Abstract
While the overwhelming majority of wounds heal rapidly, a significant proportion fail to progress through the wound‐healing process. These resultant chronic wounds cause considerable morbidity and are costly to treat. Wound bed preparation, summarised by the TIME (Tissue, Inflammation/infection, Moisture imbalance, Epithelial edge advancement) concept, is a systematic approach for assessing chronic wounds. Each of these components needs to be addressed and optimised to improve the chances of successful wound closure. We present an up‐to‐date literature review of the most important recent aspects of wound bed preparation. While there are many novel therapies that are available to the treating clinician, often, there are limited data on which to assess their clinical value, and a lack of appreciation for adequate wound bed preparation needed before expensive therapy is used to heal a wound.
Keywords: Chronic wounds, Wound bed preparation, Wound healing
Introduction
The vast majority of wounds progress through the normal process of wound healing (haemostasis, inflammation, proliferation, maturation) uninhibited. However, a significant minority fail to progress through these steps, resulting in a chronic wound with associated morbidity and cost. Wound bed preparation is defined as the management of a wound in order to promote natural healing or to facilitate alternative methods to achieve healing, such as skin grafting, dermal matrices or other skin coverage products. It is of particular value in systematically assessing chronic wounds to promote the chance of healing.
Schultz et al. 1 first published the concept of wound bed preparation in 2003, which is a structured framework for use in the management of wounds. The TIME (Tissue, Inflammation/infection, Moisture imbalance, Epithelial edge advancement) acronym, published the following year 2, describes four aspects of wound bed preparation that need to be systematically addressed in order for wound healing to take place. This acronym has since been widely accepted in clinical practice in both the assessment and management of chronic wounds. The value of timely and meaningful intervention of a chronic wound is being increasingly recognised as the chance of achieving successful wound closure decreases the longer the wound has been present 3.
The TIME concept (Figure 1) consists of:
Tissue
Figure 1.
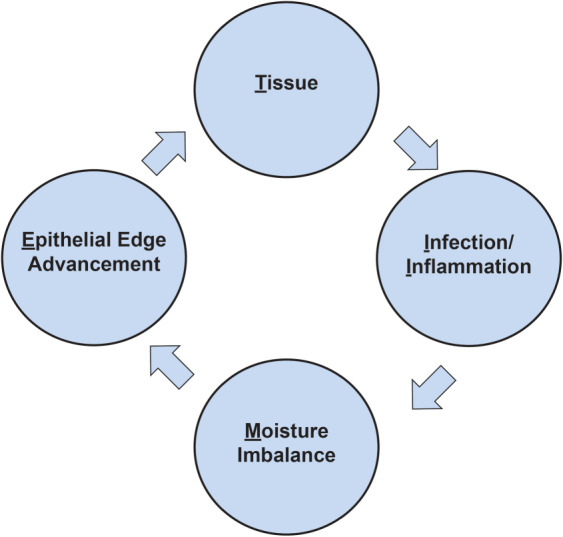
The TIME concept.
This involves assessing for the presence of non‐viable or necrotic tissue; callus, foreign bodies; and exudate, biofilm or slough. Intervention consists of debridement, for which there is a wide range of techniques available; wound cleansing; and negative pressure wound therapy (NPWT).
Infection/inflammation
This involves assessing the aetiology of the wound and treating infection or inflammation unrelated to infection. Intervention includes topical antimicrobials and systemic antibiotics.
Moisture imbalance
This involves the assessment and management of wound fluid/exudate.
Epithelial edge advancement
This involves the assessment and management of non‐advancing or undermining wound edges and the condition of the surrounding skin.
It is imperative that the TIME concept be considered part of a comprehensive approach to each patient. This includes assessment of underlying pathology, patient comorbidities and the health care delivery setting.
Over the last 13 years, numerous novel wound care diagnostics, developments and therapies have been developed. This review paper will provide an up‐to‐date summary of the key research findings relating to wound bed preparation and the TIME approach to chronic wounds.
Tissue
Chronic wounds often result in the build up of necrotic tissues, which require treatment to facilitate healing. The purpose of wound bed debridement is the removal of necrotic tissue, reduction of pressure, inspection of underlying tissue, elimination of dead space harbouring bacteria, drainage of pus and optimisation for topical preparations in an attempt to stimulate healing. Debridement has long been recognised as necessary for the management of chronic wounds 4 and consists of a range of methods, including surgical (or sharp), autolytic, chemical, larval, mechanical, hydrosurgery and ultrasonic methods, or a combination of these (Table 1). Surgical debridement is traditionally perceived to represent the gold standard form of debridement; however, no form of debridement has been proven superior over another, and there are insufficient data from randomised controlled trials (RCTs) in surgical wounds, venous leg ulcers and diabetic foot ulcers on which to base current practice 5, 6, 7. When deciding on the appropriate debridement technique, consideration needs to be given to patient factors, wound appearance, environmental factors and practitioner competence.
Table 1.
Descriptions of debridement techniques
| Debridement method | Description |
|---|---|
| Surgical (or sharp) | An invasive method using either a curette or scalpel, which involves the removal of callus, non‐viable tissue, biofilm, slough and/or foreign bodies as well as debridement of the wound edges and base down to healthy bleeding tissue. Traditionally, surgical debridement is regarded as the gold standard form of debridement; however, it requires a competent practitioner to perform it and appropriate local anaesthesia and carries a risk of bleeding or tissue damage. Caution should be exercised in patients on anticoagulants or who are immunosuppressed 60. |
| Autolytic | A method using moisturisation to allow degradation by phagocytic cells, softening of necrotic tissue and liquefaction of slough. It includes moist dressings such as hydrocolloid and alginate dressings, honey dressings, hydrogels and polyarylates 61, 62, 63. Wounds with high exudate output may not be suitable for this method. |
| Chemical | The use of antiseptics such as silver, povidone‐iodine, chlorhexidine, PHMB or octenidine can achieve debridement 64. Hydrogen peroxide or sodium hydrochlorite have a limited role because of the toxic effects and pain experienced with their use. |
| Larval | Larval therapy is a form of atraumatic selective removal of moist slough using larvae from the green bottle fly (Lucilia sericata or Lucilia cuprina); they can ingest pathogenic organisms but cannot remove callus 65. |
| Mechanical | Traditionally, mechanical debridement used wet to dry gauze that adhered to the top layer of the wound bed on drying, with debridement taking place on removal of the dressing. Debridement or monofilament pads have become popular in clinical use, which comprise a fleece‐like contact layer, which is used to remove debris, slough, exudate and necrotic tissue 66, 67. |
| Hydrosurgery | Hydrosurgery consists of wound lavage through a pressurised hand piece 68, 69 or whirlpool 70. It is relatively painless and has been shown to reduce bioburden 71. |
| Ultrasonic | Low‐frequency, low‐dose ultrasonic‐assisted debridement can be undertaken with either contact 72 or non‐contact 73 devices. Contact devices work by cavitation and acoustic streaming, which directly agitates the wound bed. Non‐contact devices work in conjunction with atomised saline. They are relatively painless, but the equipment can be expensive and not often readily available. |
Wound cleansing is defined as the removal of surface contaminants, bacteria and remnants of previous dressings from the wound surface and its surrounding skin 8. There are various wound‐cleansing solutions in clinical use – potable tap water, sterile water, sterile normal saline and antiseptics solutions such as polyhexanide with betaine (PHMB), povidone‐iodine and octenidine with ethylhexyl glycerine. International consensus recommends that infected chronic wounds require cleansing on each dressing change 9. Results from a single‐blind RCT supported the use of propylbetaine‐polihexanide solution when compared to normal saline to accelerate the healing of vascular leg ulcers and pressure ulcers 10. However, a Cochrane review found that there is no strong evidence that wound cleansing either speeds healing or decreases infection risk 11.
NPWT is a widely used technology that is predominantly utilised as an adjunct therapy to standard wound care. NPWT involves the application of a wound dressing through which a negative pressure is applied. NPWT is thought to work through numerous actions: removing wound exudate and infectious materials, reducing oedema, promoting granulation tissue formation and perfusion, and drawing the wound edges together 12, 13, 14. However, NPWT may be unacceptable to patients (because of pump noise and lack of portability) and can be associated with high costs. Despite the wide use of NPWT, there is currently limited evidence to support its use, and the efficacy and cost‐effectiveness has yet to be established in a range of wounds 15, 16, 17.
Infection/inflammation
Many chronic wounds fail to progress past the ‘Inflammation’ stage of wound healing because of imbalances of inflammatory cells, cytokines, growth factors and/or proteases, such as matrix metalloproteinases (MMPs) 19, 20, 21. Specialised microscopic techniques have shown that 60–90% of chronic wounds have wound biofilm present 22, 23. A biofilm is defined as ‘a structured consortium of microbial cells surrounded by a self‐produced polymer matrix’ 24. In addition to microorganisms, components such as fibrin, platelets or immunoglobulins may be integrated into the biofilm matrix. Biofilms are characterised by persisting and progressive pathology, primarily because of the inflammatory response surrounding the biofilm 25. Identifying the presence of a biofilm can be difficult as it is not always detected with the naked eye. A tissue biopsy may reveal a biofilm, but searching for biofilms in tissue biopsies from clinical samples can be time‐consuming and may result in false negative results 25. Currently, the only definitive method of identifying a biofilm involves advanced microscopy or specialised culture techniques 26. However, certain clinical indicators should raise suspicion to the presence of a biofilm 26:
Antibiotic failure
Infection of >30 days duration
Friable granulation tissue
A gelatinous material easily removed from wound surface that quickly rebuilds
Strategies for treating biofilm include debridement and cleansing to physically disrupt and remove the biofilm and topical antimicrobials to kill planktonic microorganisms and prevent further wound contamination.
Wound infection refers to a spectrum of microbial burden ranging from simple colonisation to systemic infection (Figure 2) 27. The investigation and management required is dependent on the degree of wound infection. As a result of poor biofilm penetration, altered tissue perfusion in the base of chronic wounds and risk of antibiotic resistance, systemic antibiotic treatment is not advocated for localised infection. In most cases of local infection wound cleansing, debridement and topical antimicrobials will treat the bioburden sufficiently (Table 2). There is little evidence to suggest that one antimicrobial is superior to another; however, some may be more acceptable to patients 28, 29, 30, 31. If there are signs of systemic infection, spreading cellulitis or lymphangitis, then these treatments should be combined with oral or intravenous antibiotic therapy. Antibiotic therapy should be prescribed according to local microbiology guidelines and should be based on any available sensitivities from wound cultures. It is important for the assessing clinician to exercise caution in immunosuppressed or comorbid patients as they may not exhibit classic signs or symptoms of local or systemic infection.
Figure 2.
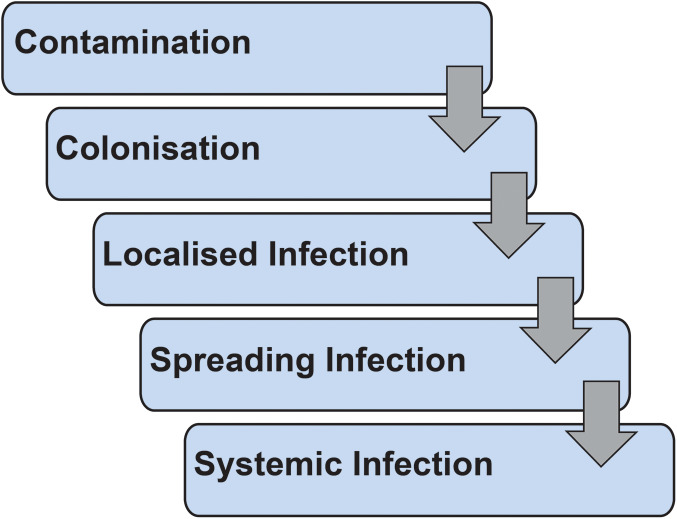
Wound infection spectrum.
Table 2.
Topical antimicrobials in clinical use for chronic wounds
| Topical antimicrobial | Delivery |
|---|---|
| Potassium permanganate | Soaks |
| Acetic acid | Soaks |
| Polyhexamethylene biguanide (PHMB) | Wound cleansing, gel or dressing |
| Chlorhexidine | Wound cleansing |
| Iodine (povidone‐iodine or cadexomer iodine) | Dressing or ointment |
| Octenidine | Wound cleansing |
| Medicinal grade honey | Dressing |
| Silver | Dressing or ointment |
| Dialkylcarbamoyl chloride (DACC) | Dressing |
Moisture imbalance
Exudate is an essential component of wound healing, necessary in activating the complement system (a sequence of proteins in serum and extracellular fluid that destroys pathogens) and aiding autolytic debridement 32. However, in chronic wounds with either excessive or insufficient exudate production, wound‐healing processes may be inhibited. Excessive levels of exudate can cause damage to the surrounding skin (maceration) and is also thought to promote biofilm formation as a potential nutrient source 33, whereas low levels of exudate promotes eschar formation and inhibits cellular activities. However, it is not just the volume of exudate that is important as there is evidence that chronic wound fluid composition is as important as exudate amount. In comparison to acute wound fluid, chronic wound fluid has been shown to inhibit the growth of fibroblasts (required for the deposition and organisation of collagen) 34 and has increased levels of pro‐inflammatory cytokines, free oxygen radicals and proteases (prolonging the inflammatory stage of wound healing) 35.
Dressing choice is important in managing exudate levels and should provide appropriate moisture balance, avoid maceration of the skin edges, prevent leakage and be easy to apply and remove. Protease‐modulating dressings may be appropriate to control wound proteases found in highly exuding wounds, which subsequently denature growth factors and the extracellular matrix 36. The development of these dressings has focused on reducing levels of MMPs by absorbing wound exudate and holding proteases within the dressing structure and inactivating the excess MMPs 20. There is evidence that collagen/oxidised protease‐modulating dressings may increase healing rates in diabetic foot ulcers 37. Dressing with super‐absorbent properties and skin barrier creams may be necessary to avoid peri‐wound maceration. NPWT has also been advocated for exudate control because of the action of physically removing fluid from the wound bed, as discussed earlier in this paper.
There may be other factors to consider in a patient with high levels of exudate, including medical comorbidities such as congestive cardiac failure, hepatic failure, renal failure and malnourishment. Where these medical comorbidities are suspected, referral should be made to an appropriate practitioner. Failure of the lymphatic system or underlying venous disease may also be a contributory factor, and treatment should be aimed at the removal of the oedema through compression therapy 38. Compression therapy should always be performed by a competent practitioner following a satisfactory vascular examination. For patients with lymphoedema, referral to a lymphoedema team for specialist compression therapies may be useful.
Epithelial edge advancement
Wound edge assessment can indicate the progress of wound contraction and epithelialisation and confirm if current wound treatment is effective. A 20–40% reduction in wound area after 2 and 4 weeks of treatment has been shown to be a reliable predictor of healing 39. It is also important to assess the condition of the surrounding skin as dry or macerated edges can hinder healing. Consideration should be given for corrective therapies, such as debridement, skin grafting, acellular dermal matrices and adjunctive therapies, to achieve advancement of epidermal margins. There have been recent developments in edge advancement therapies, which will be discussed below.
Acellular dermal matrices are tissue‐engineered products advocated for wound healing that are devoid of living cells and biologically inert. They can be derived from a range of products, including animal or human tissue, synthetic or a composite product. Their mode of action is by either replicating the extracellular matrix or by acting as a temporary skin substitute. Recent systematic reviews have concluded that while data are limited, there is some evidence to support their use in chronic wounds of the extremities 40, 41.
Epidermal cell harvesting has been advocated as a novel therapy as a substitute for skin grafting, which may be better tolerated in comorbid or elderly patients as it potentially has less morbidity 42, 43. However, to date, there is limited evidence to support its use.
Electromagnetic therapy provides a continuous or pulsed electromagnetic field, which is thought to induce cell proliferation; however, there is currently a lack of evidence to support its benefit in venous leg ulcers or pressure ulcers 44, 45.
Low‐level gas laser therapy (helium neon or gallium arsenide) has been used to increase cellular proliferation and migration. There is limited evidence to support its use currently 46.
Phototherapy is a relatively new, non‐invasive and pain‐free treatment that has received clearance from the United States Food and Drug Administration for its beneficial effects on tissue healing and has been proposed as a therapy for wound healing. However, there is no evidence to support its benefit and safety 47.
Ultrasonic therapy delivers mechanical energy, hypothesised to stimulate cellular activity within the wound bed. There is limited evidence to support its use in venous leg ulcers; however, the authors concluded that further larger‐scale trials are required 48. There was no evidence of benefit when used on pressure ulcers 49.
Hyperbaric oxygen therapy (HBOT) is short‐term, high‐dose oxygen inhalation and diffusion, achieved by breathing concentrated oxygen at a pressure higher than at sea level in hyperbaric chambers 50. It has been suggested in the management of chronic wounds in order to increase the supply of oxygen to the wound. However, HBOT has limited availability in many countries, requires frequent visits to the facility and often can not be tolerated in certain patient groups, such as the elderly. Two recent systematic reviews have concluded that it was not possible to establish the benefits of the treatment for diabetic foot ulcers, including the cost benefit 51, 52.
Topical oxygen has been hypothesised to help improve angiogenesis, reduce infection rates and increase wound‐healing rates 53. An ongoing RCT is assessing its effect on healing rates for chronic diabetic foot ulcers 54.
Growth factors are secreted by regulatory proteins, which effect cell survival, proliferation and differentiation. Recombinant human platelet‐derived growth factor (Becaplermin) is the only growth factor product licensed for use in wound healing to date. Evidence from three RCTs in diabetic foot ulcers has confirmed that it is safe to use, superior to a placebo gel but inferior to an acellular dermal matrix 55, 56, 57.
Stem cells have been theorised to help promote wound healing by migrating across the wound bed and secreting chemokines and growth factors to induce angiogenesis and extracellular matrix remodelling 58. However, further work is required to determine their use in human subjects.
Autologous platelet‐rich plasma gel consists of cytokines, growth factors and a fibrin scaffold derived from the patient's own blood. A recent systematic review showed some increase in the rate of wound healing compared to a placebo gel or standard care; however, the authors noted that the RCTs included were of low quality 59.
NPWT has been advocated for wound edge advancement and has been described earlier in this paper.
Conclusion
Wound bed preparation is a widely utilised tool for assessing and treating chronic wounds. Its value lies in providing the treating clinician with a systematic approach to chronic wounds, which can ensure that logical treatments are given, and their responses are noted and acted upon. While there are many novel therapies that have become available over the past 13 years, to date, only a few have a significant evidence base on which practice can be based. Until such data emerges, it is likely that the vast majority of wounds are best managed with simple therapies combined with regular debridement. Well‐conducted RCTs are required for both novel products and how to objectively measure adequacy/completeness of wound bed preparation.
Acknowledgements
Dr. Harding served as a consultant to KCI, an ACELITY Company, and presented as a faculty member at an ACELITY symposium, parallel to the 2016 World Union of Wound Healing Societies (WUWHS) conference. No other author has any conflicts of interests to declare. This article is part of an ACELITY‐funded supplement based on the 2016 WUWHS ACELITY symposium presentations. ACELITY provided editorial assistance.
References
- 1. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1–28. [DOI] [PubMed] [Google Scholar]
- 2. Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of TIME. Int Wound J 2004;1:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosanquet DC, Harding KG. Wound duration and healing rates: cause or effect? Wound Repair Regen 2014;22:143–50. [DOI] [PubMed] [Google Scholar]
- 4. European Wound Management Association (EWMA) . Position document: wound bed preparation in practice. XXX: European Wound Management Association, 2004. [Google Scholar]
- 5. Gethin G, Cowman S, Kolbach DN. Debridement for venous leg ulcers. Cochrane Database Syst Rev 2015;9:CD008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev 2010;1:CD003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith F, Dryburgh N, Donaldson J, Mitchell M. Debridement for surgical wounds. Cochrane Database Syst Rev 2013;9:CD006214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodeheaver GT. Wound cleansing, wound irrigation, wound disinfection. In: Krasner D, Kane D, editors. Chronic wound care: a clinical source book for healthcare professionals. Wayne, PA: Health Management Publications, Inc,, 1997:97–108. [Google Scholar]
- 9. World Union of Wound Healing Societies . Principles of best practice: wound infection in clinical practice. An international consensus. London: MEP Ltd, 2008. [Google Scholar]
- 10. Bellingeri A, Falciani F, Traspedini P, Moscatelli A, Russo A, Tino G, Chiari P, Peghetti A. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single‐blind RCT. J Wound Care 2016;25:162–6; 168. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev 2012;2:CD003861. [DOI] [PubMed] [Google Scholar]
- 12. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 14. Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dumville JC, Land L, Evans D, Peinemann F. Negative pressure wound therapy for treating leg ulcers. Cochrane Database Syst Rev 2015;7:CD011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumville JC, Webster J, Evans D, Land L. Negative pressure wound therapy for treating pressure ulcers. Cochrane Database Syst Rev 2015;5:CD011334. [DOI] [PubMed] [Google Scholar]
- 17. Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, Peinemann F. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2013;10:CD010318. [DOI] [PubMed] [Google Scholar]
- 18. Hodde JP, Johnson CE. Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol 2007;8:61–6. [DOI] [PubMed] [Google Scholar]
- 19. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence‐‐implications for treatment. Int Wound J 2005;2:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibson D, Cullen B, Legerstee R, Harding KG, Schultz G. MMPs made easy. Wounds Int 2010;1:1–6. [Google Scholar]
- 21. Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol 1998;8:437–41. [DOI] [PubMed] [Google Scholar]
- 22. Thomson CH. Biofilms: do they affect wound healing? Int Wound J 2011;8:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Attinger C, Wolcott R. Clinically addressing biofilm in chronic wounds. Adv Wound Care 2012;1:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall‐Stoodley L, Stoodley P, Kathju S, Hoiby N, Moser C, Costerton JW, Moter A, Bjarnsholt T. Towards diagnostic guidelines for biofilm‐associated infections. FEMS Immunol Med Microbiol 2012;65:127–45. [DOI] [PubMed] [Google Scholar]
- 25. Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall‐Stoodley L, Hola V, Imbert C, Kirketerp‐Moller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015;21:S1–25. [DOI] [PubMed] [Google Scholar]
- 26. Keast D, Swanson T, Carville K, Fletcher J, Schultz G, Black J. Ten top tips. Understanding and managing wound biofilm. Wounds Int 2014;5:22–4. [Google Scholar]
- 27. Fletcher J, Harding K, Richards A. Treatment strategies for wound infection. In: Edwards‐Jones V, editor. Essential microbiology for wound care. Oxford: Oxford University Press, 2016:149–63. [Google Scholar]
- 28. Norman G, Dumville JC, Mohapatra DP, Owens GL, Crosbie EJ. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst Rev 2016;3:CD011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst Rev 2006;1:CD005082. [DOI] [PubMed] [Google Scholar]
- 30. Vermeulen H, van Hattem JM, Storm‐Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007;1:CD005486. [DOI] [PubMed] [Google Scholar]
- 31. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LG, Martyn‐St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014;1:CD003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones V, Harding K, Stechmille J, Schultz G. Acute and chronic wound healing. In: Baranoski S, Ayello EA, editors. Wound care essentials practice principles. Philadelphia, PA: Lippincott, Williams and Wilkins, 2007:64–76. [Google Scholar]
- 33. Hurlow J, Bowler PG. Potential implications of biofilm in chronic wounds: a case series. J Wound Care 2012;21:109–10, 112, 114, 116, 118. [DOI] [PubMed] [Google Scholar]
- 34. Phillips TJ, al‐Amoudi HO, Leverkus M, Park HY. Effect of chronic wound fluid on fibroblasts. J Wound Care 1998;7:527–32. [DOI] [PubMed] [Google Scholar]
- 35. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25. [DOI] [PubMed] [Google Scholar]
- 36. Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc 2002;92:12–8. [DOI] [PubMed] [Google Scholar]
- 37. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–7. [DOI] [PubMed] [Google Scholar]
- 38. European Wound Management Association . Position document: understanding compression therapy. London: Medical Education Partnership LTD., 2003. [Google Scholar]
- 39. Dowsett C. Exudate management: a patient‐centred approach. J Wound Care 2008;17:249–52. [DOI] [PubMed] [Google Scholar]
- 40. Iorio ML, Shuck J, Attinger CE. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast Reconstr Surg 2012;130:232S–41. [DOI] [PubMed] [Google Scholar]
- 41. Reyzelman AM, Bazarov I. Human acellular dermal wound matrix for treatment of DFU: literature review and analysis. J Wound Care 2015;24:128–34. [DOI] [PubMed] [Google Scholar]
- 42. Richmond NA, Lamel SA, Braun LR, Vivas AC, Serena T, Kirsner RS. Epidermal grafting using a novel suction blister‐harvesting system for the treatment of pyoderma gangrenosum. JAMA Dermatol 2014;150:999–1000. [DOI] [PubMed] [Google Scholar]
- 43. Kirsner RS, Bernstein B, Bhatia A, Lantis J, Le L, Lincoln K, Liu P, Rodgers L, Shaw M, Young D. Clinical experience and best practices using epidermal skin grafts on wounds. Wounds 2015;27:282–92. [PubMed] [Google Scholar]
- 44. Aziz Z, Bell‐Syer SE. Electromagnetic therapy for treating pressure ulcers. Cochrane Database Syst Rev 2015;9:CD002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aziz Z, Cullum N. Electromagnetic therapy for treating venous leg ulcers. Cochrane Database Syst Rev 2015;7:CD002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flemming K, Cullum N. Laser therapy for venous leg ulcers. Cochrane Database Syst Rev 2000;2:CD001182. [DOI] [PubMed] [Google Scholar]
- 47. Chen C, Hou WH, Chan ES, Yeh ML, Lo HL. Phototherapy for treating pressure ulcers. Cochrane Database Syst Rev 2014;7:CD009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cullum NA, Al‐Kurdi D, Bell‐Syer SE. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst Rev 2010;6:CD001180. [DOI] [PubMed] [Google Scholar]
- 49. Baba‐Akbari Sari A, Flemming K, Cullum NA, Wollina U. Therapeutic ultrasound for pressure ulcers. Cochrane Database Syst Rev 2006;3:CD001275. [DOI] [PubMed] [Google Scholar]
- 50. Londahl M. Hyperbaric oxygen therapy as adjunctive treatment of diabetic foot ulcers. Med Clin North Am 2013;97:957–80. [DOI] [PubMed] [Google Scholar]
- 51. O'Reilly D, Pasricha A, Campbell K, Burke N, Assasi N, Bowen JM, Tarride JE, Goeree R. Hyperbaric oxygen therapy for diabetic ulcers: systematic review and meta‐analysis. Int J Technol Assess Health Care 2013;29:269–81. [DOI] [PubMed] [Google Scholar]
- 52. Game FL, Hinchliffe RJ, Apelqvist J, Armstrong DG, Bakker K, Hartemann A, Londahl M, Price PE, Jeffcoate WJ. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2012;28:119–41. [DOI] [PubMed] [Google Scholar]
- 53. Chambers AC, Leaper DJ. Role of oxygen in wound healing: a review of evidence. J Wound Care 2011;20:160–4. [DOI] [PubMed] [Google Scholar]
- 54. Health Research Authority . Topical oxygen and diabetic foot ulcers 2 (TODFU2). London: Health Research Authority, 2015. URL http://www.hra.nhs.uk/news/research-summaries/topical-oxygen-and-diabetic-foot-ulcers-2-todfu2/ [accessed on 25 May 2016]. [Google Scholar]
- 55. Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16‐week pilot study. Int Wound J 2006;3:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Landsman A, Agnew P, Parish L, Joseph R, Galiano RD. Diabetic foot ulcers treated with becaplermin and TheraGauze, a moisture‐controlling smart dressing: a randomized, multicenter, prospective analysis. J Am Podiatr Med Assoc 2010;100:155–60. [DOI] [PubMed] [Google Scholar]
- 57. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 58. Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract 2012;96:1–9. [DOI] [PubMed] [Google Scholar]
- 59. Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta‐analysis. Eplasty 2011;11:e38. [PMC free article] [PubMed] [Google Scholar]
- 60. Sieggreen MY, Maklebust J. Debridement: choices and challenges. Adv Wound Care 1997;10:32–7. [PubMed] [Google Scholar]
- 61. Cutting KF. Honey and contemporary wound care: an overview. Ostomy Wound Manage 2007;53:49–54. [PubMed] [Google Scholar]
- 62. Kennedy K, Tritch D. Debridement. In: Krasner D, Kane D, editors. Chronic wound care: a clinical source book for healthcare professionals. Wayne, PA: Health Management Publications, Inc,, 1997:227–35. [Google Scholar]
- 63. Vermeulen H, Ubbink DT, de Zwart F, Goossens A, de Vos R. Preferences of patients, doctors, and nurses regarding wound dressing characteristics: a conjoint analysis. Wound Repair Regen 2007;15:302–7. [DOI] [PubMed] [Google Scholar]
- 64. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999;12:147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Effective debridement in a changing NHS: a UK consensus. London: Wounds UK, 2013. [Google Scholar]
- 66. Gray D, Acton C, Chadwick P, Fumarola S, Leaper D, Morris C, Stang D, Vowden K, Vowden P, Young T. Consensus guidance for the use of debridement techniques in the UK. Wounds UK 2011;7:77–84. [Google Scholar]
- 67. Bahr S, Mustafi N, Hattig P, Piatkowski A, Mosti G, Reimann K, Abel M, Dini V, Restelli J, Babadagi‐Hardt Z, Abbritti F, Eberlein T, Wild T, Bandl K. Clinical efficacy of a new monofilament fibre‐containing wound debridement product. J Wound Care 2011;20:242–8. [DOI] [PubMed] [Google Scholar]
- 68. Granick MS, Posnett J, Jacoby M, Noruthun S, Ganchi PA, Datiashvili RO. Efficacy and cost‐effectiveness of a high‐powered parallel waterjet for wound debridement. Wound Repair Regen 2006;14:394–7. [DOI] [PubMed] [Google Scholar]
- 69. Caputo WJ, Beggs DJ, DeFede JL, Simm L, Dharma H. A prospective randomised controlled clinical trial comparing hydrosurgery debridement with conventional surgical debridement in lower extremity ulcers. Int Wound J 2008;5:288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tao H, Butler JP, Luttrell T. The role of whirlpool in wound care. J Am Coll Clin Wound Spec 2012;4:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Allan N, Olson M, Nagel D, Martin R. 050: the impact of VERSAJET hydrosurgical debridement on wounds containing bacterial biofilms. Wound Repair Regen 2010;18:A88. [Google Scholar]
- 72. Wendelken ME, Markowitz L, Alvarez OM. A closer look at ultrasonic debridement. Podiatry Today 2010;23:42–8. [Google Scholar]
- 73. Ennis WJ, Valdes W, Gainer M, Meneses P. Evaluation of clinical effectiveness of MIST ultrasound therapy for the healing of chronic wounds. Adv Skin Wound Care 2006;19:437–46. [DOI] [PubMed] [Google Scholar]


