Abstract
Lipoedema is a rare painful disorder of the adipose tissue. It essentially affects females and is often misdiagnosed as lymphoedema or obesity. It is globally misdiagnosed or underdiagnosed, and the literature is lacking appropriate guidance to assist clinicians towards this diagnosis. However, the need to recognise this disorder as a unique entity has important implications to establish proper treatment and, therefore, its tremendous effect on patients. Early diagnosis and treatment can turn these patients’ lives upside down. The aim of this review is to focus on the clinical guidance, differential diagnosis, and management strategies. In addition, other aspects of lipoedema, including epidemiology and pathogenesis, are also being discussed here. Lipoedema is distinct from obesity and distinct from lymphoedema, although it might progress to involve the venous and lymphatic system (venolipedema or lympholipedema or both). Late diagnosis can leave the patient debilitated. Management of lipoedema includes weight loss, control of oedema, complex decongestive physiotherapy, liposuction, and laser‐assisted lipolysis. However; there are increasing reports on tumescent liposuction as the preferred surgical option with long‐lasting results. The role of more randomised controlled studies to further explore the management of this clinical entity remains promising.
Keywords: lipoedema, lymphoedema, lympholipedema (in German lipo‐lymphedema), tumescent liposuction
1. INTRODUCTION
Lipoedema is thought to be an uncommon disorder of adipose tissue. It is a symmetrical, disproportional distribution of fat deposited in the lower limbs and/or upper limbs featuring frequent ecchymosis because of even minor traumatic injuries. The pathophysiology of this group of disease with disproportional fatty distribution lipodystrophies, such as multiple symmetric lipomatosis, Dercum's disease (DD), and lipoedema, is not clearly elucidated.
Lipoedema almost exclusively affects women, and it is commonly misdiagnosed as lymphoedema (Figures 1 and 2). The phonetic sound of both diagnoses is quite similar, which might even add to the misconception and common error between the two. However, lipoedema is a distinct entity with some differences in comparison with lymphoedema. The former has a possible inheritance of X‐linked dominant or autosomal dominant pattern with sex limitation.1 It is bilaterally symmetrically distributed, and it is associated with pain, oedema, and easy bruising. It commonly affects females after puberty but may also develop at other periods of hormonal change, such as pregnancy or menopause.1, 2, 3 It has a significant physical and psychological effect on its patients. The diagnosis of lipoedema is quite frequently missed because of unfamiliarity of clinicians with this condition and, perhaps, the over‐diagnosis of lymphoedema or, sometimes, obesity instead.4, 5 Lymphoedema is more commonly asymmetric (Figure 2), without associated pain or bruising. However, lipoedema is a chronic progressive disease, and advanced cases may deteriorate to involve either the lymphatic system (lympholipedema) or the venous system (venolipedema) (Figure 2), or both, which will add to the confusion in the diagnosis. While progressing to either lympholipedema or to venolipedema, complication such as recurrent infections and ulceration might persist.
Figure 1.
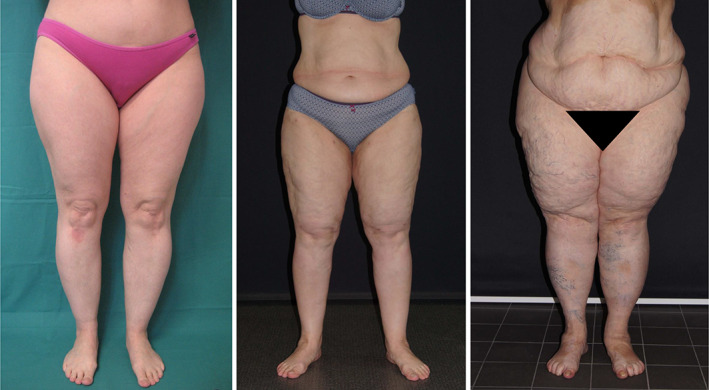
Stages of lipoedema. (From left to right) stages I‐II‐III [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
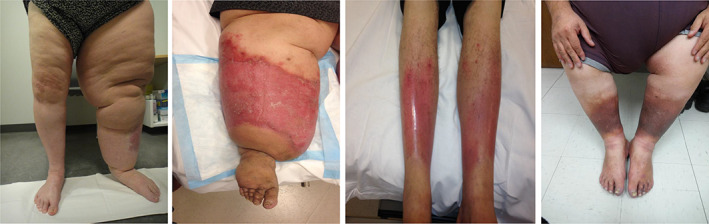
Differential diagnosis: (from left to right) unilateral lymphedema (notice the asymmetry), venolipoedema‐notice the “ankle pad” sign is prominent (also secondary changes of lipodermatosclerosis), acute lipodermatosclerosis and chronic lipodermatosclerosis (right picture)‐notice the sharp margination and sparing of the feet [Colour figure can be viewed at wileyonlinelibrary.com]
Obesity is another common misdiagnosis, but in obesity, the distribution of fat is symmetrical with the involvement of the central body and common involvement of arms. Even though patients with lipoedema commonly have a high body mass index (BMI), because of localised distribution, dieting and exercises have limited success for weight loss. Oddly, the English literature is sparse regarding the pathogenesis, epidemiology, diagnosis, and management strategies for this disorder,6 although the number of textbooks and publications dealing with lipoedema is extensive in the German literature.7 The need to recognise this disorder as a unique entity is universal and has important implications as late diagnosis can delay proper treatment and lead to true debilitation of these patients. The aim of this review is to increase the awareness of lipoedema as a distinct entity and to provide clues for the diagnosis; therefore, we have emphasised the clinical guidance and differential diagnosis for clinicians as well as revised the knowledge about epidemiology, pathogenesis, and management strategies for lipoedema.
2. METHODOLOGY
We have conducted a database search of PubMed and EMBASE for published articles on the subject of lipoedema. Using the keywords “lipedema” and lipoedema”, all studies and review from 1995 to October 2017 were included. We have included papers published in the English and German languages. Our search has demonstrated 112 reports; 4 were excluded as a result of being in another language (2 in French and 2 in Serbian), and 14 did not pertain directly to the topic of lipoedema, which led to a total of 94 articles.
3. EPIDEMIOLOGY AND PATHOGENESIS
The aetiology of lipoedema is unknown. The disorder was originally described by Allen and colleagues in 1940 as “large legs due to the subcutaneous deposition of fat in the buttocks and lower extremities and the accumulation of fluid in the legs”.8 Based on a study of 330 family members, a possible autosomal‐dominant inheritance with incomplete penetrance was suspected; however, the exact genes involved in lipoedema have not been identified. The VEGFR‐3 heterozygote‐inactivating missense mutation and mutation of PIT‐1 gene have been reported.
Lipoedema is encountered exclusively in female patients, during or following puberty, during the second to the third decades of life. However, there have been reports of onset after pregnancy or even menopause.8 Although exclusive to females, there have been 8 case reports of lip0edema in males in the context of pronounced hormonal imbalance.1, 9
Oestrogen has a direct effect on white fat through its oestrogen receptors, but the exact change in oestrogen receptors in lipoedematous tissue and the exact role of oestrogen is not clear. Most patients with lipoedema have a high BMI, which can be either because they are overweight or obese; however, many of these patients have a normal appearance above their waist, accounting for a disfigurement between their lower and upper extremities, and this might falsely elevate their BMI levels.
A positive family history suggesting lipoedema is linked to a genetic component has been described in up to 60% of cases.1, 10, 11 The overall prevalence of lipoedema in the general population is uncertain, and it has been reported to be as low as 0.1%; however, some studies conducted on outpatient's clinics estimate the prevalence to be 7% to 10%.11 Whereas studies conducted on hospitalised patients demonstrated prevalence percentages between 8% and 18%.10, 11, 12, 13 In a trial in southern Germany, the prevalence was 10% among adult women.14 Despite the wide range of prevalence documented in various studies, we still believe that lipoedema is an uncommon disease.
The histopathology of lipoedema is not pathognomonic and cannot serve as a diagnostic tool. With a greater amount of fat cells and occasional hypertrophy, the interstitial space demonstrates numerous capillary vessels. The infiltration surrounding the capillaries includes macrophages, fibroblasts, and mast cells and increased fibrosis with disease progression.15
Microangiopathy is one of the earliest changes in lipoedematous tissue that could be related to endothelial barrier function. Adipocyte hyperproliferation because of hypoxia may lead to adipocyte necrosis, production of inflammatory cytokines, and macrophage infiltration.
Immunohistochemical analyses of lipoedematous tissue demonstrated necrotising adipocytes surrounded by infiltrating CD68+ macrophages, which is a feature commonly seen in obese adipose tissue. Furthermore, there was a proliferation of adipose‐derived stem cells, progenitor cells, and stromal cells (Ki67+ CD34+ cells). Such findings suggest that the possible mechanism leading to the development of lipoedema may involve increased adipogenesis, leading to hypoxia, further adipocyte necrosis, and macrophage recruitment.5, 15, 16, 17 However, in a recent study, proteins from adipose tissue of lipoedema patients were harvested and did not indicate tyrosine‐phosphorylated proteins in lipoedema tissue and controls. These results suggest the absence of activated growth factor receptors in the pathways of adipogenesis of the lipoedema patient.18 Another interesting finding was that stromal vascular fraction cells (CD90+, CD146+) were significantly enhanced in lipoedematous adipose tissue compared with normal adipose tissue. On the other hand, the adipogenic differentiation potential of these cells was significantly reduced when compared with healthy controls.19
Functional lymphoscintigraphy, which study the lymphatic system, have been conducted comparing patients with lipoedema, normal subjects, and patients with lymphoedema. In the early stages of the disease, increased lymph flow (high‐volume insufficiency) has been reported, along with decreased lymphatic flow with pathological lymph node uptake at later stages,20, 21 whereas some cases showed no significant changes.22 These contradicting studies suggest that, unlike lymphoedema, lipoedema may not be related to lymphatic disturbance and may rather be a form of lipodystrophy or lipomatosis.
4. CLINICAL PRESENTATION
Lipoedema starts almost imperceptibly after puberty; it persists over the lifetime and progresses gradually. There are three stages to this disease as listed in Table 1. Stage III of lipoedema may develop after several decades without appropriate therapy and leads to impairment of gait and disability of these patients.23 Therefore, it is crucial to establish early and correct diagnosis.
Table 1.
Stages of lipoedema
| Stages | Characteristics |
|---|---|
| Stage 1 | Smooth skin; soft homogenous increase in subcutaneous tissue, cool skin in certain areas because of functional vascular imbalance |
| Stage 2 | Irregular skin surface, nodular changes of the subcutaneous tissue |
| Stage 3 | Tender subcutaneous nodules, pronounced increase in circumference with loose skin/tissue, bulging protrusion of fat mainly at inner thighs and knees |
The changes associated with lipoedema occur symmetrically on the legs and arms (gynoid distribution). The typical presentation is of a woman with bilateral “stovepipe” enlargement of the legs and without involvement of the feet with a sharp demarcation between normal and abnormal tissue at the ankle, referred to as the “cuff sign.” This is often combined with a symmetrical involvement of arms, particularly the upper arms, with sparing of hands. Lipoedema may be isolated to the arms without involvement of the legs, but it is extremely rare.11 A characteristic feature of lipoedema is pain; it is described as dull, heavy, and pressing. The pain can be elicited by touch or pressure, or it can occur after standing or sitting for a protracted period, as well as at the end of the day.11, 24 This tenderness makes high‐compression therapy challenging, particularly in cases where lipoedema is associated with the involvement of veins or lymphatics. The nosology of oedema is divided according to the substance or fluid involved; while the oedema content of lymphoedema consists of lymph fluid, in lipoedema, swelling a result of the deposition of fatty tissue.
5. DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
The diagnosis of lipoedema is clinical and mainly relies only on history and physical examination. Even though lipoedema is commonly mistaken to be obesity and lymphoedema, striking clinical features, sparing feet and hands, filling of retromalleolar sulci with fat, and pressure sensitivity of the below‐knee medial fat pad is helpful for diagnosis when clinicians are familiar with the disease.
Considering the enlargement of lower extremities, the first step towards the diagnosis is to determine whether this enlargement is unilateral or bilateral (see Figure 3). Lipoedema is always bilateral and non‐pitting, and other diagnoses that might cause bilateral lower limb with pitting oedema include early lipodermatosclerosis, dependent oedema, limb swelling because of internal (such as thyroid, heart, kidney) diseases, and oedema because of certain medications.25
Figure 3.
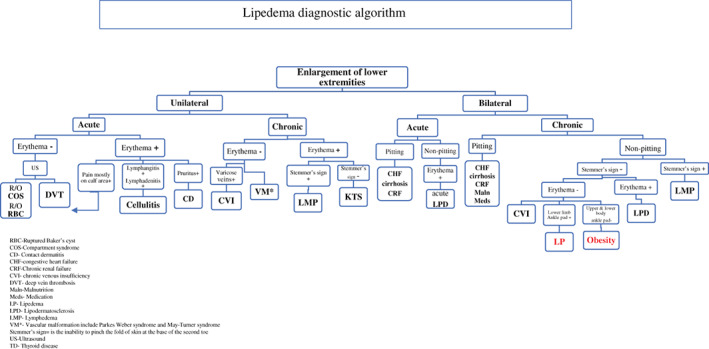
Lipoedema diagnostic algorithm (and approach to enlargement of lower extremities) [Colour figure can be viewed at wileyonlinelibrary.com]
Lipoedema is almost always bilateral, whereas lymphoedema may be unilateral or bilateral, but the involvement may be dominant on one side. Lipoedema typically presents in women bilaterally and symmetrically with a sharp demarcation at the ankle, referred to as the “cuff sign” or “reverse shouldering” (see Figure 2). The skin is normal, particularly in the early phases, and as mentioned before, the patients may complain of “painful lower limbs.”
Lipodermatosclerosis presents as bilateral swelling of the shins associated with erythematous changes, and the skin is indurated with “woody‐like” consistency. In the acute setting, acute lipodermatosclerosis will not demonstrate induration yet, instead demonstrating bilateral erythema, oedema, and increased warmth on palpation; the clinical presentation somewhat mimics cellulitis (Figure 2).26
Dependent pitting oedema can be multifactorial, and the common causes include congestive heart disease, chronic renal insufficiency, liver cirrhosis, and hypoalbuminemia. Many clues can be provided in the history and during bed side physical examination, for example, dyspnoea, jugular veins distention, pleural effusion, and ascites. Bilateral non‐pitting oedema can be caused by thyroid hormone imbalance such as pretibial myxedema, a rare autoimmune manifestation of Graves’ disease.27 Typically, bilateral, non‐pitting, doughy oedema is a result of thickening and induration because of mucin deposition. The oedema has a unique clinical feature with more predominant on anterior shin. A thyroid‐stimulating hormone (TSH) level should be set in patients with lower‐extremity non‐pitting oedema without known cause. Medications are another cause of lower limbs swelling, most commonly calcium channel blockers (such as Amlodipine), and other culprit drugs include gabapentin, non‐steroidal anti‐inflammatory drugs (such as ibuprofen), oral contraceptives, corticosteroids (prednisone), and thiazolidinediones (such as pioglitazone).28
Lymphoedema and chronic venous insufficiency can present with lower limb swelling that can either be unilateral or bilateral. Lymphoedema is more commonly presented bilaterally, whereas unilateral involvement could be related to primary lymphoedema, or the inguinal nodes are affected iatrogenically either post‐surgery (lymph node dissection because of various causes) or because of compromising the lymphatic system through recurrent infections (recurrent cellulitis/erysipelas) or in developing countries because of filariasis. Stemmer sign, or the inability to pinch the base of the second toe, is a unique characteristic symptom for lymphoedema.29 In contrast to lipoedema, lymphoedema is not typically associated with easy bruising and tenderness; Table 2 provides the differential diagnosis of lipoedema.30, 31
Table 2.
Differential diagnosis of common entities that cause lower limb swelling
| Characteristics | Lipoedema | Lymphedema | LPD | Obesity | Venous insufficiency | Dercum's disease | Myxedema |
|---|---|---|---|---|---|---|---|
| Pathophysiology | Genetic, primary | Defects in lymph vessels | Fibrinolytic abnormalities | Multifactorial | CVI | Genetic | Thyroid abnormalities |
| Incompetent valves | |||||||
| Primary or secondary | CVI | ||||||
| Other | |||||||
| Disproportion | Yes | No | No | No | Yes | No | |
| Age of onset | Puberty | Any age | Adults mostly elderly | Any age | Adults | PML | Any age |
| Gender | Female | Both genders | Both genders | Both genders | Both genders | Female | More common in female |
| Skin consistency | Firm | Soft | Wooden hard | Firm | Depending on the stage | Firm | Firm |
| Skin colour | Normal | Brown, warty, sclerotic | Early‐red | Normal | Depending on the stage | Normal | Reddish‐orange |
| Sometimes ecchymosis | Late‐brownish | Sometimes ecchymosis | |||||
| Extent of involvement | Bilateral | Unilateral or bilateral most commonly on legs and arms | Shins | Bilateral | Unilateral or bilateral lower limbs | Bilateral legs, arms, trunk | Bilateral shins |
| Mainly legs | Unilateral or bilateral most commonly bilateral | Mainly central body | |||||
| Symmetry | Symmetric | May be asymmetric | May be asymmetric | Symmetric | May be symmetric | Symmetric | Symmetric |
| Clinical clues | “Cuff sign” ankle pad fatty retromalleolar sulcus or lack of Achilles tendon definition | Verruca papillomatosis pebbly stone skin positive stemmer signa | Hard consistency, colour change, other changes of CVI may be present | Central obesity | Other signs of venous disease, stasis dermatitis, varicose veins | Painful lipomas | Reddish‐brown plaques on both shins “peau d'orange look” |
| Involvement of arms and trunk | |||||||
| Involvement of feet | No | Yes | No | No | Yes | Yes | No |
| Response to compression therapy | No | Yes | Yes | No | Yes | Yes | No |
| Common associations | Anxiety/depression | Venous disease | CVI | Metabolic syndrome | CVI | Mood disorder | Thyroid disease |
| DVT | |||||||
| Recurrent cellulitis | Diabetes | Other autoimmune diseases | |||||
| Hypermobility | |||||||
| Easy bruising | Yes | No | No | No | No | Yes | No |
Abbreviations: CVI, chronic venous insufficiency; DVT, deep venous thrombosis; LPD, lipodermatosclerosis; PML, postmenopausal.
A positive Stemmer sign is the inability to pinch the fold of skin at the base of the second toe or finger, indicating the presence of lymphoedema.
DD is an uncommon painful lipodystrophy with autosomal‐dominant inheritance that mainly involves post‐menopausal females. DD can be further classified into nodular, diffuse, and mixed type. Both diffuse and mixed are in included in the differential diagnosis of lipoedema. Although DD and lipoedema share several characteristics, patients with DD have more systemic involvement. In a study by Beltran et al. 94 patients with DD, 120 patients with lipoedema, and 18 with both conditions were studied. Patients with lipoedema had a significantly lower prevalence of type 2 diabetes and a higher prevalence of hypermobility. The location of fat, high average daily pain, presence of lipomas, and comorbid painful disorders help in the differentiation of DD from lipoedema.31
We have established an algorithmic approach to guide clinicians towards the diagnosis of lipoedema. An algorithm for the diagnosis of lipoedema is given in Figure 3.
6. TREATMENT
The goal of therapy is to improve symptoms and prevent secondary complications, particularly to reduce pain and decrease the bulk of fat deposition. Conservative treatment should be employed to control associated oedema, and options include: combined decongestive therapy, manual lymphatic drainage, compression garments or bandages, and mobilisation.32
Compression therapy is a challenge in these patients because of lower leg tenderness. In a study from the United Kingdom, the two main reasons for non‐adherence in these patients included discomfort and difficulty in putting on the compression garments.33 The effectiveness of compression therapy is also minimal, but it is beneficial for the associated oedema in these patients through the reduction in interstitial fluid. The better outcome is associated with the introduction of compression therapy to these patients at the early stage of the disease.34, 35, 36 A good support measure can include manual lymphatic drainage and intermittent pneumatic compression if the pneumatic compression has at least 6 to 12 chambers.34 Diet has very limited effect in lipoedema patients on the surplus weight accumulated in the lower extremities that would eliminate the obvious disproportion between the relatively thin upper half of the body and the large lower extremities. This may contribute to some frustration, reduction in self‐esteem, and mental health consideration.2, 37 The role of diet is much more relevant in the case of combined obesity. Surgical options are used to permanently reduce the amount of subcutaneous fatty tissue from the affected areas. Liposuction can be performed under tumescent local anaesthesia. Presently, liposuction under tumescent local anaesthesia has become an established and low‐risk surgical procedure; in fact, the largest study to date has demonstrated tumescent liposuction to be the most effective option.38, 39 This procedure is associated with a pronounced improvement in pain, tenderness to pressure, oedema ,and easy bruising.39 In a study by Baumgartner et al., 85 patients with lipoedema had bilateral leg liposuction and made comparisons between baseline and the 4‐ and 8‐year follow‐up periods in terms of pain, oedema, bruising, restriction of movement, cosmetic impairment, quality of life, overall impairment, and the necessity of decongestive therapy. The result of the study confirmed the role of liposuction as the main stay of treatment for this challenging condition and preservation of the long‐term effect of the procedure in any stage of the disease. Early recognition of these cases and appropriate intervention will decrease the burden of this disease for patients and health care professionals.40
Liposuction can be performed either manually or laser‐assisted. Due to numerous advantages, liposuction preferably performed under tumescent local anesthesia, using the wet technique with blunt cannulas. Supportive techniques in the form of vibration‐assisted or water jet‐assisted liposuction can be used in this context. In tumescent liposuction, large amounts of a mixture of saline, lidocaine, adrenaline, and sodium bicarbonate are injected in to the tissue, and with a blunt micro cannula, up to 10 to 20 L of liquid fat can be removed during multiple sessions. This leads to significant reductions of subcutaneous fat tissues. However, studies comparing the outcome of each technique do not favour one of the procedures considering the efficacy and safety for the patient, although laser‐assisted liposuction provides better tissue tightening.41, 42
Other available options in the literature include laser surgery and lipectomy (see Table 3 for other therapeutic options). Lipectomy, however, bears the risk of relapse and always produces long scars. Lipectomy should be reserved to the juxtarticular lipoedema of the knee. The procedure is indispensable in advanced cases of fibrotic tissue conversion.49 Liposuction in lipoedema does not aim to remove giant amounts of adipose tissue but improve quality of life and significantly reduce pain. If there is concomitant morbid obesity, this should be treated first.
Table 3.
Various treatments deployed
| Author | Sample study | Study type | Treatment | Length of follow up and recurrence | Outcome |
|---|---|---|---|---|---|
| Dadras et al39 | 25 patients | Longitudinal study questionnaire | Liposuction | 16 and 37 mo | Significant reductions in spontaneous pain, sensitivity to pressure, feeling of tension, bruising, cosmetic impairment, and general impairment to quality of life |
| No recurrence reported | |||||
| Baumgartner et al43 | 85 patients | Single‐Centre study. A mail questionnaire—often in combination with clinical controls | Liposuction | 4 and 8 y | Improvement in spontaneous pain, sensitivity to pressure, oedema, bruising, and restriction of movement persisted |
| No recurrence reported | |||||
| Couto JA et al44 | 1 patient | Retrospective | Liposuction | 8 y | Suction‐assisted tissue removal |
| No recurrence reported | |||||
| Leclère et al45 | 30 patients | Satisfaction questionnaire | Laser‐assisted lipolysis | 3 mo | Homogeneous reduction of fatty tissue with skin tightening and overall satisfaction reported in 29 of 30 patients |
| Wollina et al41 | 24 patients | Open trial comparing liposuction (n = 12) with complex decongestive therapy (n = 12) | Laser‐assisted liposuction | 3 to 60 mo | Short operation time and early mobilisation, reduction of pain and improved mobility |
| No recurrence reported | |||||
| Schmeller et al40 | 164 patients | Monocentric standardised questionnaire | Tumescent liposuction | 1 to 8 y (mean 3 y and 4 mo) | Significant reduction of subcutaneous fatty tissue, improvement of shape, pain, sensitivity to pressure, oedema, bruising, restriction of movement, and cosmetic impairment |
| No recurrence reported (19% reported to need MLD and compression as before) | |||||
| Rapprich et al46 | 25 patients | Case series | Tumescent liposuction | 6 mo | Measurement of the volume of the legs using visual analogue scales (VAS, scale 0‐10). Results: The volume of the leg was reduced by 6.9 %. Pain was the predominant outcome. For symptom, quality of life as a measure of the psychological strain was assessed |
| No recurrence reported | |||||
| Peled et al47 | 1 patient | Case report | Suction‐assisted lipectomy | 4 y | Successful treatment of the lipodystrophy and maintenance of improved aesthetic results at 4‐y postoperative follow up |
| No recurrence reported | |||||
| Szolnoky et al48 | 38 patients | Case‐control (21 vs 17) | Complex decongestive physiotherapy | Not available | Significant reduction of petechiae and capillary fragility in treatment group |
| Szolnoky et al35 | 24 patients | Prospective, randomised trial | Complex decongestive physiotherapy with or without IPC | Not available | No significant reduction in lower extremity volume with IPC |
Abbreviation: IPC, intermittent pneumatic compression; MLD, manual lymphatic drainage.
In conclusion, healthy life style, weight control, oedema reduction, and other supportive therapies are recommended in the management of patients with lipoedema. However, tumescent liposuction is the treatment of choice in case of progression despite consequent conservative therapy.
Conflict of interest
No conflict of interest was declared.
Shavit E, Wollina U, Alavi A. Lipoedema is not lymphoedema: A review of current literature. Int Wound J. 2018;15:921–928. 10.1111/iwj.12949
REFERENCES
- 1. Child AH, Gordon KD, Sharpe P, et al. Lipedema: an inherited condition. Am J Med Genet A. 2010;152A:970‐976. [DOI] [PubMed] [Google Scholar]
- 2. Fife CE, Maus EA, Carter MJ. Lipedema: a frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv Skin Wound Care. 2010;23:81‐92. [DOI] [PubMed] [Google Scholar]
- 3. Okhovat JP, Alavi A. Lipedema: a review of the literature. Int J Low Extrem Wounds. 2015;14(3):262‐267. [DOI] [PubMed] [Google Scholar]
- 4. Fonder MA, Loveless JW, Lazarus GS. Lipedema, a frequently unrecognized problem. J Am Acad Dermatol. 2007;57(2 suppl):S1‐S3. [DOI] [PubMed] [Google Scholar]
- 5. Godoy Mde F, Buzato E, Brigidio PA, Pereira de Godoy JM. Is lymphostasis an aggravant of lipedema? Case Rep Dermatol. 2012;4:222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmeller W, Meier‐Vollrath I. Lipedema and liposuction. In: Weissleder H, Schuchhardt C, eds. Lymphedema. Diagnosis and Therapy. 4th ed. Essen, Germany: Vivavital; 2008:294, 473‐323, 89. [Google Scholar]
- 7. Schmeller W, Meier‐Vollrath I. Tumescent liposuction: a new and successful therapy for lipedema. J Cutan Med Surg. 2006;10:7‐10. [DOI] [PubMed] [Google Scholar]
- 8. Allen EVN, Hines EA. Lipedema of the legs: a syndrome characterized by fat legs and orthostatic edema. Proc Staff Meet Mayo Clin. 1940;15:184‐187. [Google Scholar]
- 9. Chen SG, Hsu SD, Chen TM, Wang HJ. Painful fat syndrome in a male patient. Br J Plas Surg. 2004;57:282‐286. [DOI] [PubMed] [Google Scholar]
- 10. Langendoen SI, Habbema L, Nijsten TE, Neumann HA. Lipoedema: from clinical presentation to therapy. A review of the literature. Br J Dermatol. 2009;161:980‐986. [DOI] [PubMed] [Google Scholar]
- 11. Reich‐Schupke S, Schmeller W, Brauer WJ, et al. S1 guidelines: Lipedema. J Dtsch Dermatol Ges. 2017;15(7):758‐767. [DOI] [PubMed] [Google Scholar]
- 12. Herpertz U. Lipedema. Z Lymphol. 1995;19(1):1‐11. [PubMed] [Google Scholar]
- 13. Lulay G. Lymphologische Akutklinik. Lymphol Forsch Praxis. 2010;14:90‐95. [Google Scholar]
- 14. Marshall M, Schwahn‐Schreiber C. Prävalenz des Lipödems bei berufstätigen Frauen in Deutschland (Lipödem‐3‐Studie). Phlebologie. 2011;40:127‐134. [Google Scholar]
- 15. Suga H, Araki J, Aoi N, Kato H, Higashino T, Yoshimura K. Adipose tissue remodeling in lipedema: adipocyte death and concurrent regeneration. J Cutan Pathol. 2009;36:1293‐1298. [DOI] [PubMed] [Google Scholar]
- 16. Földi M, Földi E, eds. Lehrbuch der Lymphologie: für Mediziner, Masseure und Physiotherapeuten. Stuttgart, Gemany: Urban & Fischer; 2005:374‐378. [Google Scholar]
- 17. de Godoy JM, Barufi S, Godoy Mde F. Lipedema: is aesthetic cellulite an aggravating factor for limb perimeter? J Cutan Aesthet Surg. 2013;6:167‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schneble N, Wetzker R, Wollina U. Lipedema—lack of evidence for the involvement of tyrosine kinases. J Biol Regul Homeost Agents. 2016;30(1):161‐163. [PubMed] [Google Scholar]
- 19. Priglinger E, Wurzer C, Steffenhagen C, et al. The adipose tissue‐derived stromal vascular fraction cells from lipedema patients: are they different? Cytotherapy. 2017. Jul;19(7):849‐860. [DOI] [PubMed] [Google Scholar]
- 20. Bilancini S, Lucchi M, Tucci S, Eleuteri P. Functional lymphatic alterations in patients suffering from lipedema. Angiology. 1995;46:333‐339. [DOI] [PubMed] [Google Scholar]
- 21. Boursier V, Pecking A, Vignes S. Comparative analysis of lymphoscintigraphy between lipedema and lower limb lymphedema. J Mal Vasc. 2004;29:257‐261. [DOI] [PubMed] [Google Scholar]
- 22. Bräutigam P, Földi E, Schaiper I, Krause T, Vanscheidt W, Moser E. Analysis of lymphatic drainage in various forms of leg edema using two compartment lymphoscintigraphy. Lymphology. 1998;31:48‐55. [PubMed] [Google Scholar]
- 23. JJ S, D K. Water jet‐assisted liposuction for patients with lipoedema: histologic and immunohistologic analysis of the aspirates of 30 lipoedema patients. Aesth Plast Surg. 2009;33:153‐162. [DOI] [PubMed] [Google Scholar]
- 24. Schmeller W, Meier‐Vollrath J. Schmerzen beim Lipödem. LympForsch. 2008;12:8‐12. [Google Scholar]
- 25. Schellong SM, Wollina U, Unger L, Machetanz J, Stelzner C. [Leg swelling] (German: Das geschwollene Bein). Internist (Berl). 2013;54(11):1294‐1303. [DOI] [PubMed] [Google Scholar]
- 26. Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part II. Conditions that simulate lower limb cellulitis. J Am Acad Dermatol. 2012;67(2):177. [DOI] [PubMed] [Google Scholar]
- 27. Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves' disease: a 2014 update. J Endocrinol Invest. 2014;37(8):691‐700. [DOI] [PubMed] [Google Scholar]
- 28. Ratchford EV, Evans NS. Approach to lower extremity edema. Curr Treat Options Cardiovasc Med. 2017;19(3):16. [DOI] [PubMed] [Google Scholar]
- 29. Stemmer R. A clinical symptom for the early and differential diagnosis of lymphedema. Vasa. 1976;5:261‐262. [PubMed] [Google Scholar]
- 30. Porter JM, Moneta GL. Reporting standards in venous disease: an update. International consensus committee on chronic venous disease. J Vas Surg. 1995;21:635‐645. [DOI] [PubMed] [Google Scholar]
- 31. Beltran K, Herbst KL. Differentiating lipedema and Dercum's disease. Int J Obes (Lond). 2017;41(2):240‐245. [DOI] [PubMed] [Google Scholar]
- 32. Heinig B, Wollina U. Komplexe Entstauungstherapie. Hautarzt. 2015;66(11):810‐818. [DOI] [PubMed] [Google Scholar]
- 33. Alavi A, Sibbald RG, Phillips TJ, et al. What's new: management of venous leg ulcers: approach to leg ulcers. J Am Acad Dermatol. 2016;74(4):627‐640. [DOI] [PubMed] [Google Scholar]
- 34. Fetzer A. Specialist approaches to managing lipoedema. Br J Community Nurs. 2016;21(Suppl):S30‐S35. [DOI] [PubMed] [Google Scholar]
- 35. Szolnoky G, Borsos B, Barsony K, Balogh M, Kemeny L. Complete decongestive physiotherapy with and without pneumatic compression for treatment of lipedema: a pilot study. Lymphology. 2008;4:40‐44. [PubMed] [Google Scholar]
- 36. Szolnoky G, Varga E, Varga M, Tuczai M, Dósa‐Rácz E, Kemény L. Lymphedema treatment decreases pain intensity in lipedema. Lymphology. 2011;44:178‐182. [PubMed] [Google Scholar]
- 37. Reich‐Schupke S, Altmeyer P, et al. Pilotstudie zur Kompressionsversorgung bei Patienten mit Lipödem. Lymphödem und Lipolymphödem LymphForsch. 2012;16:65‐69. [Google Scholar]
- 38. Hodson S, Eaton S. Lipoedema management: gaps in our knowledge. J Lymphoedema. 2013;8:30‐34. [Google Scholar]
- 39. Dadras M, Mallinger PJ, Corterier CC, Theodosiadi S, Ghods M. Liposuction in the treatment of lipedema: a longitudinal study. Arch Plast Surg. 2017;44(4):324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmeller W, Hueppe M, Meier‐Vollrath I. Tumescent liposuction in lipoedema yields good long‐term results. Br J Dermatol. 2012;166:161‐168. [DOI] [PubMed] [Google Scholar]
- 41. Wollina U, Heinig B. Tumsescent microcannular (laser‐assisted) liposuction in painful lipedema. Eur J Aesthet Med Dermatol. 2012;2(2):56‐69. [Google Scholar]
- 42. Goldman A, Wollina U, de Mundstock EC. Evaluation of tissue tightening by the subdermal Nd: YAG laser‐assisted liposuction versus liposuction alone. J Cutan Aesthet Surg. 2011;4(2):122‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baumgartner A, Hueppe M, Schmeller W. Long‐term benefit of liposuction inpatients with lipoedema: a follow‐up study after an average of 4 and 8 years. Br J Dermatol. 2016;174(5):1061‐1067. [DOI] [PubMed] [Google Scholar]
- 44. Couto JA, Maclellan RA, Greene AK. Management of vascular anomalies and related conditions using suction‐assisted tissue removal. Plast Reconstr Surg. 2015;136(4):511e‐514e. [DOI] [PubMed] [Google Scholar]
- 45. Leclere FM, Moreno‐Moraga J, Mordon S, et al. Laser assisted lipolysis for ankle remodelling: a prospective study in 30 patients. Lasers Med Sci. 2014;29:131‐136. [DOI] [PubMed] [Google Scholar]
- 46. Atiyeh B, Costagliola M, Illouz YG, Dibo S, Zgheib E, Rampillon F. Functional and therapeutic indications of liposuction: personal experience and review of the literature. Ann Plast Surg. 2015;75(2):231‐245. [DOI] [PubMed] [Google Scholar]
- 47. Peled AW, Slavin SA, Brorson H. Long‐term outcome after surgical treatment of lipedema. Ann Plast Surg. 2012;68(3):303‐307. [DOI] [PubMed] [Google Scholar]
- 48. Szolnoky G, Nagy N, Kovacs RK, et al. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology. 2008;41:161‐166. [PubMed] [Google Scholar]
- 49. Wollina U, Heinig B, Schönlebe J, et al. Debulking surgery for elephantiasis nostras with large ectatic podoplanin‐negative lymphatic vessels in patients with lipo‐lymphedema. Eplasty. 2014;14:e11. [PMC free article] [PubMed] [Google Scholar]


