Abstract
The care and the management of the healing of difficult wounds at the level of the skull‐facial face many problems related to patient compliance and the need to perform multiple dressings, with long periods of healing and, occasionally, a very long hospitalisation period. The introduction and evolution of negative pressure wound therapy (NPWT) in the treatment of difficult wounds has resulted in better healing, with a drastic reduction in terms of time and biological costs to the patient and cost to the health care system. The main aim of this study is to describe and discuss, using out our experience, the usefulness of NPWT in the cranial‐facial‐cervical region. We studied 16 patients with complex wounds of the cranial‐facial‐cervical region treated with NPWT. We divided clinical cases in four groups: cervicofacial infectious disease, healing complications in oncological‐reconstructive surgery, healing complications of injury with exposure of bone and/or internal fixations and healing complications in traumatic injury with loss of substance. We evaluated complete or incomplete wound healing; application time, related also to hospitalisation time; days of intensive care unit (ICU) stay; management of the upper airways; timing of medication renewal; and patient comfort and compliance (on a scale of 1–5). Depression values were always between −75 and −125 mmHg in a continuous aspiration pattern. For every patient, we used the ActiVAC Therapy Unit, derived from the vacuum‐assisted closure system (Kinetic Concepts Inc., San Antonio, TX). Medication renewals were performed every 48–72 hours. The NPWT application time ranged from 4 to 22 days (mean of 11·57 day). Therapy was effective to gain a complete restitutio ad integrum in every patient included in the group of cervicofacial infectious disease. Therapy has, however, been well tolerated in our series; this is probably due to the decreased number of applications, the ease of use and the comfort of the system relative to traditional dressing. Results were satisfactory for most of cases treated; faster and more effective wound healing was achieved. The lower number of NPWT applications, relating to standard dressings, led to an increase in patient comfort and compliance and a decrease in the use of medical, and in some cases economic, resources according to international literature.
Keywords: Negative pressure wound therapy, VAC Therapy cervical wounds, Cranio‐maxillofacial wounds, Vacuum‐assisted closure
Introduction
‘Time heals all wounds’ is a sentence‐serving but quite true assertion due to the abilities of the human body.
Historically, the management of complex wounds included thorough cleansing, surgical preparation of the infected site and removal of necrotising tissue, as well as postoperative accurate dressing. Although cleansing, debridement, frequent renewal of medication and medical therapy still represent the gold standard in complex wounds healing, negative pressure wound therapy (NPWT) could be used to achieve better and faster recovery.
The evolution of NPWT started with the suction drainage, introduced in the 1930s to evacuate fluid in excess 1; first, Silvis and Moloney developed a method for the removal of excess liquid during head and neck surgery, but it was only in the 1960s that Von Leden and Kaplan 2, as well as McLean, described the use of a portable suction drainage system (the Hemo Vac System) that successfully improved the outcome of cervical surgery 3, 4, 5. Morbidity, salivary fistula and infections were described to decrease, while histological examinations confirmed far less interstitial exudation and tissue necrosis 2, 6, 7.
The practice of exposing a wound to subatmospheric pressure for an extended period to promote debridement and healing was first described by Fleischmann et al. 8, following the successful use of this technique in 15 patients with open fractures. They reported that the treatment resulted in ‘efficient cleaning and conditioning of the wound, with marked proliferation of granulation tissue’. 8
In early studies, negative pressure within the wound was achieved through the use of conventional methods, such as a wall suction apparatus or surgical vacuum bottles. Both these methods were associated with practical problems in terms of the delivery, control and maintenance of the required levels of negative pressure, as discussed by Banwell 3
In the early 1990s, Morykwas and Argenta conducted a series of animal studies focusing on a polyurethane foam as an interface between the wound surface and the vacuum source, this foam proved essential in uniform pressure distribution over the entire wound surface. That stimulated the development of vacuum‐assisted closure (V.A.C. Advanced Therapy System) by Kinetic Concepts Inc. of San Antonio, TX 8, 9, 10.
In 1995, a commercial system for promoting VAC was introduced into the United States market. This equipment, called V.A.C., was designed to overcome some of the problems described by Banwell. The heart of the system is a microprocessor‐controlled vacuum unit that is capable of providing controlled levels of continuous or intermittent subatmospheric pressure ranging from 25 to 200 mmHg.
The objectives of NPWT include the removal of excessive exudate, catabolic products and necrotic debris while stimulating the wound bed to produce granulation tissue; improving local vascularisation; and mechanically stabilising the wound or any skin grafts and flaps. In comparison to conventionally used medications, this system eliminates most of the oedema and interstitial exudate, increases local tissue perfusion and reduces the bacterial load; this leads to more rapid and efficient healing of complex wounds 9, 11.
Only recently have articles appeared regarding NPWT applied to the cranial‐facial‐cervical region. In 2006, Schuster and Adrews published the first clinical trials related to V.A.C. application to both complicated cranial‐facial lesions and in cervical infected wounds that in exposure of cranial plank 12, 13.
The main aim of this study is to describe and discuss, using out our experience, the usefulness of NPWT in the cranial‐facial‐cervical region.
Materials and methods
Our study included 16 patients with complex cranial‐facial‐cervical wounds treated between March 2010 and April 2016 with NPWT at the Department of Maxillo Facial Surgery and Neurosurgery of ASST San Gerardo Hospital – Monza and Department of Maxillo Facial Surgery of Niguarda Hospital – Milan. Inclusion criteria followed the European Wound Management Associations guidelines 14. Before the treatment, patients underwent surgical debridement of wounds, while NPWT was continued during hospitalisation in all but two cases; if not hospitalised in our unit, the patient was subject to strict follow up.
We divided the clinical cases into four groups:
cervicofacial infectious disease,
healing complications in oncological‐reconstructive surgery,
healing complications of injury with bone and/or internal fixations exposed,
healing complications in traumatic injury with loss of substance,
Some of these patients had comorbidities such as HIV or HCV infections, diabetes, vasculopathy or obesity (Table 1).
Table 1.
Patients treated with Negative Pressure Wound Therapy
| Patient no. | Age | Sex | Diagnosis | Comorbility |
|---|---|---|---|---|
| 1 | 25 | Male | Cervicofacial infectious disease originated by odontogenic abscess | – |
| 2 | 43 | Male | Cervicofacial infectious disease originated by odontogenic abscess | HIV |
| 3 | 47 | Female | Cervicofacial infectious disease originated by odontogenic abscess | Diabetes mellitus, obesity |
| 4 | 38 | Male | Cervicofacial infectious disease originated by odontogenic abscess | Obesity |
| 5 | 23 | Male | Facial infectious disease originated by odontogenic abscess | – |
| 6 | 49 | Male | Cervicofacial infectious disease originated by odontogenic abscess | – |
| 7 | 55 | Female | Healing complications in oncologic‐reconstructive surgery (surgical wound infection in lingual resection for squamous cell carcinoma) | Hypertension |
| 8 | 78 | Male | Healing complications in oncologic‐reconstructive surgery (surgical wound infection in resection for squamous cell carcinoma of the orbito‐zygomatic region) | Diabetes mellitus |
| 9 | 65 | Male | Healing complications in oncologic‐reconstructive surgery (surgical wound infection in resection for relapse of squamous cell carcinoma of the tongue) | Previous radiotherapy |
| 10 | 54 | Female | Healing complications in oncologic‐reconstructive surgery (surgical wound infection in resection lingual squamous cell carcinoma) | Liver disease, anorexia , vasculopathy |
| 11 | 10 months | Female | Healing complications of injury with exposure of bone and/or internal fixations (exposure of cranioplasty internal fixation plates) | Apert Syndrome |
| 12 | 64 | Female | Healing complications of injury with exposure of bone and/or internal fixations (dural exposure after removal of atypical frontotemporal meningioma) | Previous radiotherapy |
| 13 | 9 months | Male | Healing complications of injury with exposure of bone and/or internal fixations (wound infection after Dandy‐Walker Hydrocephalous surgical tratment) | Pierre‐Robin sequence |
| 14 | 50 | Male | Healing complications in traumatic injury with loss of substance (internal fixation plate exposure) | HCV |
| 15 | 30 | Male | Healing complications in traumatic injury with loss of substance (internal fixation plate exposure) | – |
| 16 | 26 | Female | Healing complications in traumatic injury with loss of substance (infected injury with loss of substance in temporal region in polytrauma) | HCV, Hirschsprung disease |
We evaluated the following criteria:
Complete or incomplete wound healing,
Application time, related also to hospitalisation time,
Days of intensive care unit (ICU) stay and management of the upper airways,
Timing of medication renewal,
Patient comfort and compliance (on a scale of 1–5)
The ActiVAC Therapy Unit, derived from a V.A.C System (Kinetic Concepts Inc.), was applied to every patient; we used hydrophobic polyurethane black open pore (400–600 microns) foam, designed with a length–width ratio of 3:1, in order to effectively propagate the depression coefficient. Depression values were always between −75 and −125 mmHg in a continuous aspiration pattern.
Medication renewals were performed depending on local and general clinical condition every 48–72 hours; when foam was situated in deep neo‐formed space, procedures took place in the operating room under sedation. Otherwise, dressing that was placed superficially was changed with minimal discomfort in the ward.
In patients affected by cervicofacial infectious disease, V.A.C. Therapy was initiated immediately after surgical toilette, during the first hospitalisation day, as a result of dangerous and rapidly worsening pathology. Four patients stayed in ICU units and underwent endotracheal intubation for severe respiratory failure; one of them needed a tracheotomy on the seventh postoperative day. V.A.C. Therapy was renewed every 48–72 hours (Figures 1, 2, 3, 4, 5).
Figure 1.
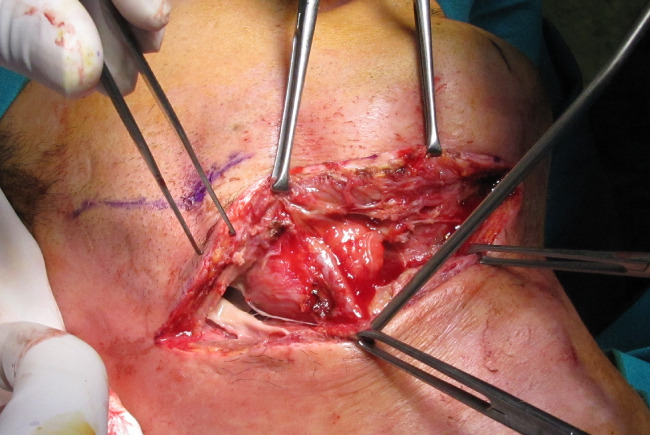
Sub‐mandibular dissection to evacuate colliquation in cervicofacial necrotising fasciitis.
Figure 2.
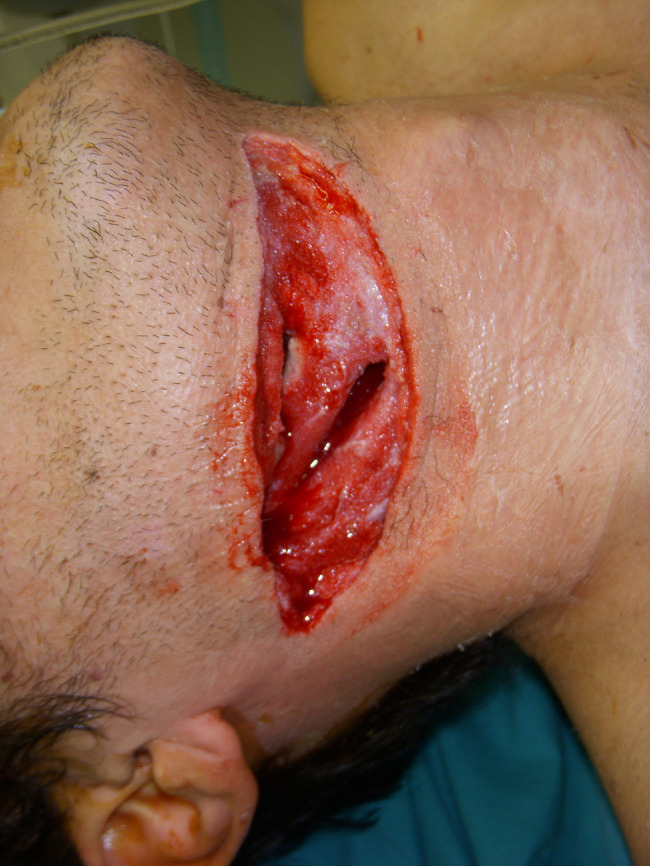
Debridement and removal of necrotising tissue after V.A.C. application.
Figure 3.
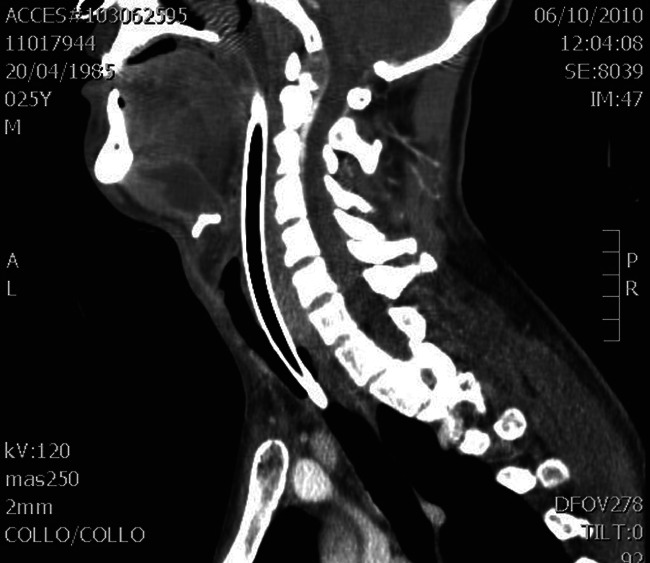
CT scan showing deep cervical space abscess.
Figure 4.
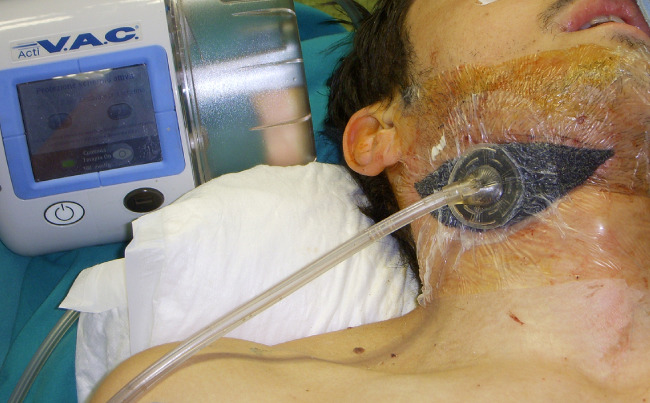
Operating V.A.C. Therapy.
Figure 5.
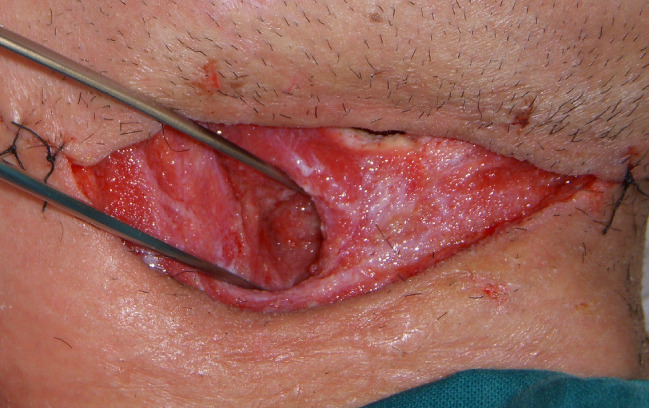
Neck wound after 14 days of V.A.C. Therapy.
NPWT application was really effective over scalp wounds but was technically challenging owing to consistent difficulties in film adhesion due to complex surface, hair bulbs and complex anatomical structures, such as pinna. Our experience highlighted the necessity for a wide and well‐shaved area surrounding the injury; film has to be trimmed around anatomical structures, in order to gather the seal. Finally, hair must be shaved and carefully removed at every dressing renewal.
In the group of patients affected by healing complications after oncological‐reconstructive surgery, three cases presented dehiscence, with no salivary fistula after lateral neck dissection (Figures 6 and 7). Another patient showed partial necrosis of cutaneous graft and temporalis muscle flap with preauricolar bone exposure after exenteratio orbitae. V.A.C. Therapy was administered between the 21st and 33rd day after surgery after the failure of traditional wound dressing. Therapy was renewed every 48–72 hours. Every oncological case was disease‐free at V.A.C. application; therapy itself has been applied as indicated by the European Wound Management Association (EWMA).
Figure 6.
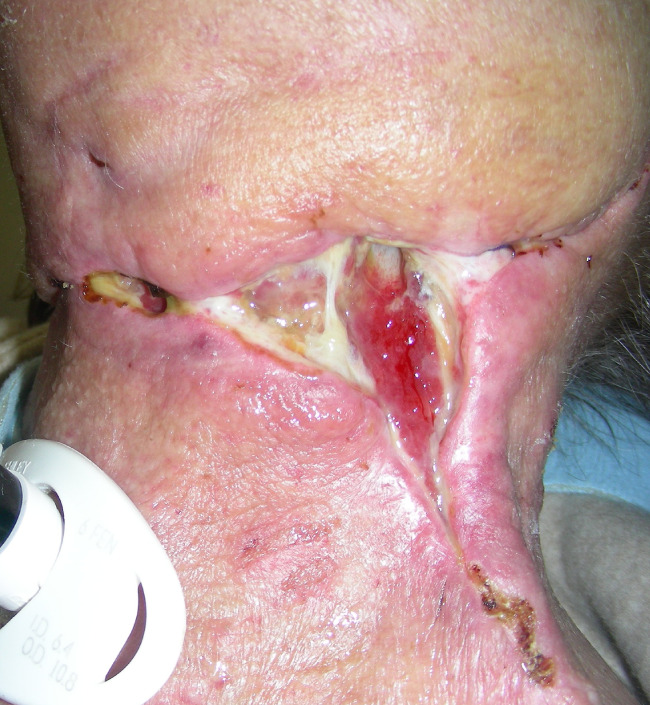
Lateral neck dehiscence.
Figure 7.
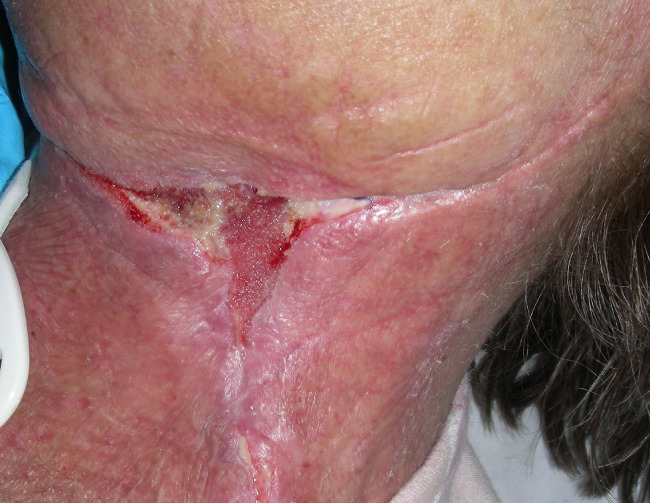
Wound healing after 15 days of V.A.C. Therapy.
We treated three patients affected by surgical outcomes with exposure of underlying osteosynthesis devices: the first, a 10‐month Apert syndrome, showed coronal dehiscence of the surgical wound after cranioplasty (Figure 8, 9, 10). The second patient underwent c1–c4 surgical stabilisation in anterolisthesis, with surgical wound dehiscence. The third case was an exposed plurifocal and comminuted jaw fracture in a suicide attempt: open reduction and internal stabilisation ended with a sub‐mandibular purulent gathering. It was evacuated on the ninth day after surgical treatment, and V.A.C. Therapy was immediately initiated in the virtual space. In every case, V.A.C. Therapy was initiated in the Operating Room (OR) and was renewed every 48–72 hours.
Figure 8.
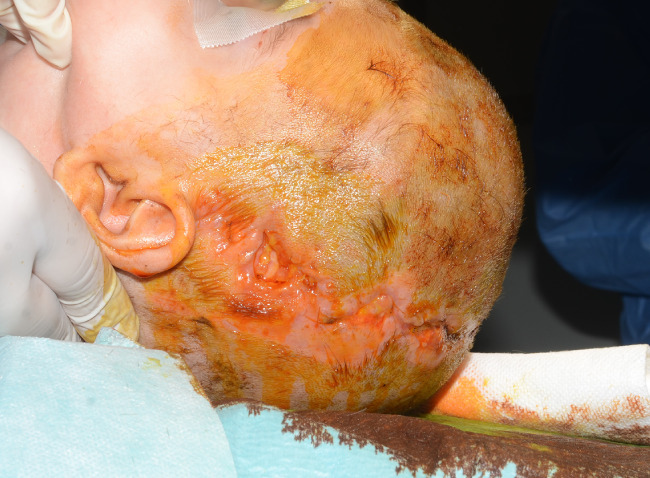
Postoperative coronal dehiscence cranioplasty in Apert Syndrome.
Figure 9.
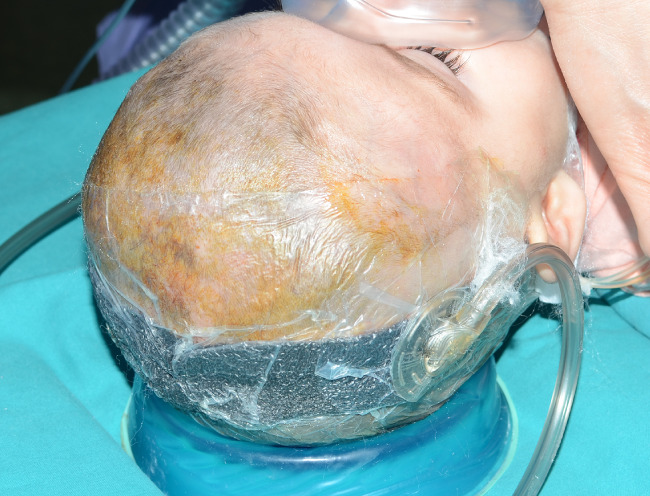
Operating V.A.C.
Figure 10.
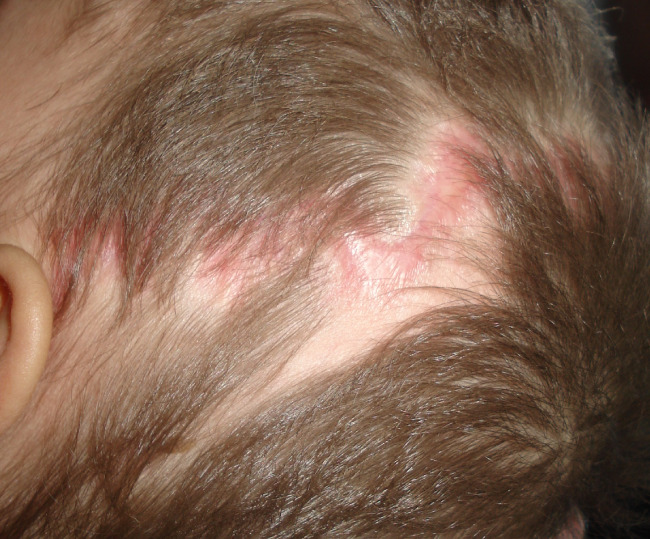
Coronal dehiscence 10 days after V.A.C. Therapy.
One patient experienced severe traumatic injuries, with loss of substance in the zygomatic‐temporal region, not resolvable with local flaps. V.A.C. Therapy was immediately initiated after surgical treatment of multiple facial fractures and complicated skin tears. Even here, we renewed medication every 48–72 hours.
In patients at a high risk of surgical wound complications, NPWT was applied to prevent inconveniences. The therapy was initiated in situ in the operating room immediately after surgical treatment; it was renewed once, if necessary, after about 7 days.
Results
The study group consisted of 11 males and 5 females, aged between 9 month and 78 years; two paediatric patients were included (Table 2).
Table 2.
Evaluation parameters in patients treated with Negative Pressure Wound Therapy
| Patient no. | Healing | Application/hospitalisation time (days) | Timing of medication renewal | ICU stay (days) | Patient comfort and compliance |
|---|---|---|---|---|---|
| 1 | Complete | 22/25 | 9 | 9 | 3 |
| 2 | Complete | 21/25 | 9 | 9 | 3 |
| 3 | Complete | 14/33 | 6 | 18 | 4 |
| 4 | Complete | 5/16 | 2 | – | 5 |
| 5 | Complete | 6/13 | 3 | – | 3 |
| 6 | Partial | 14/24 | 5 | 14 | 5 |
| 7 | Complete | 7/37 | 4 | 2 | 5 |
| 8 | Complete | 5/17 | 7 | – | 5 |
| 9 | Partial | 14/43 | 6 | 2 | 4 |
| 10 | Partial | 17/56 | 6 | 5 | 4 |
| 11 | Partial | 8/40 | 3 | 2 | 5 |
| 12 | Complete | 15/20 | 6 | – | 3 |
| 13 | Complete | 10/24 | 3 | 7 | 5 |
| 14 | Complete | 4/25 | 1 | – | 4 |
| 15 | Complete | 5/31 | 1 | – | 5 |
| 16 | Complete | 14/42 | 5 | – | 3 |
Of 16 patients with complex wounds in the cervical‐cranial‐facial region, 12 (75%) achieved a full recovery. V.A.C. Therapy was effective in gaining a complete restitutio ad integrum in every patient included in the group of cervicofacial infectious disease; according to the literature, it demonstrated high efficiency in decreasing local oedema, exudate and bacterial load 8, 15, 16.
In cases where NPWT could not achieve complete recovery, it was useful to reduce wound area and prepare the wound bed for further treatments. Four patients underwent local advanced dressing over the residual defect, whereas a cutaneous graft was necessary in only two cases.
The precautionary use of NPTW showed no local signs of dehiscence, liquid gathering or infection; total restoration was achieved.
V.A.C. Therapy application time ranged from 4 to 22 days (mean of 11·57 day), with 4.6 average renewals in every study group; the hospitalisation period included between 5 and 56 days, with an average of 28·0.19 days. Therefore, V.A.C. Therapy application time averagely occupied 42% of time of hospital stay, excluding cases with prosecution of treatment after discharge.
Patients affected by infectious disease or necrotising fasciitis underwent V.A.C. Therapy from 5 to 22 days (average time of 12·0.64 days); hospital stay ranged from 13 to 33 days, with a mean time of 30·0.1 days. Three patients were admitted to the ICU, with length of hospital stay of between 9 and 18 days, with a mean of 12 days. Ratio of hospitalisation and days of NPWT was 60·7%.
Oncological patients with complex wounds were treated in a range of 7–17 days (mean of 10.75), while length of hospital stay was between 17 and 56 days (38·25 average), often prolonged by the general state of health complications.
There is no evidence of heterogeneity in the remaining clinical cases.. V.A.C. Therapy has, however, been well tolerated in our series; this is probably due to the decreased number of application, the ease of use and the comfort of the system relative to traditional dressing.
Discussion
Physiological wounds healing passes through several stages; it can be simplified in the processes of haemostasis, inflammation, cell proliferation and differentiation. Steady scientific research has led to a greater comprehension of those courses through the years, allowing the development of new medication techniques simultaneously. Despite these developments, best outcomes result from a synergistic surgical, medical and nursing approach 17.
Complex wounds are lesions whose healing is made difficult by several factors: patient health status, local injury conditions and anatomical area of the wound. It has been clearly demonstrated how an effective vascular provision is a favourable prognostic factor for healing; so, it is obvious how reduced peripheral blood flow in diabetic, vasculopatic or radiotherapic patients constitutes a concrete worsening of the healing course. In a similar way, infection jeopardises the recovery process: a high bacterial load prolongs inflammatory phase, hindering the formation of granulation tissue. Again, adverse local circumstances, such as unfavourable temperature or humidity and wound edges strain, resulting in ischaemia, hematomas or seromas compromising anatomical planes adhesion, are the most frequent local complications 18, 19.
Traditional dressings aim to cover and protect wounds, absorbing exudate and restricting any bleeding; advanced medications biologically interact with underlying tissue by means of their biocompatibility. Alginates, hydrocolloids and hydrofibres allow optimum moisture, leading to quicker tissue repair. Although there is no standard dressing suitable for every injury, the general indication is to use a simpler but more durable medication; NPWT aims to fill the shortcomings of traditional and advanced medications when a complicated treatment is needed.
Increasingly technical and demanding surgical treatments are performed on older patients, synergistically enhancing the management. Furthermore, in serious head and neck infectious diseases, few other therapeutic options have the intrinsic valuable characteristics of NPWT: it continuously removes exudate and cellular debris quickly and considerably reduces oedema and excellently insulates wound 20, 21, 22, 23, 24, 25.
Finally, with osteosynthesis exposure, NPWT leads to plentiful granulation tissue, richer vascularisation and less shear stress on wounds edge 24, 26, 27, 28.
Our study proposed to assess an advanced medication system on the healing of complex wounds, which is able to cope with aggravating circumstances that slow or place doubt on outcomes. Our results argue for the benefit of NPWT use in traumatic, oncological, malformative and, above all, infectious complex wounds.
A complete healing was achieved in most cases, whereas remaining patients reached a more‐than‐adequate clinical improvement; average application times in every study group appear to be lower, compared to traditional dressing, both in oncological surgery and in infectious disease (based on authors' personal experience due to ethical implications opposed to the establishment of a control group). This value, strictly related to this technique's cost, has to be compared to savings in hospitalisation days.
NPWT was, together with medical and surgical therapy, a treatment mainstay for those affected by cervical infectious disease: application of NPWT appears to have been crucial for the prognosis. Disease rapidly regressed, and only one patient needed a surgical tracheostomy 29, 30.
ICU stay was necessary for continuous monitoring of cardiovascular homeostasis in patients susceptible to septic shock, in addition to maintaining pharyngo‐laryngeal patency. It should be emphasised that dressing changes were carried out at more deferred time ranges, compared with traditional methods; this is due to exudate and necrotic debris removal, decrease of bacterial load and maintenance of a favourable environment at the site of injury. Moreover, painfulness decreased, and symptoms resolution occurred earlier 31, 32.
Considering, whenever possible, the comfort of NPWT. and its logistical implications, NPWT application constitutes an undeniable advantage in terms of both in‐hospital and home handling care. Therefore, our results are in agreement with international literature 33.
There are few economic assessments about wound management, owing to few clinical or longitudinal studies; moreover, many factors are relevant to treatment choice but become insignificant for economic evaluations. Research linking intermediate‐ to long‐term outcomes are now emerging 34, but data are insufficient to carry out economic studies.
Long‐term evaluations will contribute to determining economic viability; an analysis made by Harding et al. shows how dressing can affect up to 29% of the whole economic costs of pressure ulcers 35. It has been shown that dressing with the lowest acquisition cost has led to the highest total expenditure at the end of the healing process because of the relative effectiveness.
Analysing dressing costs, frequency of dressing changes, nursing time, healing rates and impact on hospitalisation are far more relevant and must be considered.
Conclusion
This study evaluated outcomes of NPWT in cranio‐maxillofacial‐cervical region, in different types of lesion and comorbidities of the subject.
Results were satisfactory for most of cases treated; faster and more effective wound healing was achieved. The adherence of a negative pressure gradient led to an acceptable decrease in exudate and bacterial load, in addition to an increase of wound bed vascularisation; this was logically anticipated by an adequate medical therapy besides the surgical debridement of necrotic or contaminated tissue. Furthermore, we obtained an early primary intention wound closure, passing through a rapid formation of the granulation tissue and a consequent decrease of the injury volume.
The lower number of NPWT application, relating to standard dressings, led to an increase of patients comfort and compliance and less use of medical, and in some cases economic, resources, according to international literature
Acknowledgements
The authors have no proprietary or commercial interest in materials discussed in this article. None of the authors has any conflicts of interest to disclose.
References
- 1. Chaffin R. Drainage. Am J Surg 1934;40:100. [Google Scholar]
- 2. Von Leden H, Kaplan J. Closed wound suction in head and neck surgery. Arch Otolaryngol 1962;75:103–7. [DOI] [PubMed] [Google Scholar]
- 3. Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg 1998;51:79. [DOI] [PubMed] [Google Scholar]
- 4. Moloney GE. Apposition and drainage of large skin flaps by suction. Aust N Z J Surg 1957;26:173–9. [DOI] [PubMed] [Google Scholar]
- 5. Silvis RS, Potter LE, Robinson DW, Hughes WF. The use of continuous suction negative pressure instead of pressure dressing. Ann Surg 1955;142:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breslau RC, Pories WJ, Schwartz SI. A portable technique for the maintenance of constant sterile postoperative wound suction. Surgery 1959;46:711–7. [PubMed] [Google Scholar]
- 7. McLean WC. The role of closed wound negative pressure suction in radical surgical procedures of the head and neck. Laryngoscope 1964;74:70–94. [DOI] [PubMed] [Google Scholar]
- 8. Fleischmann W, Strecker W, Bombelli M, Kinzi L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurgr 1993;96:488–92. [PubMed] [Google Scholar]
- 9. Morykwas MJ, Argenta LC, Schelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 10. Orgill DP, Bayer L, Neuwalder J, Felter R. Microdeformational wound therapy – a new era in wound healing. Business Briefing: Global Surgery – Future directions. 2005:1–3.
- 11. De Franzo AJ, Argenta LC, Marks MW. The use of vacuum assisted closure therapy for the treatment of lower extremity wounds with exposed bone. Plast Reconstr Surg 2001;108:1184. [DOI] [PubMed] [Google Scholar]
- 12. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 13. Schuster Moradzadeh A, Waxman K. The use of vacuum assisted closure therapy for the treatment of a large infected facial wound. Am Surg 2006;129:31. [PubMed] [Google Scholar]
- 14. Mendez‐Eastman S. Guidelines for using negative pressure wound therapy. Adv Skin Wound Care 2001;14:314–22. [DOI] [PubMed] [Google Scholar]
- 15. Dosluoglu HH, schimpf DK, Schultz R, Cherr GS. Preservation of infected and exposed vascular grafts using vacuum assisted closure without muscle flap coverage. J Vasc Surg 2005;42:989–92. [DOI] [PubMed] [Google Scholar]
- 16. Mehbod AA, Ogilvie JW. Postoperative deep wound infections in adults after spinal fusion: management with vacuum assisted closure. J Spinal Disord 2005;18:14–7. [DOI] [PubMed] [Google Scholar]
- 17. Fisher E, Frodel JL. Wound healing, 2nd edn. New York: Thieme, 2002. [Google Scholar]
- 18. Riou JP, Cohen JR, Johnson H. Factors influencing wound dehiscence. Am J Surg 1992;163:324–30. [DOI] [PubMed] [Google Scholar]
- 19. Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care 2004;17:426–35. [DOI] [PubMed] [Google Scholar]
- 20. Adamkova M, Tymonova J, Kladlcick M, Klosova H. First experience with the use of vacuum assisted closure in treatment of skin defects at the burn center. Acta Chir Plast 2005;47:24–7. [PubMed] [Google Scholar]
- 21. Borgquist O, Ingemansson R, Malmsjo M. The influence of low and high pressure levels during negative pressure wound therapy on wound contraction and fluid evacquation. Plast Reconstr Surg 2011;127:551–9. [DOI] [PubMed] [Google Scholar]
- 22. Lubanaris AP, Polykandriotis E, Horch RE. The effect of vacuum assisted closure in lymph vessels in chronic wounds. J Plast Reconstr Aesthet Surg 2009;62:1068–75. [DOI] [PubMed] [Google Scholar]
- 23. Novelli G, Catanzaro S, Canzi G, Sozzi D, Bozzetti A. Vacuum assisted closure therapy in the management of cervico‐facial necrotizing fasciitis: a case report and review of the literature. Minerva Stomatol 2014;63:135–44. [PubMed] [Google Scholar]
- 24. Orgill DP, Manders EK, Sumpio BE. The mechanism of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 25. Yang CC, Chang DS, Web LX. Vacuum assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Orthop Adv 2006;15:19–23. [PubMed] [Google Scholar]
- 26. Kairinos M, Solomons M, Hudson DA. The paradox of negative pressure wound therapy – in vitro studies. J Plast Reconstr Aesth Surg 2010;63:174–9. [DOI] [PubMed] [Google Scholar]
- 27. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 28. Scherer SS, Pietramaggriori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- 29. Asher SA, White HN, Golden JB, Magnuson JS, Carroll WR, Rosenthal EL. Negative pressure wound therapy in head and neck surgery. JAMA Facial Plast Surg 2014;16:120–6. [DOI] [PubMed] [Google Scholar]
- 30. Palm HG, Hauer T. Vacuum assisted closure of head and neck wounds. HNO 2011;59:819–30. [DOI] [PubMed] [Google Scholar]
- 31. Huang WS, Hsieh SC. Use of vacuum assisted wound closure to manage limb wounds in patients suffering from acute necrotiziong fasciitis. Asian J Surg 2006;29:135–9. [DOI] [PubMed] [Google Scholar]
- 32. Oczenski W, Waldenberger F. V.A.C. for the treatment of cervical and mediastinal necrotizing fasciitis. J Cardiothorac Anesth 2004;18:336–8. [DOI] [PubMed] [Google Scholar]
- 33. Jeroen DD, Vuerstaek MD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State of the art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–38. [DOI] [PubMed] [Google Scholar]
- 34. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med 2003;115:627–31. [DOI] [PubMed] [Google Scholar]
- 35. Harding K, Cutting K, Prinece P. The cost‐effectiveness of wound management protocols of care. Br J Nurs 2000;9(19 Suppl):S6–24. [DOI] [PubMed] [Google Scholar]


