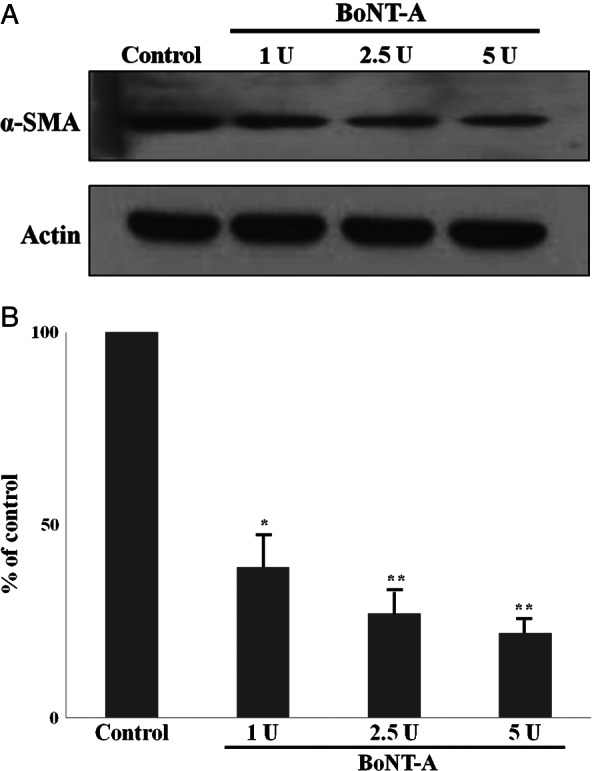
The protein levels of alpha‐smooth muscle actin (α‐SMA) were measured using a standard Western blotting technique. The protein expression of the α‐SMA was significantly decreased in botulinum‐neurotoxin‐type A‐treated dermal fibroblasts than serum‐free‐treated fibroblasts (P < 0·05). In addition, this effect was dose‐dependent. *P < 0·05 versus control; **P < 0·01 versus control.
Figure 6.
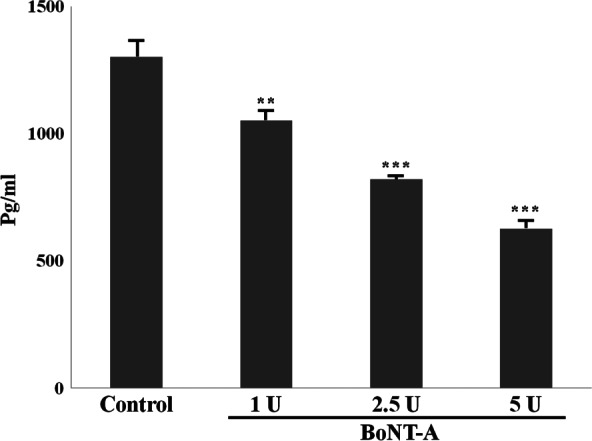
TGF‐β1 protein expression levels after treated botulinum neurotoxin type A (BoNT‐A) using enzyme‐linked immunosorbent assay. The results showed decrease in TGF‐β1 treated with BoNT‐A. In addition, this effect was dose dependent. *P < 0·05 versus control; **P < 0·01 versus control ; ***P < 0·001 versus control.
Discussion
In the early 1980s, BoNT‐A was first introduced for medical indications such as strabismus and blepharospasm 11, 12. BoNT‐A induces chemodenervation by preventing the release of acetylcholine by presynaptic neurons, leading to functional denervation of striated muscle for 2–6 months after injection. This results in muscle fibre atrophy and subsequent clinical flaccid paralysis 13. The cosmetic use of BoNT‐A for treating wrinkles induced by muscle hyperactivity has become widespread 14, 15.
Currently, many off‐label cosmetic applications of BoNT are under evaluation. Some physicians have observed improved cosmetic appearance of scarring, as BoNT infiltration effectively minimises the tension of the healing wound edges by inducing temporary paralysis of the muscle underlying a wound 16, 17, 18. The development of a capsule around an implanted device is a normal response of the body to a foreign object, and a mature capsule is typically constructed of cells similar to those found in scar tissue 19, 20. One study demonstrated that the use of BoNT‐A in breast augmentation with silicone implants was effective in reducing the incidence of capsular contracture 21. In addition to improving the appearance of facial scars and reducing hypertrophic scars, BoNT was recently reported to inhibit the proliferation of fibroblasts by affecting the cell cycle distribution of cells 22, 23.
Our histological results demonstrated that mice treated with BoNT‐A had a less intense inflammatory response surrounding the implant compared with the response in untreated control mice. The capsules around the implants in the BoNT‐A‐treated groups had less fibrous tissue deposition and significantly thinner walls compared with the capsules in the control group. These results suggest that BoNT decreases capsule formation. With increasing doses of BoNT‐A, the preventative effect increased, although there were no significant differences between mice treated with 2·5 U and 5 U of BoNT‐A.
It is generally accepted that myofibroblasts represent key players in the physiological reconstruction of connective tissue after injury and in the deformation of pathological tissue observed in fibrosis 24, 25. Myofibroblasts are also thought to participate in capsular contracture, despite little information to support such a mechanism 26. Our in vivo studies demonstrated a reduction in the myofibroblast population and density within the capsules in the experimental groups. Our in vitro experiments showed that protein expression of α‐SMA, a widely used myofibroblast marker, was significantly decreased in BoNT‐A‐treated dermal fibroblasts compared with untreated fibroblasts, and this effect was dose‐dependent. Taken together, these results suggest that BoNT‐A decreases capsule formation by preventing differentiation of fibroblast to myofibroblast.
Capsule formation is a type of inflammation‐related healing reaction around an implant, and different cell subsets secrete numerous mediators that orchestrate the overlapping phases. Cytokines and growth factors contribute to cell‐to‐cell crosstalk and are markers of the functionality of the transduction pathways in inflammation. We evaluated the expression of three cytokines (TGF‐β1, TNF‐α and VEGF receptor) involved in tissue inflammation in the wound healing process. Of these, only TGF‐β1 was significantly underexpressed in the experimental groups. Additionally, in vitro analysis showed decreased TGF‐β1 protein levels in cultured fibroblasts treated with BoNT‐A. TGF‐β1 is considered the major growth factor directly promoting myofibroblast development by inducing the expression of α‐SMA 27, 28.
In this study, BoNT‐A prevented capsular formation around silicone implants by interrupting the differentiation of fibroblasts to myofibroblasts, probably by blocking TGF‐β1 signalling. Further investigations regarding TGF‐β1 signalling are necessary.
Acknowledgment
This study was financially supported by research fund of Chungnam National University in 2011. The authors declare that there are no conflicts of interests.
References
- 1. Benlier E, Unal Y, Usta U, Top H, Aygit AC. Effect of verapamil on reduction of peri‐implant capsular thickness. Aesthetic Plast Surg 2009;33:570–5. [DOI] [PubMed] [Google Scholar]
- 2. Embrey M, Adams EE, Cunningham B, Peters W, Young VL, Carlo GL. A review of the literature on the etiology of capsular contracture and a pilot study to determine the outcome of capsular contracture interventions. Aesthetic Plast Surg 1999;23:197–206. [DOI] [PubMed] [Google Scholar]
- 3. Minami E, Koh IH, Ferreira JC, Waitzberg AF, Chifferi V, Rosewick TF, Pereira MD, Saldiva PH, de Fiqueiredo LF. The composition and behavior of capsules around smooth and textured breast implants in pigs. Plast Reconstr Surg 2006;118:874–84. [DOI] [PubMed] [Google Scholar]
- 4. Zimman OA, Toblli J, Stella I, Ferder L, Inserra F. The effects of angiotensin‐converting‐enzyme inhibitors on the fibrous envelope around mammary implants. Plast Reconstr Surg 2007;120:2025–33. [DOI] [PubMed] [Google Scholar]
- 5. Adams WP Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg 2009;36:119–26. [DOI] [PubMed] [Google Scholar]
- 6. Bern S, Burd A, May JW Jr. The biophysical and histologic properties of capsules formed by smooth and textured silicone implants in the rabbit. Plast Reconstr Surg 1992;89:1037–42 discussion 43–4. [PubMed] [Google Scholar]
- 7. Ibrahim Canter H, Konas E, Bozdogan O, Vargel I, Ozbatir B, Oner F, Erk Y. Effect of slow‐release 5‐Fluorouracil on capsule formation around silicone breast implants: an experimental study with mice. Aesthetic Plast Surg 2007;31:674–9. [DOI] [PubMed] [Google Scholar]
- 8. Poeppl N, Schreml S, Lichtenegger F, Lenich A, Eisenmann‐Klein M, Prantl L. Does the surface structure of implants have an impact on the formation of a capsular contracture? Aesthetic Plast Surg 2007;31:133–9. [DOI] [PubMed] [Google Scholar]
- 9. Moreira M, Fagundes DJ, de Jesus SM, de Oliveira MC, Dos Santos Previdelli IT, Moreira AC. Zafirlukast pocket delivery impairs the capsule healing around textured implants in rats. Aesthetic Plast Surg 2009;33:90–7. [DOI] [PubMed] [Google Scholar]
- 10. Vieira VJ, d'Acampora AJ, Marcos AB, Di Giunta G, de Vasconcellos ZA, Bins‐Ely J, d'Eca Neves R, Fiqueiredo CP. Vascular endothelial growth factor overexpression positively modulates the characteristics of periprosthetic tissue of polyurethane‐coated silicone breast implant in rats. Plast Reconstr Surg 2010;126:1899–910. [DOI] [PubMed] [Google Scholar]
- 11. Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980;87:1044–9. [DOI] [PubMed] [Google Scholar]
- 12. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol 1985;103:347–50. [DOI] [PubMed] [Google Scholar]
- 13. Fagien S. Botox for the treatment of dynamic and hyperkinetic facial lines and furrows: adjunctive use in facial aesthetic surgery. Plast Reconstr Surg 1999;103:701–13. [DOI] [PubMed] [Google Scholar]
- 14. Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol Surg 1998;24:1189–94. [DOI] [PubMed] [Google Scholar]
- 15. Rohrich RJ, Janis JE, Fagien S, Stuzin JM. Botulinum toxin: expanding role in medicine. Plast Reconstr Surg 2003;112(5 Suppl):1S–3S. [DOI] [PubMed] [Google Scholar]
- 16. Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 2000;105:1948–53 discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 17. Sherris DA, Gassner HG. Botulinum toxin to minimize facial scarring. Facial Plast Surg 2002;18:35–9. [DOI] [PubMed] [Google Scholar]
- 18. Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg 2006;117:1758–66 discussion 67–8. [DOI] [PubMed] [Google Scholar]
- 19. Brohim RM, Foresman PA, Hildebrandt PK, Rodeheaver GT. Early tissue reaction to textured breast implant surfaces. Ann Plast Surg 1992;28:354–2. [DOI] [PubMed] [Google Scholar]
- 20. Carpaneda CA. Inflammatory reaction and capsular contracture around smooth silicone implants. Aesthetic Plast Surg 1997;21:110–4. [DOI] [PubMed] [Google Scholar]
- 21. Xiao Z. Effect of botulinum toxin type A on the capsule around a subpectoral implant for breast augmentation. Aesthetic Plast Surg 2009;33:782–3. [DOI] [PubMed] [Google Scholar]
- 22. Chuang YC, Chiang PH, Huang CC, Yoshimura N, Chancellor MB. Botulinum toxin type A improves benign prostatic hyperplasia symptoms in patients with small prostates. Urology 2005;66:775–9. [DOI] [PubMed] [Google Scholar]
- 23. Zhibo X, Miaobo Z. Botulinum toxin type A affects cell cycle distribution of fibroblasts derived from hypertrophic scar. J Plast Reconstr Aesthet Surg 2008;61:1128–9. [DOI] [PubMed] [Google Scholar]
- 24. Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res 1981;54:183–4. [PubMed] [Google Scholar]
- 25. Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 2005;13:7–12. [DOI] [PubMed] [Google Scholar]
- 26. Baker JL Jr, Chandler ML, LeVier RR. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast Reconstr Surg 1981;68:905–12. [DOI] [PubMed] [Google Scholar]
- 27. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor‐beta 1 induces alpha‐smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronnov‐Jessen L, Petersen OW. Induction of alpha‐smooth muscle actin by transforming growth factor‐beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 1993;68:696–707. [PubMed] [Google Scholar]


