Abstract
Acral lentiginous melanoma affects the palms, soles, and nail apparatus. Around 3–15% of all cutaneous melanomas are located on the foot and have a poorer prognosis than melanoma elsewhere. Possible reasons for this prognostic difference may be omitting this area during routine skin check by both the patient and the physicians, in addition to misdiagnosis of melanoma as other benign skin lesions. We describe here an elderly female patient treated for a non‐healing foot ulcer interpreted as a diabetic ulcer, which after 2 years was diagnosed as acral melanoma with satellitosis. Histopathological examination of the amputated distal phalanx revealed an advanced stage melanoma with 1·2 cm Breslow thickness and of Clark level 5. Dermoscopy of the bluish papulonodules scattered on the dorsal foot showed characteristic findings described for metastasis of skin melanoma.
This case underlines the importance of considering skin malignancies in case of chronic, non‐healing ulcers in diabetic patients. Furthermore, we point out the critical significance of skin examination as a whole, and dermoscopy being an important tool in the diagnosis of melanoma and/or cutaneous melanoma metastasis.
Keywords: Acral lentiginous melanoma, Diabetic foot, Dermoscopy, Foot ulcer
Introduction
Acral melanoma affects the palms, soles and nail apparatus and is relatively rare in the white populations. Around 3–15% of all cutaneous melanomas are located on the foot and have a poorer prognosis than melanoma elsewhere 1. Possible reasons for this prognostic difference may be omitting this area during routine skin check both by the patient and the physicians, in addition to misdiagnosis of melanoma for other benign skin lesions 1, 2. We describe here a female diabetic patient who presented with acral melanoma with satellitosis masquerading as a diabetic ulcer for 2 years.
Case report
A 87‐year‐old diabetic woman was referred to the wound healing outpatient clinic of our hospital, with a non‐healing, painless ulcer on her left foot of 2·5 years duration. Her long‐standing type 2 diabetes was well‐controlled with oral antidiabetics. The lesion had begun as a dark spot under the nail, which the patient had related with a fall, and then a small ulcer had developed nearby. The ulcer was managed with local wound care, and systemic and topical antibiotics for nearly 2 years by non‐dermatologist physicians and nurses from the local health services. Physical examination by the cardiovascular surgeon from the wound clinic revealed a necrotic ulcer on the dorsal surface of the fifth toe. The lesion was restricted to the distal phalanx, destructing the nail unit completely (Figure 1A). Arterial Doppler ultrasonography detected atherosclerotic irregularities of vascular walls but no overt occlusion or stenosis. On prediagnosis of refractory diabetic ulcer, distal phalanx was amputated and sent to the pathology laboratory for histological evaluation. During this period some papules and nodules scattered on the dorsal foot attracted attention and dermatological consultation was requested. The patient declared that these lesions had developed slowly over the previous year. On dermatological examination, several greyish blue, hard, asymptomatic papules and nodules of 3–10 mm in diameter were seen on the distal dorsal foot, neighbouring the ulcer (starting from close proximity to the ulcer, and extending to dorsal foot) (Figure 1B). Dermoscopy of these lesions revealed homogenous, globular and structureless areas (Figure 2). Histopathological examination of the amputated phalanx showed ulcerated nodular melanoma with a maximum width of 3 cm, Clark level 5, Breslow thickness 1·2 cm, mitosis rate > 4/mm2 and Ki‐67 rate 20%. While lymphatic invasion and a non‐brisk tumoural lymphocytic infiltration were positive; regression, perineural invasion and microsatellites were negative (Figure 3). Staining with HMB45 (DAKO, code IS052, California, USA) and S‐100 protein Ab‐1 (Lab Vision Corporation, Fremont, USA) revealed strong positivity. Acral melanoma with macroscopic satellitosis was diagnosed. Complete blood cell count and blood biochemistry showed profound anaemia (Hb: 8·72 g/dl, Htc: 26·6%, erythrocytes 3·2 M/µl), leukocytosis, neutrophilia, high blood urea nitrogen (76 mg/dl), high CRP (61·84 mg/l) and high serum creatinine level (3·7 mg/dl). Thoracic computerised tomography revealed hilar and mediastinal multiple lymph nodes. During the time of these investigations, the patient developed pneumonia and acute renal insufficiency, requiring hospitalisation at the intensive care unit. These severe intervening problems prevented further examinations and treatment for melanoma. At the time of this writing, the patient was still under treatment in hospital for several underlying systemic conditions.
Figure 1.
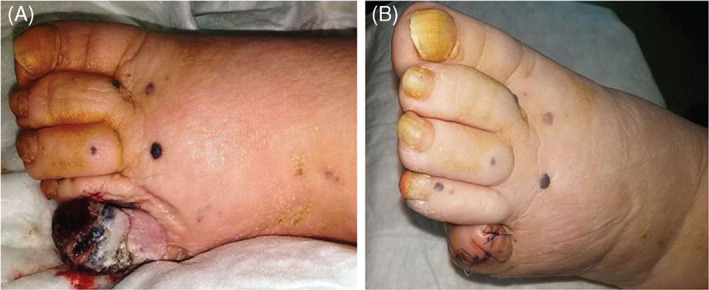
(A and B) Clinical presentation of the lesions before and after digital amputation.
Figure 2.
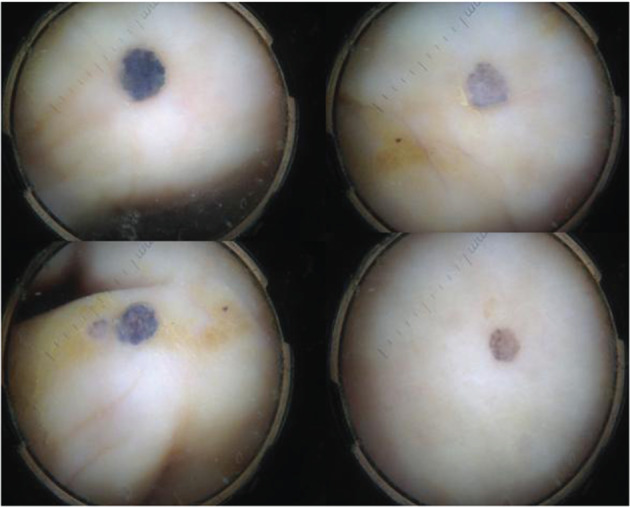
Dermoscopic features of satellite lesions revealed homogenous, globular and structureless blue‐gray areas.
Figure 3.
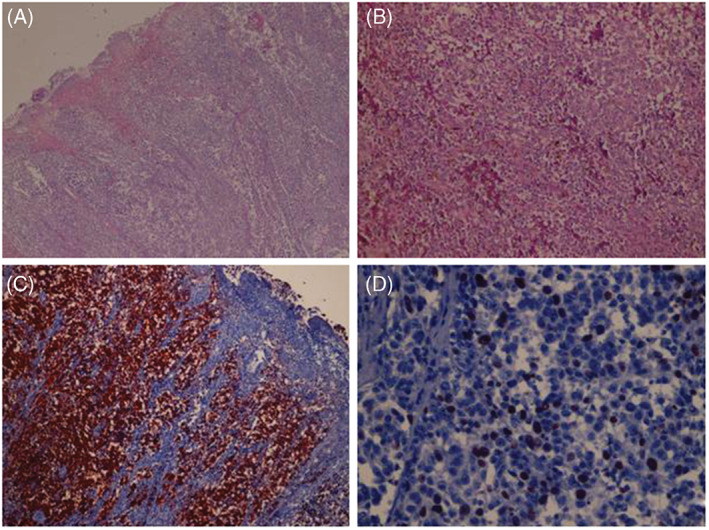
(A) Nodular tumoural lesion causing destruction of the surface epithelium and ulceration (H & E, ×50). (B) Closer view shows large neoplastic cells with prominent nucleoli and brownish cytoplasmic melanin pigment (H&E, ×200). (C) Neoplastic cells reveal diffuse HMB45 positivity (immunoperoxidase, HMB45, ×200). (D) 20% of neoplastic cells demonstrate Ki‐67 positivity (immunoperoxidase, Ki‐67, ×200).
Discussion
Misdiagnosis of melanoma is a relatively common feature. Of the various melanoma types, acral melanoma has been the one with a high reported rate of misdiagnosis between 25% and 36% 3. Its location and atypical clinical presentation, in addition to ignorance of the early signs by both the patients and physicians may account for this high rate. Age is another risk factor, and misdiagnosed cases occur predominantly in the sixth decade, reflecting the relative frequency of underlying vascular and neurological disorders in older ages. Moreover, this type of melanoma is commonly amelanotic, frequently ulcerates and lacks the classic signs of melanoma associated with the ‘ABCD’ (asymmetry, border, colour, diameter) criteria 4, 5. Reported misdiagnoses include onychomycosis, ingrowing toe nail, paronychia, warts, callus, benign tumours, subungual hematoma, traumatic lesions and ischemic or neuropathic ulcers 1. Misdiagnosis is particularly likely in cases of amelanotic melanoma. There are less than 20 melanoma cases misdiagnosed as diabetic foot ulcers in the literature 1, 2, 5, 6, 7, 8, 9, 10, 11. Most of them are plantar ulcers, mimicking mal perforans 4. This may be due to the fact that the most common site for foot melanomas is plantar aspect 1, 3.
In our case, because of underlying diabetes mellitus and old age, the ulcer was attributed to ischemic necrosis due to microangiopathy, precipitated by diabetes and atherosclerosis. As the physicians taking care of the patient were not dermatologists, the nature of the ulcer was not recognised properly. Moreover, although our patient had been having satellite lesions on the foot for nearly a year, neither the patient nor the physicians paid attention to them. Similar to the primary lesions, these satellite lesions were asymptomatic, probably another reason for being neglected. Satellite lesions indicated a late stage of disease and a thick primary tumour, which was confirmed by histopathological findings.
Dermoscopy of pigmented lesions provided a great assistance for accurate diagnosis. Dermoscopic criteria for the diagnosis of melanoma metastasis have been described in recent years, in several reports on this issue 12, 13. According to Bono et al., homogenous pattern, saccular pattern and vascular pattern (polymorphic atypical vessels and windings vessels), together with pigmentary halo and peripheral grey spots are the most significant features for diagnosis of melanoma metastases 12. Focusing on the analyses of dermoscopic patterns, Costa et al. described six dermoscopic patterns of melanoma metastases [blue nevus‐like, angioma‐like, nevus‐like (globular and non‐globular), vascular and unspecific patterns] 13. Our patient showed a relatively similar dermoscopic pattern in all these satellite cutaneous metastases, with a homogenous, blue nevus pattern and/or nevus‐like globular pattern. The clinical and dermoscopic similarities supported the diagnosis of metastasis from the primary tumour.
Early and accurate diagnosis and treatment of melanoma are paramount components of a successful treatment, and improve the survival rate of the patients. Unfortunately this was not the case in this patient. Melanomas misdiagnosed as foot ulcer share some features such as aggressive course and rapidly occurring metastases, probably due to delayed diagnosis.
To prevent misdiagnosis of foot melanoma, a high index of suspicion is necessary. As the prevalence of diabetes mellitus has been increasing steadily, foot care of these patients are usually carried on by primary care providers. Clinicians treating diabetic foot wounds including podiatrists, family practitioners and general physicians should be aware of the possibility of malignancy, including melanoma. Especially in ulcers with atypical clinical features that resist standard treatment modalities, biopsy is required. We also suggest biopsy of those ulcers without signs of neuropathia or ischaemia, and ulcers showing pigmentation.
References
- 1. Soon SL, Solomon AR Jr, Papadopoulos D, Murray DR, McAlpine B, Washington CV. Acral lentiginous melanoma mimicking benign disease: the Emory experience. J Am Acad Dermatol 2003;48:183–8. [DOI] [PubMed] [Google Scholar]
- 2. Kong MF, Jogia R, Jackson S, Quinn M, McNally P, Davies M. Malignant melanoma presenting as a foot ulcer. Lancet 2005;366:1750. [DOI] [PubMed] [Google Scholar]
- 3. Fortin PT, Freiberg AA, Rees R, Sondak VK, Johnson TM. Malignant melanoma of the foot and ankle. J Bone Joint Surg Am 1995;77:1396–403. [DOI] [PubMed] [Google Scholar]
- 4. Bristow IR, Acland K. Acral lentiginous melanoma of the foot and ankle: a case series and review of the literature. J Foot Ankle Res 2008;1:11. DOI: 10.1186/1757-1146-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers LC, Armstrong DG, Boulton AJ, Freemont AJ, Malik RA. Malignant melanoma misdiagnosed as a diabetic foot ulcer. Diabetes Care 2007;30:444–5. [DOI] [PubMed] [Google Scholar]
- 6. Yesil S, Demir T, Akinci B, Pabuccuoglu U, Ilknur T, Saklamaz A. Amelanotic melanoma misdiagnosed as a diabetic foot ulcer. J Diabetes Complications 2007;21:335–7. [DOI] [PubMed] [Google Scholar]
- 7. Hussin P, Loke SC, Noor FM, Mawardi M, Singh VA. Malignant melanoma of the foot in patients with diabetes mellitus – a trap for the unwary. Med J Malaysia 2012;67:422–3. [PubMed] [Google Scholar]
- 8. Mrozikiewicz‐Rakowska B, Bizon M, Chojnowska N, Karnafel W. Malignant melanoma or diabetic foot syndrome? Diabetic Foot Canada 2014;2:36–8. [Google Scholar]
- 9. Scalvenzi M, Palmisano F, Costa C. Misdiagnosed, mistreated and delay diagnosed acral melanoma: the atypical presentations. J Clin Exp Dermatol Res 2012;S6:002. [Google Scholar]
- 10. Torres T, Rosmaninho A, Caetano M, Selores M. Malignant melanoma misdiagnosed as a diabetic foot ulcer. Diabet Med 2010;27:1302–3. [DOI] [PubMed] [Google Scholar]
- 11. Thomas S, Meng YX, Patel VG, Strayhorn G. A rare form of melanoma masquerading as a diabetic foot ulcer: a case report. Case Rep Endocrinol 2012;2012:502806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bono R, Giampetruzzi AR, Concolino F, Puddu P, Scoppola A, Sera F, Marchetti P. Dermoscopic patterns of cutaneous melanoma metastases. Melanoma Res 2004;14:367–73. [DOI] [PubMed] [Google Scholar]
- 13. Costa J, Ortiz‐Ibañez K, Salerni G, Borges V, Carrera C, Puig S, Malvehy J. Dermoscopic patterns of melanoma metastases: interobserver consistency and accuracy for metastasis recognition. Br J Dermatol 2013;169:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


