Abstract
Venous thromboembolism (VTE) is a common complication after surgical treatment of fractures, which is associated with significant morbidity and mortality. Identifying the risk factors for VTE is important for preventive strategies to reduce the incidence of VTE. Therefore, we conducted a meta‐analysis to evaluate the incidence of VTE and the risk factors influencing the development of VTE in patients who underwent surgery for fractures below the hip. PubMed, Embase, Web of Science, SinoMed (Chinese BioMedical Literature Service System, China) and CNKI (National Knowledge Infrastructure, China) databases were systematically searched to identify cohort or case–control studies that investigated the incidence and risk factors for VTE following surgical treatment of fractures below the hip. VTE risk ratios (RRs) were pooled by use of a fixed‐effect model or a random‐effect model, depending on the heterogeneity among the included studies. Heterogeneity between the studies was assessed by I2 statistics. Twenty‐three studies with a total of 191 294 patients who met the inclusion criteria were included in this meta‐analysis. Our results demonstrated that age (≥60 years) (RR = 1·85, 95% confidence interval (CI): 1·34, 2·55; P = 0·000), previous VTE(RR = 5·25, 95% CI: 2·77, 9·96; P = 0·000), heart failure (RR = 1·74, 95% CI: 1·34, 2·27; P = 0·000), current smoking status (RR = 1·23, 95% CI: 1·07, 1·41; P = 0·004), hypertension (RR = 1·62, 95% CI: 1·27, 2·06; P = 0·000), hyperlipidaemia (RR = 2·16, 95% CI: 1·79, 2·62; P = 0·000), diabetes mellitus (RR = 1·46, 95% CI: 1·27, 1·68; P = 0·000), obesity (RR = 1·58, 95% CI: 1·35,·1·85; P = 0·000), multiple fractures (RR = 2·14, 95% CI: 1·00, 4·60; P = 0·050), varicose veins (RR = 3·07, 95% CI: 1·12, 8·47; P = 0·030), prolonged operation time (weighted mean differences (WMD) = 1·22, 95% CI: 0·63, 1·81; P = 0·000) and prolonged bed rest time (WMD = 3·12, 95% CI: 2·96, 3·29; P = 0·000) were associated with an increased risk of developing VTE. The other variables, including age (<60 years), previous smoking, immobility, pregnancy, cancer, open fractures and combination with trauma were not identified as significant risk factors for VTE. Almost all the risk factors mentioned above are in line with the known risk factors for VTE following surgery for fractures below the hip. Thus, surgeons should pay close attention to patients with these medical conditions in order to reduce the incidence of VTE following surgical treatment of fractures below the hip.
Keywords: Fractures, Low extremity to the hip, Meta‐analysis, Risk factors, Venous thromboembolism
Introduction
Venous thromboembolism (VTE), which encompasses asymptomatic and symptomatic deep vein thrombosis (DVT) and pulmonary thromboembolism (PE), is a potentially serious complication of operatively treated fractures in patients undergoing major orthopaedic surgery 1. It has been reported that the incidence of VTE in these patients ranges from 8% to 70% for DVT and 1% to 10% for PE 2, 3, 4. Moreover, compared with patients without VTE, VTE patients incur ten times the health care costs and more than twice the length of hospital stay 5. The American College of Chest Physicians (ACCP) guidelines recommend that efficient strategies should be implemented in medical and surgical patients to identify the risk factors for VTE and then prevent the occurrence of VTE‐related morbidity and mortality 6.
It is a crucial and challenging task to identify risk factors for VTE because there are a large number of potential VTE risk factors that are worthy of attention. Several researches focusing on patient demographic factors, such as increased body mass index and previous history of VTE, have found consistent association between these factors and increased VTE 7, 8, 9. However, for other risk factors, including cancer, age and sex, there are controversial results among researches 10, 11, 12. These might be attributed to the several design issues including small sample size 7, 9, selected patient populations 7, patients recruited from a single institution 10 and insufficient control of confounding factors. Identifying risk factors for VTE is very important for clinical workers to apply efficient strategies to prevent the occurrence of VTE, improve survival and decrease health care costs.
To extend the knowledge of VTE risk, we conducted a meta‐analysis based on eligible studies to identify the risk factors for VTE in patients who underwent surgery for fractures below the hip. Based on these identified risk factors, we could provide more information and improved guidance for clinical workers to manage this subgroup of patients and reduce the morbidity, mortality and health care costs.
Materials and methods
Search strategy and review strategy
Two researchers independently conducted a comprehensive literature review. Multiple databases, including PubMed, Embase, Web of Science, SinoMed (Chinese BioMedical Literature Service System, China) and CNKI (National Knowledge Infrastructure, China), were searched for data from 6 May 2015 to 15 July 2015. The structured search strategies used were listed as follows: (‘fractures, bone’ [MeSH Terms]) OR (‘fractures’ [All Fields] AND ‘bone’ [All Fields]) OR (‘bone fractures’ [All Fields]) OR (‘bone’ [All Fields] AND ‘fracture’ [All Fields]) OR (‘bone fracture’ [All Fields] AND ‘veins’ [MeSH Terms]) OR (‘veins’ [All Fields]) OR (‘venous’ [All Fields] AND ‘thrombosis’ [MeSH Terms]) OR (‘thrombosis’ [All Fields]) OR (‘thrombus’ [All Fields] AND ‘risk factors’ [MeSH Terms]) OR (‘risk’ [All Fields] AND ‘factors’ [All Fields]) OR (‘risk factors’ [All Fields]). The search was limited to human subjects and no language restriction was imposed. Moreover, we also manually searched the reference lists of the included studies and reviews to identify potential eligible studies until no additional articles could be found.
Endnote (version X, Thomson Reuters, Inc., Philadelphia, PA) bibliographic software was used to establish an electronic library of citations identified in the database searches. Two reviewers independently performed the title/abstract screening and then the full‐text screening. Disagreements between the two reviewers were resolved by consulting with a third reviewer.
Study inclusion and exclusion criteria
All studies investigating the incidence and risk factors for VTE in a series of patients with fractures below the hip were considered eligible for analysis. To be included, publications had to meet the following inclusion criteria: (i) the study population was composed of adult patients who underwent an operative procedure for a fracture below the hip; (ii) the study was performed with a case–control or cohort design and (iii) results reported data on the incidence and risk factors for VTE. Reports were excluded from the final analysis if the participants were younger than 18 years or underwent non‐operative treatment. Reviews, editorials, comments and letters were excluded from this analysis.
Data extraction and quality assessment
We estimated a data extraction sheet based on the Cochrane Consumers and Communication Review Group data extraction template. Two independent investigators extracted the following information: author, age, patients in each group, sex, number of DVT/PE, sociodemographic risk factors for VTE after the surgical treatment of fractures and study design. For the studies without directly available data in the paper, we contacted the corresponding author for this information.
The modified Newcastle–Ottawa Scale (NOS) was used to evaluate the methodological quality of the studies included in this meta‐analysis 13. The scale consists of three items: patient selection, comparability of intervention/control group and outcome assessment 13. The total score of NOS is 9 points, and articles with a total score of more than 5 points are considered as high quality 13.
Statistical analysis
We investigated the incidence and risk factors for VTE following surgical treatment of fractures based on the data from the studies included in this meta‐analysis. For dichotomous variables (i.e., incidence and risk factors for VTE), the number of case events and control number of patients were extracted from the included studies. Afterwards, the risk ratio (RR) with 95% confidence intervals (95% CIs) was calculated. For continuous outcomes (i.e., duration of operation and bed rest), the number of total patients, mean time and standard error were extracted, and they were expressed as weighted mean differences (WMD) with 95% CIs.
Pooled estimates of RR or WMD were calculated using a fixed‐effects model (Mantel‐Haenszel method) 14.When substantial heterogeneity existed, a random‐effects model (DerSimonian‐Laird method) 15 was used to pool the data.
I 2 statistics, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance, was used to test for heterogeneity. Studies with an I 2 value of <25%, ∼50%, ∼75% and ∼100% were considered to have no, low, moderate and high heterogeneity, respectively 16.
The presence of publication bias was evaluated using the Begg's and Egger's tests 17, 18. A P value <0·05 was judged as statistically significant. All statistical analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX).
Results
Identification of eligible studies
The initial search yielded a total of 2361 articles. After screening title/abstract and full‐text information, 728 were excluded because of duplicated publications, and 1610 were excluded because the data they provided was not available or unrelated to our topic. Finally, 23 articles that met the inclusion criteria were included in this meta‐analysis 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41. The flow chart of the search strategy is shown in Figure 1.
Figure 1.
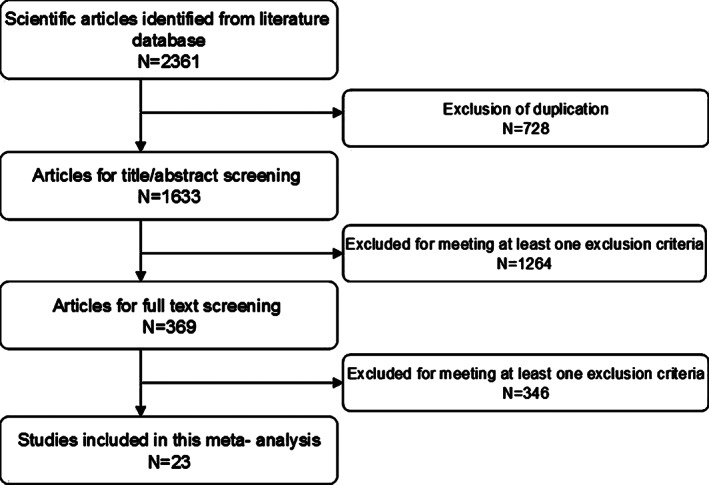
Eligibility of studies for inclusion in meta‐analysis.
Characteristics of eligible studies
The 23 studies provided a total of 191 294 patients, 3459 of whom developed VTE after surgical treatment. Of the 23 studies, 15 were carried out in China, two in Denmark, one in USA, one in Korea and one in Turkey. The mean age at the time of surgery was 47·0 years (range 18–96 years), and 103 681(54·2%) patients were male. Of the 191 294 patients, 1046 (0·5%) were treated prior to the initiation of chemoprophylaxis. The modified NOS scale for these studies ranged from 7 to 8 (median scale 8), indicating high quality. The baseline characteristics of the included studies are presented in Table 1.
Table 1.
Characteristics of included studies to assess risk factors for surgical site infection following spinal surgery
| Study | Year | Country | Study design | Sample size | Case | control | Risk factors | NOS scale |
|---|---|---|---|---|---|---|---|---|
| Wahlsten et al. 19 | 2015 | Denmark | Cohort | 57619 | 594 | 57025 | Heart failure/previous DVT/previous PE/cancer/hypertension/diabetes/obesity/ current smoking | 8 |
| Akpinar et al. 20 | 2013 | Turkey | Case–control | 1306 | 55 | 1251 | Age/obesity/immobility/cancer/previous VTE/heart failure | 8 |
| Park et al. 21 | 2015 | Korea | Cohort | 901 | 38 | 863 | Age/lung disease/neurologic disease/cardiovascular disease/open fractures/isolated fractures/multiple fractures | 7 |
| SooHoo et al. 22 | 2013 | USA | Case–control | 57183 | 29 | 57154 | Age/lateral fractures/open fractures/diabetes | 8 |
| Pedersen et al. 23 | 2010 | Denmark | Case–control | 67469 | 1390 | 66079 | Age/diabetes/cancer/previous VTE/cardiovascular disease | 8 |
| Sen et al. 24 | 2011 | India | Case–control | 56 | 24 | 32 | Previous VTE/proximal thrombi/immobility | 7 |
| Riou et al. 25 | 2007 | Case–control | 2761 | 178 | 2582 | Age/previous VTE/diabetes/current smoking/severe injury/moderate injury/immobilisation | 8 | |
| Xu and Xu 26 | 2008 | China | Case–control | 136 | 24 | 112 | Age/previous VTE/diabetes/cardiovascular disease | 7 |
| Mantilla et al. 27 | 2003 | USA | Case–control | 232 | 116 | 116 | Previous VTE/cardiovascular disease/diabetes/current smoking | 7 |
| Wang 28 | 2013 | China | Case–control | 103 | 52 | 51 | Diabetes/hypertension/hyperlipidaemia/isolated fractures/multiple fractures | 7 |
| Long 29 | 2013 | China | Case–control | 145 | 73 | 72 | Isolated fractures/multiple fractures/hypertension/hyperlipidaemia/diabetes | 8 |
| Li and Xu 30 | 2013 | China | Case–control | 100 | 58 | 42 | Hyerlipidaemia/hypertension/diabetes/current smoking | 7 |
| Guo 31 | 2013 | China | Case–control | 180 | 98 | 82 | Hyerlipidaemia/hypertension/diabetes/current smoking | 8 |
| Huang 32 | 2014 | China | Case–control | 160 | 80 | 80 | Hyerlipidaemia/hypertension/diabetes/isolated fractures/multiple fractures/age | 8 |
| Yao 33 | 2012 | China | Case–control | 459 | 64 | 395 | Hyerlipidaemia/hypertension/diabetes | 8 |
| Yang et al. 34 | 2010 | China | Case–control | 515 | 58 | 457 | Current smoking/obesity/ | 7 |
| Wang et al. 35 | 2015 | China | Case–control | 336 | 58 | 278 | Age/hypertension/open fractures/multiple fractures | 8 |
| Hou et al. 36 | 2014 | China | Case–control | 329 | 98 | 231 | Hypertension/diabetes/failure disease/cancer/ pregnancy/hyperlipidaemia/varicose veins | 8 |
| Zhang et al. 37 | 2012 | China | Case–control | 319 | 161 | 158 | Age/previous VTE/cancer/pregnancy | 7 |
| Zhu et al. 38 | 2012 | China | Case–control | 448 | 79 | 369 | Age/current smoking/combination with trauma | 8 |
| Gu et al. 39 | 2007 | China | Case–control | 102 | 18 | 84 | Age/obesity/current smoking/alcohol consumption/varicose veins/diabetes | 8 |
| Li et al. 40 | 2014 | China | Case–control | 287 | 37 | 250 | Age/current smoking/combination with trauma | 8 |
| Yi 41 | 2011 | China | Case–control | 148 | 77 | 71 | Failure disease/diabetes/hypertension/ hyperlipidaemia/cancer | 7 |
DVT, deep venous thrombosis; NOS, Newcastle–Ottawa Scale; PE, pulmonary embolism; VTE, venous thromboembolism.
Incidence of VTE
All studies reported data on the incidence of VTE 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41. The pooled estimates, using a random‐effect model, indicated that the incidence of VTE in patients who underwent surgery for fractures below the hip was 21% (95%CI: 17%, 24%; P = 0·000) (Figure 2).
Figure 2.
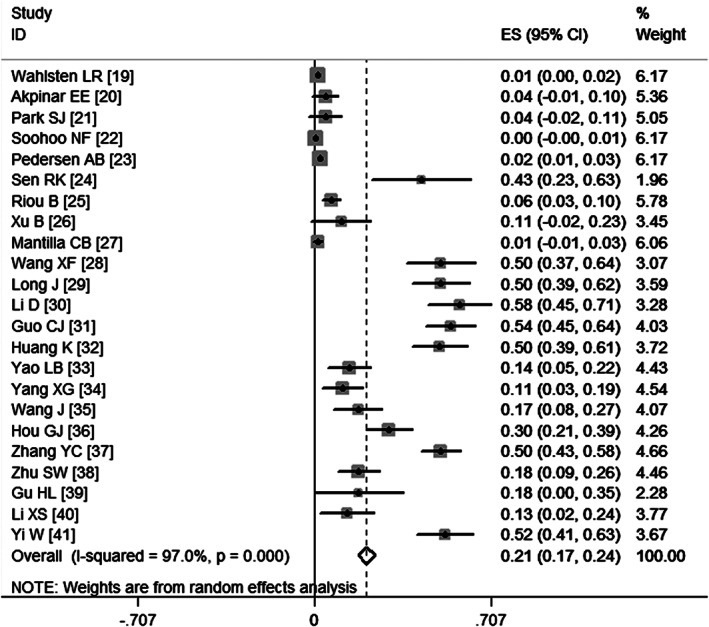
Meta‐analysis of the incidence of venous thromboembolism.
Egger's test (P = 0·438) and Begg's test (P = 0·553) revealed publication bias.
Demographic factors of DVT
Age
Table 2 illustrates the aggregated RRs for the most important risk factors for VTE. Ten studies 20, 21, 22, 23, 25, 32, 37, 38, 39, 40 examined age as a risk factor for VTE. Pooled estimates using a random‐effects model (P = 0·000) showed that patients younger than 60 years had a decreased risk of VTE (RR = 0·53, 95% CI: 0·40, 0·71; P = 0·000) (Figure 3), whereas patients older than 60 years of age had almost twice the higher risk of developing VTE (RR = 1·85, 95% CI: 1·34, 2·55; P = 0·000) (Figure 4). Egger's test (P = 0·19) and Begg's test (P = 1·00) revealed no publication bias.
Table 2.
Pooled estimates of risk ratios obtained from meta‐analysis of risk factors for venous thromboembolism following surgical treatment of fracture below the hip
| Risk factors | RR | 95% Confidence interval (CI) | P value |
|---|---|---|---|
| Age (≥60 years) | 1·85 | 1·34–2·55 | 0·000 |
| Age (<60 years) | 0·53 | 0·40–0·71 | 0·000 |
| Previous smoking | 1·35 | 0·99–1·86 | 0·059 |
| Current smoking | 1·23 | 1·07–1·41 | 0·004 |
| Immobility | 1·38 | 0·97–1·95 | 0·069 |
| Pregnancy | 1·09 | 0·42–2·80 | 0·864 |
| Previous VTE | 5·25 | 2·77–9·96 | 0·000 |
| Heart failure | 1·74 | 1·34–2·27 | 0·000 |
| Hypertension | 1·62 | 1·27–2·06 | 0·000 |
| Hyperlipidaemia | 2·16 | 1·79–2·62 | 0·000 |
| Cancer | 1·69 | 0·99–2·87 | 0·054 |
| Diabetes mellitus | 1·46 | 1·27–1·68 | 0·000 |
| Obesity | 1·58 | 1·35–1·85 | 0·000 |
| Open fractures | 0·97 | 0·74–1·27 | 0·0848 |
| Isolated fractures | 0·50 | 0·30–0·85 | 0·010 |
| Multiple fractures | 2·14 | 1·00–4·60 | 0·050 |
| Varicose veins | 3·07 | 1·12–8·47 | 0·030 |
| Combination with trauma | 1·22 | 1·05–1·41 | 0·628 |
Figure 3.
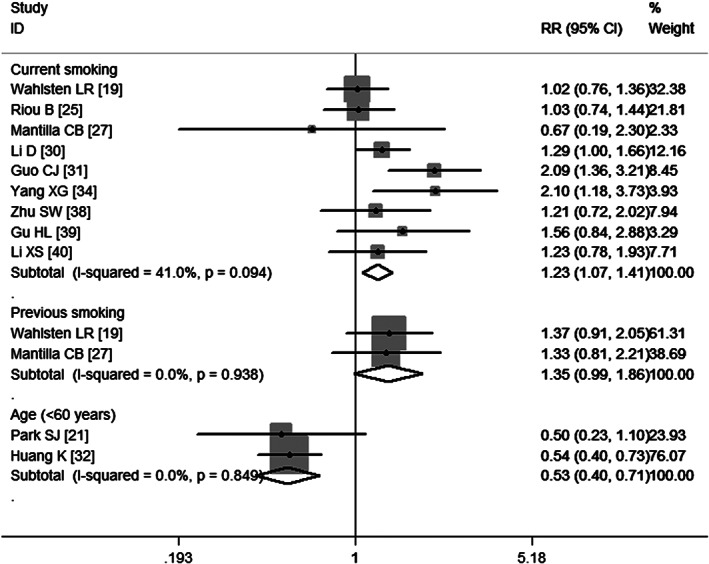
Meta‐analysis of the association between current smoking, previous smoking, age (<60 years) and venous thromboembolism.
Figure 4.

Meta‐analysis of the association between age (≥60 years) and venous thromboembolism.
Smoking
Nine studies 19, 25, 27, 30, 31, 34, 38, 39, 40 have assessed the association between smoking and VTE. Pooled results using a fixed‐effects model (P = 0·094) suggest that patients who had a previous history of smoking were not at a significant risk of VTE (RR = 1·35, 95%CI: 0·99, 1·86; P = 0·059) (Figure 3), whereas patients who were currently smoking were at a significant risk of developing VTE (RR = 1·23, 95%CI: 1·07, 1·41; P = 0·004) (Figure 3).Egger's test (P = 0·566) and Begg's test (P = 0·754) revealed no publication bias.
Immobility
Four studies 20, 25, 38, 40 have evaluated the association between immobility and the development of VTE. The aggregated results obtained using a random‐effects model (P = 0·000) show that immobility was not a significant risk factor of VTE (RR = 1·38, 95%CI: 0·97, 1·95; P = 0·069) (Figure 5). As the number of included studies was less than five, the assessment of publication bias was not performed.
Figure 5.
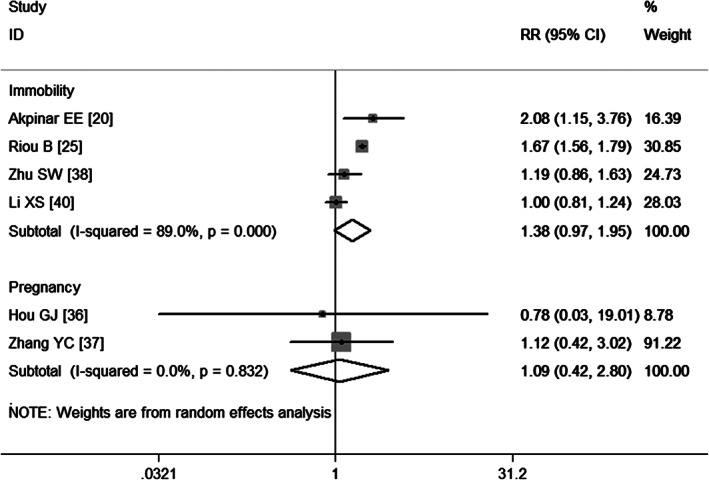
Meta‐analysis of the association between immobility, pregnancy and venous thromboembolism.
Pregnancy
Two studies 36, 37 report pregnancy as a risk factor for VTE. The aggregated results obtained using a fixed‐effects model (P = 0·832) suggest that pregnancy was not a risk factor for VTE (RR = 1·09, 95% CI: 0·42, 2·80; P = 0·864) (Figure 5).
Clinical factors of VTE
Previous VTE
Six studies 19, 20, 23, 25, 27, 37 have evaluated the association between previous history of VTE and the postoperative VTE. The pooled estimates obtained using a random‐effects model (P = 0·000) demonstrate that a previous history of VTE was a high risk factor for the development of postoperative VTE (RR = 5·25, 95% CI: 2·77, 9·96; P = 0·000) (Figure 6). Egger's test (P = 0·439) and Begg's test (P = 1·000) revealed no publication bias.
Figure 6.
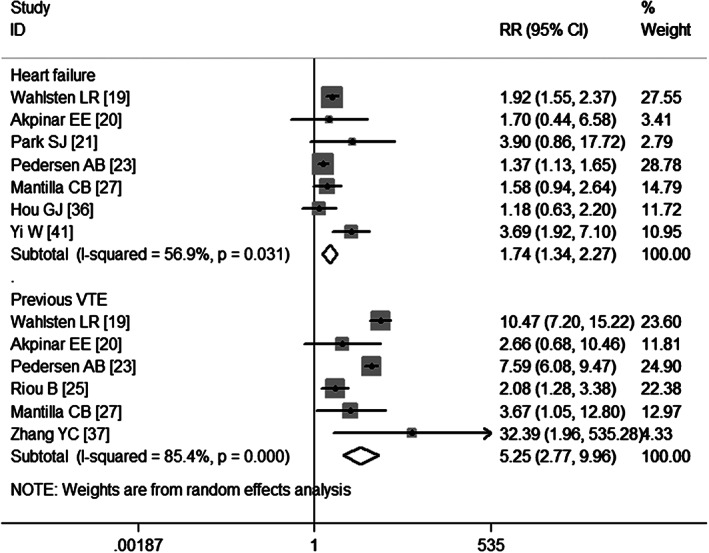
Meta‐analysis of the association between heart failure, previous venous thromboembolism (VTE) and VTE.
Heart failure
Seven studies 19, 20, 21, 23, 27, 36, 41 report heart failure as a risk factor for VTE. The aggregate results obtained using a random‐effects model (P = 0·031) show that patients with heart failure had almost twice the risk of developing postoperative VTE (RR = 1·74, 95% CI: 1·34, 2·27; P = 0·000) (Figure 6). Egger's test (P = 0·406) and Begg's test (P = 0·368) revealed no publication bias.
Hypertension
Nine studies 19, 28, 29, 30, 31, 32, 33, 35, 41 have evaluated the association between hypertension and VTE. The pooled estimates obtained using a fixed‐effects model (P = 0·024) demonstrate that hypertension was significantly associated with a higher risk of developing VTE (RR = 1·62, 95% CI: 1·27, 2·06; P = 0·000) (Figure 7). Egger's test (P = 0·353) and Begg's test (P = 0·348) revealed no publication bias.
Figure 7.
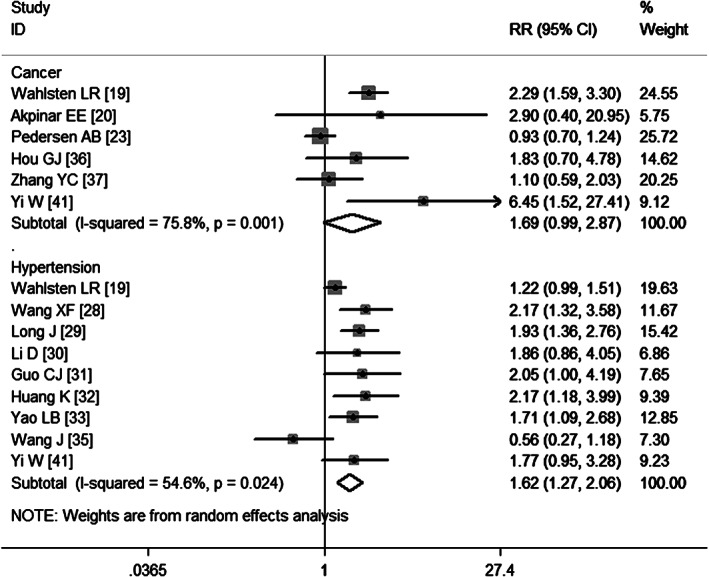
Meta‐analysis of the association between cancer, hypertension and venous thromboembolism.
Cancer
Six studies 19, 20, 23, 36, 37, 41 report cancer as a risk factor of VTE. The aggregate results obtained using a random‐effects model (P = 0·001) show that cancer was not a significant risk factor of VTE (RR = 1·69, 95%CI: 0·99, 2·87; P = 0·054) (Figure 7). Egger's test (P = 0·311) and Begg's test (P = 0·452) revealed no publication bias.
Diabetes mellitus
Fourteen studies 19, 22, 23, 25, 27, 28, 29, 30, 31, 32, 33, 36, 39, 41 have assessed the association between diabetes mellitus and VTE. Pooled results obtained using a fixed‐effects model (P = 0·472) suggest that diabetes mellitus increased the risk of developing VTE (RR = 1·46, 95%CI: 1·27, 1·68; P = 0·000) (Figure 8). Egger's test (P = 0·171) and Begg's test (P = 0·324) revealed no publication bias.
Figure 8.

Meta‐analysis of the association between diabetes mellitus and venous thromboembolism.
Hyperlipidaemia
Eight studies 28, 29, 30, 31, 32, 33, 36, 41 have assessed the association between hyperlipidaemia and VTE. Pooled results obtained using a fixed‐effects model (P = 0·582) suggest that hyperlipidaemia increased the risk of developing VTE (RR = 2·16, 95% CI: 1·79, 2·62; P = 0·000) (Figure 9). Egger's test (P = 0·205) and Begg's test (P = 0·108) revealed no publication bias.
Figure 9.
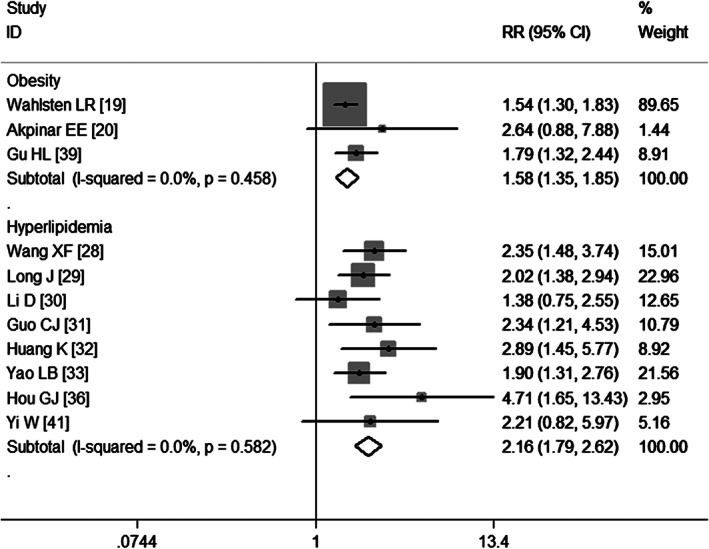
Meta‐analysis of the association between obesity, hyperlipidaemia and venous thromboembolism.
Obesity
Three studies 19, 20, 39 have assessed the association between obesity and VTE. Pooled results obtained using a fixed‐effects model (P = 0·458) suggest that obesity increased the risk of developing VTE (RR = 1·58, 95% CI: 1·35, 1·85; P = 0·000) (Figure 9).
Open fractures
Three studies 21, 22, 35 report open fractures as a risk factor of VTE. The aggregate results obtained using a fixed‐effects model (P = 0·817) show that patients with open fractures were not at a significant risk of developing VTE (RR = 0·97, 95% CI: 0·74, 1·27; P = 0·0848) (Figure 10).
Figure 10.
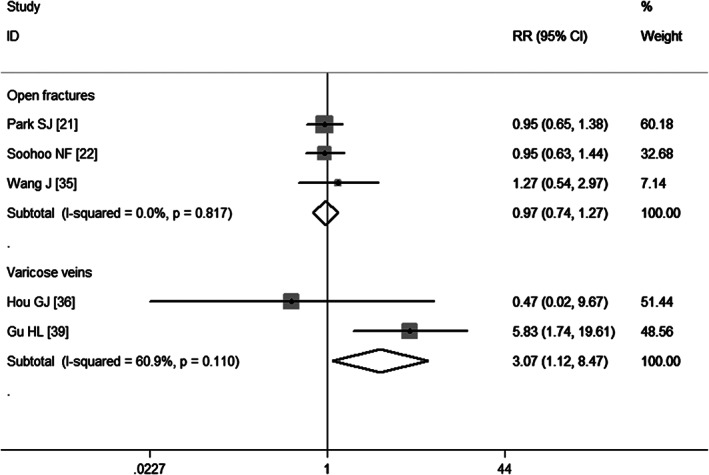
Meta‐analysis of the association between open fractures, varicose veins and venous thromboembolism.
Isolated fractures
Four studies 21, 28, 29, 32 have assessed the association between isolated fractures and VTE. Pooled results obtained using a random‐effects model (P = 0·000) suggest that isolated fractures decreased the risk of developing VTE (RR = 0·50, 95%CI: 0·30, 0·85; P = 0·010) (Figure 11).
Figure 11.
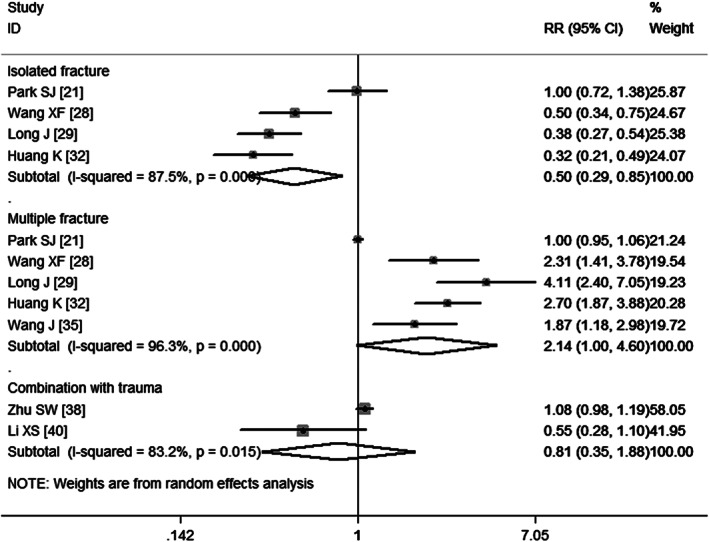
Meta‐analysis of the association between isolated fracture, multiple fractures, combination with trauma and venous thromboembolism.
Multiple fractures
Five studies 21, 28, 29, 32, 35 report multiple fractures as a risk factor of VTE. The aggregate results obtained using a random‐effects model (P = 0·000) show that patients with multiple fractures were at a higher risk of developing VTE (RR = 2·14, 95%CI: 1·00, 4·60; P = 0·050) (Figure 11). Egger's test (P = 0·10) and Begg's test (P = 0·462) revealed no publication bias.
Varicose veins
Two studies 36, 39 have assessed the association between varicose veins and VTE. Pooled results obtained using a fixed‐effects model (P = 0·110) suggest that varicose veins increased the risk of developing VTE (RR = 3·07, 95% CI: 1·12, 8·47; P = 0·030) (Figure 10).
Combination with trauma
Two studies 38, 40 report the combination with trauma as a risk factor of VTE. The aggregate results obtained using a random‐effects model (P = 0·015) show that patients who had combined with trauma were not at a significantly higher risk of developing VTE (RR = 0·81, 95% CI: 0·35, 1·88; P = 0·628) (Figure 11).
Duration of operation and duration of bed rest
Four studies 30, 31, 33, 36 report data on the duration of operation. Pooled estimates obtained using a random‐effects model revealed that patients who had postoperative DVT had 1·22 hours of operative duration more than those who had no postoperative DVT (WMD = 1·22, 95%CI: 0·63, 1·81; P = 0·000) (Figure 12), which indicated that prolonged operation time increased the risk of developing VTE.
Figure 12.
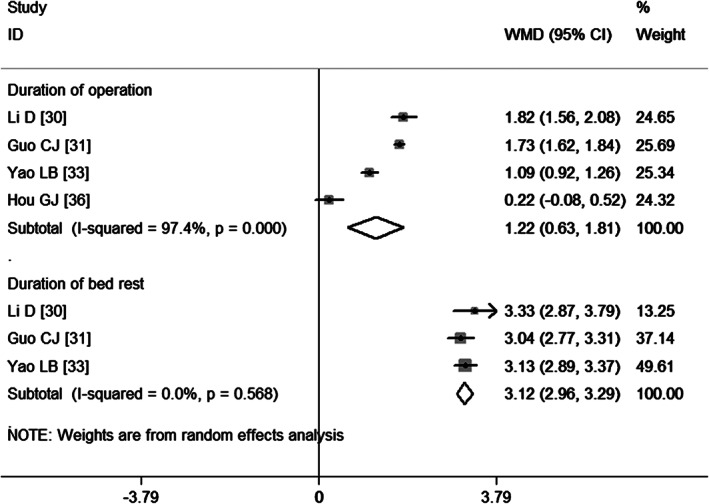
Meta‐analysis of the association between duration of operation, duration of bed rest and venous thromboembolism.
Three studies 30, 31, 33 provide data on the duration of bed rest. Pooled results obtained using a random‐effects model suggest that patients who had postoperative DVT had 3·12 hours of bed rest more than those who had no postoperative DVT (WMD = 3·12, 95%CI: 2·96, 3·29; P = 0·000) (Figure 12), indicating that prolonged bed rest time increased the risk of developing VTE.
Discussion
In this meta‐analysis, we identified independent risk factors for patients who develop VTE following surgical treatment of fractures below the hip. In our analysis of clinically significant postoperative VTEs, about 20 risk factors that might have a potential impact on the development of VTE have been identified. Using the meta‐analysis method of aggregating the data, we were able to identify 12 independent risk factors that were associated with an increased risk of developing VTE. Previous history of VTE was the strongest independent risk factor followed by varicose veins, hyperlipidaemia, multiple fractures, age (≥60 years), heart failure, obesity, hypertension, diabetes mellitus, current smoking, prolonged operation time and prolonged bed rest time.
As is commonly acknowledged, previous VTE was confirmed as a risk factor in patients who underwent surgical treatment of fractures below the hip. This conclusion was also identified in this meta‐analysis in which patients with previous history of VTE had five times higher risk of developing VTE (RR = 5·25, 95% CI: 2·77, 10·0; P = 0·000). However, among the studies included in this meta‐analysis, Akpinar et al. 20 found that previous VTE was not a risk factor of VTE, and the authors attributed this negative result to the inadequate medical records of patients with previous VTE.
Of the included studies, several have reported that patients with diabetes mellitus were at a significantly higher risk of developing postoperative VTE. Our result is consistent with that of these studies. However, some studies came to the reverse conclusion that diabetes mellitus was not a risk factor of VTE 19, 22, 23, 25. In a meta‐analysis conducted by Zhang et al. 42, the authors pooled 14 studies to identify the risk factors for VTE after total hip arthroplasty (THA) or total knee arthroplasty (TKA), and they found no correlation between diabetes mellitus and postoperative VTE (odd ratio (OR) = 1·02, 95%CI: 0·80·1.31; P = 0·88). However, these estimates were calculated based on five studies, while our result was based on 14 studies, and no heterogeneity was identified between them. Additionally, according to the previous research, diabetes mellitus is often associated with increased levels of procoagulant factors and the inhibition of endogenous fibrinolysis 43, 44.
Varicose veins have been confirmed as a risk factor of VTE in our meta‐analysis. However, this conclusion was calculated based on only two studies as others did not provide the available data for analysis. In a retrospective case–control study that was conducted by Gu et al. 39, 27·8% (5/18) of the patients in the VTE group had varicose veins compared to 4·8% (4/84) in the non‐VTE group. This result suggests that varicose veins were significantly higher in the VTE group. However, in another study that investigated varicose veins as a risk factor of VTE 36, the prevalence of varicose veins in the two groups (1·0% versus 0·87%) appeared to be lower than that in Gu's study; there was no significant difference between them (P = 0·492).
Several studies have found that obesity (body mass index (BMI) ≥ 30) was a significant risk factor for VTE, which was also observed in this meta‐analysis. However, it was not described as a significant risk factor of VTE in the study conducted by Akpinar et al. 20. In that study, obesity was not found to be an independent risk factor for VTE, but the authors did not give any explanation for this negative result. According to the previous studies, obesity was considered a higher risk factor for VTE because it could reduce antithrombin levels and fibrinolytic activity, and it was also associated with higher levels of prothrombotic factors, including fibrinogen, plasminogen activator inhibitor and factor 45, 46. Additionally, obese patients have metabolic disturbances, are less active and more likely to be hospitalised with immobilisation, which might lead to the development of VTE 45, 46, 47.
Contrary to some reports 19, 41, cancer was not identified as an independent risk factor for VTE in this meta‐analysis (RR = 1·69, 95% CI: 0·99, 2·88; P = 0·054). Akpinar et al. 20 reported that 4·7% (28/594) of patients in the VTE group had cancer compared with 2·1% (1174/57 025) in the non‐VTE group (hazard ratio = 1·65, 95% CI: 1·12, 2·42), indicating that cancer was a significant risk factor for VTE. Conversely, in another study conducted by Pedersen et al. 23, 3·9% (46/1185) of the patients who developed VTE had cancer compared with 1·1% (2768/66 284) in those without VTE (RR = 0·93, 95% CI: 0·68, 1·28), which suggested that cancer was not a significant risk factor for VTE. In a recently published meta‐analysis, Zhang et al. 42 found that ‘active’ cancer was a marginally significant risk factor for the development of VTE after THA or TKA (OR = 1·28, 95% CI: 1·01, 1·62; P = 0·04). Considering the inconsistent results of these studies 20, 23, 42, more research is necessary before definite conclusions can be drawn.
Both heart failure and hypertension were found to be significantly associated with an increased risk of developing VTE in this meta‐analysis. These might be explained by the blood's hypercoagulable state 48, 49, 50. Patients with heart failure were always less active, and they had haemostatic abnormalities that might predispose them to the occurrence of VTE 50.
One of the main limitations of this meta‐analysis is the heterogeneity among the included studies, which might influence the finally pooled estimates of all the meta‐analyses. This could be explained by the following factors: the characteristics of patients, study design (cohort study or case–control study), the diagnostic criteria, the duration of follow‐up, type of fracture and time of surgery. Because all of the included studies were of observational design, the study was performed based on hospital diagnoses. Therefore, patients with asymptomatic VTE were not registered even as an outcome, which would lead to an underestimated incidence of VTE. For some risk factors, the predictive variables may not have sufficient statistical power because of the limited number of included studies (<5). In some studies, chemoprophylaxis was administered to the patients, which confounded or strengthened the risk factors. However, a major strength of this study is the large number of patients and the high quality of the included studies, which greatly enhances the statistical power of pooled results.
In conclusion, we found that age (older than 60 years), current smoking, previous history of VTE, heart failure, hypertension, hyperlipidaemia, diabetes mellitus, obesity, multiple fractures, varicose veins, prolonged operation time and prolonged bed rest time are associated with an increased risk of developing VTE. By identifying these factors, patients with a relatively higher risk of developing VTE could be treated more intensively and, therefore, the incidence of VTE would be reduced.
Acknowledgements
All the authors declare that they have no conflict of interest.
References
- 1. Lin PP, Graham D, Hann LE, Boland PJ, Healey JH. Deep venous thrombosis after orthopedic surgery in adult cancer patients. J Surg Oncol 1998;68:41–7. [DOI] [PubMed] [Google Scholar]
- 2. Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty. J Am Acad Orthop Surg 1996;4:54–62. [DOI] [PubMed] [Google Scholar]
- 3. Dahl OE, Gudmundsen TE, Haukeland L. Late occurring clinical deep vein thrombosis in joint‐operated patients. Acta Orthop Scand 2000;71:47–50. [DOI] [PubMed] [Google Scholar]
- 4. Hyers TM, Agnelli G, Hull RD, Weg JG, Morris TA, Samama M, Tapson V. Antithrombotic therapy for venous thromboembolic disease. Chest 1998;114:561S–78S. [DOI] [PubMed] [Google Scholar]
- 5. Baser O. Prevalence and economic burden of venous thromboembolism after total hip arthroplasty or total knee arthroplasty. Am J Manag Care 2011;17:S6–8. [PubMed] [Google Scholar]
- 6. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141:7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Comp PC, Spiro TE, Friedman RJ, Whitsett TL, Johnson GJ, Gardiner GA Jr, Landon GC, Jove M. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. J Bone Joint Surg Am 2001;83‐A:336–45. [DOI] [PubMed] [Google Scholar]
- 8. Kim YH, Oh SH, Kim JS. Incidence and natural history of deep‐vein thrombosis after total hip arthroplasty. A prospective and randomised clinical study. J Bone Joint Surg Br 2003;85:661–5. [PubMed] [Google Scholar]
- 9. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology 2002;96:1140–6. [DOI] [PubMed] [Google Scholar]
- 10. Schiff RL, Kahn SR, Shrier I, Strulovitch C, Hammouda W, Cohen E, Zukor D. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest 2005;128:3364–71. [DOI] [PubMed] [Google Scholar]
- 11. White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med 2000;343:1758–64. [DOI] [PubMed] [Google Scholar]
- 12. Leizorovicz A, Turpie AG, Cohen AT, Wong L, Yoo MC, Dans A. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost 2005;3:28–34. [DOI] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000: 3–5.
- 14. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wahlsten LR, Eckardt H, Lyngbaek S, Jensen PF, Fosbol EL, Torp‐Pedersen C, Gislason GH, Olesen JB. Symptomatic venous thromboembolism following fractures distal to the knee: a nationwide Danish cohort study. J Bone Joint Surg Am 2015;97:470–7. [DOI] [PubMed] [Google Scholar]
- 20. Akpinar EE, Hosgun D, Akan B, Ates C, Gulhan M. Does thromboprophylaxis prevent venous thromboembolism after major orthopedic surgery? J Bras Pneumol 2013;39:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SJ, Kim CK, Park YS, Moon YW, Lim SJ, Kim SM. Incidence and factors predicting venous thromboembolism after surgical treatment of fractures below the hip. J Orthop Trauma 2015;29:e349–54. [DOI] [PubMed] [Google Scholar]
- 22. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg 2011;17:259–62. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen AB, Sorensen HT, Mehnert F, Overgaard S, Johnsen SP. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg Am 2010;92:2156–64. [DOI] [PubMed] [Google Scholar]
- 24. Sen RK, Kumar A, Tripathy S, Aggarwal S, Khandelwal N. Risk factors of venous thromboembolism in Indian patients with pelvic‐acetabular trauma. J Orthop Surg (Hong Kong) 2011;19:18–24. [DOI] [PubMed] [Google Scholar]
- 25. Riou B, Rothmann C, Lecoules N, Bouvat E, Bosson JL, Ravaud P, Samama CM, Hamadouche M. Incidence and risk factors for venous thromboembolism in patients with nonsurgical isolated lower limb injuries. Am J Emerg Med 2007;25:502–8. [DOI] [PubMed] [Google Scholar]
- 26. Xu B, Xu HG. Analyse of the risk factors of DVT after lower extremity surgery. Zhongguo Gu Shang 2008;21:855–7. [PubMed] [Google Scholar]
- 27. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology 2003;99:552–60; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 28. Wang XF. Analyse of the risk factors of DVT in 103 patients with bone trauma. Guide China Med 2013;11:429–30. [Google Scholar]
- 29. Long J. Incidence and risk factors of DVT in patients with bone trauma. Seek Med Ask Med 2013;11:1. [Google Scholar]
- 30. Li D, Xu YS. Risk factors for deep vein thrombosis in patients with traumatic fractures. China Healthcare Nut 2013;9:737–8. [Google Scholar]
- 31. Guo CJ. Analysis on isk factors for deep vein thrombosis in patients with traumatic fractures. J North Pharm 2013;8:99–100. [Google Scholar]
- 32. Huang K. Analyse of the risk factors of DVT in 80 patients with bone trauma. For All Health 2014;8:128–9. [Google Scholar]
- 33. Yao LB. Study of the incidence and risk factors of orthopedic trauma deep vein thrombosis. Yiayao Qianyan 2012;2:107. [Google Scholar]
- 34. Yang XG, Sun Z, Wang XQ, Lu Y, Guo RL. Risk factors for deep vein thrombosis after fracture surgery. Tianjin Med J 2010;38:464–6. [Google Scholar]
- 35. Wang J, Zhao CP, Wei J, Wang CM. An analysis of risk factors for lower limb deep venous thrombosis in patients with tibial plateau fractures. Chin J Bone Joint 2015;2:86–90. [Google Scholar]
- 36. Hou GJ, Zhou F, Ji HQ, Tian G, Zhang ZS. Influencing factors and prevention of perioperative venous thromboembolism in patients with lower extremity fractures. Chin J Orthop Trauma 2014;16:690–4. [Google Scholar]
- 37. Zhang YC, Jing ZX, Zhang ZD, Bai XL, Lou T, Guo J. Analysis of risk factors of lower limbs deep venoun thrombosis: a case–control study. Chin General Prac 2012;15:3357–9. [Google Scholar]
- 38. Zhu SW, Sun X, Yang MH, Wang MY, Wu XB, Cao QY, Wu HH. Preoperative risk factors for deep venous thromboembolIsm in pafients with acetabular and pelvic fractures. Chin J Onhop Trauma 2012;14:675–8. [Google Scholar]
- 39. Gu HL, Duan JZ, Wang H. Risk factors for deep vein thrombosis after operations for acetabular fractures. Chin J Bone Joint Inju 2007;22:462–4. [Google Scholar]
- 40. Li XS, Wang YH, He YP, Xiao J, Zhu GJ, Wei CM, Wu XB. Clillical research on related factors of preoperative deep vein thrombosis of acetablar and pelvic fracture. China Mod Doc 2014;52:4–7. [Google Scholar]
- 41. Yi W. Analysis of risk factors of actue deep veins thrombosis of lower limb in elder people. Med J Chin People's Hea 2011;23:1449. [Google Scholar]
- 42. Zhang J, Chen Z, Zheng J, Breusch SJ, Tian J. Risk factors for venous thromboembolism after total hip and total knee arthroplasty: a meta‐analysis. Arch Orthop Trauma Surg 2015;135:759–72. [DOI] [PubMed] [Google Scholar]
- 43. Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care 2003;26:2181–8. [DOI] [PubMed] [Google Scholar]
- 44. Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001;24:1476–85. [DOI] [PubMed] [Google Scholar]
- 45. Zhang ZJ, Zhao XY, Kang Y, Zhang ZQ, Yang ZB, He AS, Fu M, Sheng PY, Liao WM. The influence of body mass index on life quality and clinical improvement after total hip arthroplasty. J Orthop Sci 2012;17:219–25. [DOI] [PubMed] [Google Scholar]
- 46. Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ 3rd.. Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population‐based case–control study. Arterioscler Thromb Vasc Biol 2009;29:1399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S. Elaboration of type‐1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 1996;93:106–10. [DOI] [PubMed] [Google Scholar]
- 48. Jafri SM. Hypercoagulability in heart failure. Semin Thromb Hemost 1997;23:543–5. [DOI] [PubMed] [Google Scholar]
- 49. Jafri SM, Ozawa T, Mammen E, Levine TB, Johnson C, Goldstein S. Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur Heart J 1993;14:205–12. [DOI] [PubMed] [Google Scholar]
- 50. Sbarouni E, Bradshaw A, Andreotti F, Tuddenham E, Oakley CM, Cleland JG. Relationship between hemostatic abnormalities and neuroendocrine activity in heart failure. Am Heart J 1994;127:607–12. [DOI] [PubMed] [Google Scholar]


