Abstract
Growth factor (GF) therapy has shown promise in treating a variety of refractory wounds. However, evidence supporting its routine use in burn injury remains uncertain. We performed this systematic review and meta‐analysis assessing randomised controlled trials (RCTs) to investigate efficacy and safety of GFs in the management of partial‐thickness burns. Electronic searches were conducted in PubMed and the Cochrane databases. Endpoint results analysed included wound healing and scar formation. Thirteen studies comprising a total of 1924 participants with 2130 wounds (1131 GF receiving patients versus 999 controls) were identified and included, evaluating the effect of fibroblast growth factor (FGF), epidermal growth factor (EGF) and granulocyte macrophage‐colony stimulating factor (GM‐CSF) on partial‐thickness burns. Topical application of these agents significantly reduced healing time by 5·02 (95% confidence interval, 2·62 to 7·42), 3·12 (95% CI, 1·11 to 5·13) and 5·1 (95% CI, 4·02 to 6·18) days, respectively, compared with standard wound care alone. In addition, scar improvement following therapy with FGF and EGF was evident in terms of pigmentation, pliability, height and vascularity. No significant increase in adverse events was observed in patients receiving GFs. These results suggested that GF therapy could be an effective and safe add‐on to standard wound care for partial‐thickness burns. High‐quality, adequately powered trials are needed to further confirm the conclusion.
Keywords: Growth factor, Partial‐thickness burn, Wound healing
Introduction
Burn injuries are a global public health problem, accounting for an estimated 300 000 deaths throughout the world each year 1. Partial‐thickness (grade II) burn is a common clinical burn, anatomically involving the epidermal layer as well as a varying thickness of the dermis, further subclassified into superficial (grade IIa) and deep (grade IIb) partial‐thickness 2. Although a grade II burn is generally non‐fatal and heals with standard wound care, the impaired wound healing and hypertrophic scar formation can severely undermine the quality of survival in patients 3.
Healing of a burn wound is a dynamic process, involving a series of complex cellular and molecular events with a great degree of overlap and interdependence 4. Polypeptide growth factors (GFs) are a cluster of multifunctional peptides, playing fundamental roles in this process: by stimulating cellular and chemotaxis proliferation, by providing signalling among cells of the same and different type, by controlling extracellular matrix formation and angiogenesis, by regulating the process of contraction and by reestablishing tissue integrity during tissue repair 5, 6, 7. However, the bioavailability of GFs is generally insufficient in the wound bed of burns because of diminished synthesis and/or excessive degradation 8.
Evidence from various in vivo studies supported that exogenous GFs serve in multiple capacities of burn wound healing 9. In 1986, Brown et al. 10 first demonstrated that biosynthetic human EGF accelerated epidermal regeneration in porcine models of partial‐thickness burns. Study by Danilenko et al. 11 demonstrated that application of KGF‐1 (also known as FGF‐7) in the same model displayed a more significant increase in new epithelial area (KGF‐treated versus control, P < 0·0001) than EGF, and a modest increase in reepithelialisation (KGF‐treated versus control, P = 0·09), due to its marked stimulation of both epidermal and follicular proliferation. In a recent study by Galeano et al. 12, up‐regulated expression of vascular EGF (VEGF) in deep partial‐thickness burn wounds through a recombinant adeno‐associated virus‐mediated gene delivery system (vectors‐VEGF165) was proved to increase wound content of nitrate, epithelial proliferation, angiogenesis, maturation of the extracellular matrix and activation of nitric oxide synthesis. In terms of scarring, Xie et al. 13 demonstrated that basic FGF (bFGF)‐treated scars showed a better process of skin remodelling, which may avoid the subsequent development of fibro‐proliferative disorders.
In the past 20 years, cloned recombinant form, commercially approved GF products have been used in the management of a variety of refractory wounds such as chronic venous ulcers 14, 15, 16, diabetic foot ulcers 17, 18, 19, 20 and pressure ulcers 21, 22, and have provided positive clinical benefit. A recently published review article presented a comprehensive discussion on the potential therapeutic applications of GFs on burn injuries 23. However, due to the limitation of narrative reviews, its conclusion was qualitative. Quantitative meta‐analyses of randomised controlled trials (RCT) are recommended to substantiate knowledge about the effectiveness of a treatment by pooling data from smaller studies that do not always have enough power on their own to give clear statistical significance 24. We performed this systematic review and meta‐analysis with RCT evidence of the effect of GF therapy on the management of partial‐thickness burns. The outcomes were evaluated with emphasis on efficacy and safety of GF therapy in wound healing, scar formation and adverse reaction, compared with traditional standard wound care alone.
Methods
Search strategy and eligibility criteria
All prospective RCTs of GF therapy in the management of partial‐thickness burns in patients treated for a minimum of 1 week were identified and selected. National Library of Medicine (PubMed) and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library were searched for all publications up to January 2014 using Boolean expressions combining MeSH terms without language restriction: [Burns (Mesh) OR burn injury OR burn intervention OR burn scar] AND [Growth factor (Mesh) OR biologic agent OR biologic treatment OR biologic therapy OR cytokine therapy]. Study eligibility criteria are listed in Table 1. Titles and abstracts were first screened independently by two reviewers (YZ and JH), and discrepancies were resolved by consensus after consultation with the senior author (JD). Selected articles from this screening underwent subsequent independent full‐text reviews. The references of all articles selected for full‐text review were reviewed manually to identify other potentially appropriate publications that matched our criteria. Multiple articles from the same institution and/or author were analysed carefully to ensure that no patients were duplicated in the analysis, and only the most recent or most inclusive article was included.
Table 1.
Eligibility criteria for the inclusion in the meta‐analysis
| Inclusion criteria | 1 | Randomised controlled trial comparing growth factor (GF) therapy with standard wound care alone for partial‐thickness burns |
| 2 | Sufficient data reported for at least one clinical endpoint of interests | |
| 3 | Number of patients and wounds reported for each arms | |
| Exclusion criteria | 1 | In vitro experiments or animal studies |
| 2 | Articles from the same institution and/or author with patients duplicated | |
| 3 | Articles with less than 10 cases examined for each arm |
Endpoint outcomes
Primary outcomes analysed included the average healing time in days or percentage reductions in the measured wound size. Secondary outcomes analysed were hypertrophic scars using the Vancouver Scar Scale (VSS) 25, and any potential adverse events (AEs) relative to wound therapy (i.e. toxic side effect, allergic reaction, wound infection and severe systemic reaction).
Data extraction
Data extraction was performed independently by two reviewers (YZ and TW), and discrepancies were resolved by consensus. Data extracted from each trial referred to the name of the first author, year of publication, location of the study, intention‐to‐treat (ITT) population, gender distribution, mean age (years), burn depth, wound numbers for each arms, total body surface area (TBSA) (%), intervention (agent type, dose, route, timing and duration of administration), data regarding the effectiveness and safety of compared treatments (time to healing, reduction in wound size, assessment of hypertrophic scars and any reporting AEs) and potential conflict of interests (COI) announced on the publication. The authors were contacted by phone or e‐mail when information was inadequate in the articles.
Quality scoring and risk of bias assessment
Assessment of the methodological quality of the included trials was performed according to the Jadad scoring system by methods of random allocation (up to 2 points), blinding (up to 2 points) and patient withdrawals (up to 1 point) 26. A study can obtain 0–5 points based on the criteria. A threshold of 3 points or above is considered as indicative for high quality. Risk of bias assessment in RCTs was performed according to the Cochrane Methodology under consideration of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential sources of bias 27. Each category was scored as low, unclear or high risk of bias and expressed in a summary table with a plus, question mark and minus, respectively. Publication bias was formally assessed with the Begg's test. All the scoring was performed independently by two reviewers (YZ and JH), and any disagreement was resolved by consensus.
Statistical methods
The statistical analysis was performed using STATA version 12 software (Stata Corp, College Station, TX). Continuous variables were reported as weighted mean difference (WMD) with 95% confidence intervals (CI), whereas dichotomous data were reported as odds ratios (OR) with 95% CI. The heterogeneity was tested with the chi‐square‐based Cochran's statistic and the inconsistency index (I 2) 28. Statistically significant heterogeneity was considered present with P heterogeneity < 0·05 or I 2 > 50%. In the presence of substantial heterogeneity, a random effect model (REM) was adopted as the pooling method instead of a fixed effect model (FEM) 29. Subgroup analysis was performed when at least two studies included the considered outcome. Sensitivity analysis was performed by refitting the estimated OR omitting one study at a time. Statistical significance was indicated by P‐value < 0·05.
Results
Search and study selection
A flowchart of the selection process is shown in Figure 1. A total of 224 potentially eligible articles were identified in the literature search. After application of the inclusion and exclusion criteria described previously, 190 articles were initially excluded on the basis of the title and abstract. The remaining 34 publications underwent detailed evaluation of the full text, and 21 were proved not eligible: 5 for not reporting on outcomes of interests, 7 for data unavailable, 3 for having a small sample size (<10 participants per group) and 6 for reporting on burns of higher grade instead of partial‐thickness. Thus, 13 trials were considered eligible for inclusion.
Figure 1.
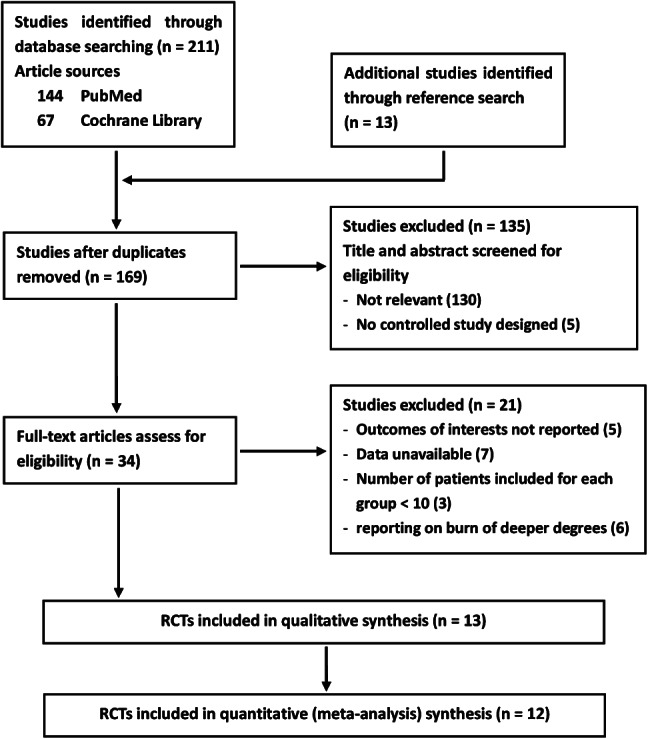
Study selection process.
Characteristics of Included RCTs
The 13 RCTs included were published between 1998 and 2012, and comprised a total of 1924 participants with 2130 wounds (1131 GF receiving patients versus 999 controls). All patients in the clinical trials included were of Asian (China and Japan) origin. The efficacy and safety of the therapy with the following three agents were evaluated and compared with standard wound care (as control) in treating partial‐thickness burn: fibroblast growth factor (FGF), epidermal growth factor (EGF) and granulocyte macrophage‐colony stimulating factor (GM‐CSF). The methodological features and outcomes measured in the studies are presented in Table 2. The evaluation of funnel plots did not suggest evidence for publication bias (Figure S1–S3, Supporting Information). Pooled analysis showed no statistically significant differences between GFs group and the control group in terms of TBSA (Figure S4). In the primary outcome, time to complete healing was investigated in 12 of the trials included, whereas in 4 trials [3 on EGF 30, 31, 32 and 1 on FGF 33], outcomes from patients of subclassified burn depths (IIa and IIb) were further investigated. Scar formation was evaluated in four studies using VSS [three on FGF 34, 35, 36 and one on EGF 37]. AEs were investigated in seven studies and reported in six [one on FGF 38, two on EGF 31, 32 and three on GM‐CSF 39, 40, 41].
Table 2.
Details of the RCTs included in the meta‐analysis
|
First author and year |
Country |
ITT (T/C) |
Gender (M/F) |
Mean age (years) |
Wound number (T/C) |
Burn depth (degree) |
TBSA (percents) |
Intervention |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
|
FGF‐treated versus control | |||||||||
|
Akita 2008 |
Japan |
51/51 |
57/45 |
57·2 |
119/122 |
II |
T: 8·9 ± 4·3 C: 9·5 ± 5·0 |
Beginning: 2–4 days after burn injury Concentration: 1 µg (120 IU) per cm2 area Frequency: daily Continuing until the wound had completely healed |
WHT: 12·0 ± 2·2 versus 15·0 ± 2·7 days (bFGF versus control, P < 0·01) VSS scores showed significant differences between bFGF‐treated and non‐bFGF‐treated scars (P < 0·01) |
|
Nie 2010 |
China |
44/41 |
60/25 |
32·7 |
59/58 |
IIb |
Comparison I T: 12·2 ± 8·9 C: 10·8 ± 9·7 Comparison II T: 13·1 ± 9·9 C: 15·5 ± 10·1 |
Beginning: immediately after thorough debridement Concentration: 150 AU (262·5 IU) per cm2 area Frequency: daily Continuing for 3 weeks |
WHT: 18·2 ± 4·8 versus 24·2 ± 5·8 days (bFGF with oxygen therapy versus oxygen therapy, P < 0·01); 22·2 ± 6·8 versus 27·3 ± 6·6 days (bFGF versus control, P < 0·01) No statistical difference was found in clinical evaluation of VSS scores between bFGF‐treated and non‐bFGF‐treated scars |
|
Hayashida 2012 |
Japan |
10/10 |
12/8 |
0·7 |
15/15 |
II |
T: 7·7 ± 2·6 C: 8·3 ± 2·9 |
Beginning: immediately after thorough debridement Concentration: 1 µg (120 IU) per cm2 area Frequency: daily Continuing until the wound had completely healed |
WHT: 13·8 ± 2·4 versus 17·5 ± 3·1 days (bFGF versus control, P < 0·01) VSS scores showed significant differences between bFGF‐treated and non‐bFGF‐treated scars (P < 0·01) |
|
Fu 1998 |
China |
300/300 |
312/282 |
34·2 |
300/294 |
IIa and IIb |
<10 |
Beginning: within 2 days of burn injury Concentration: 150 AU (262·5 IU) per cm2 area Frequency: daily Continuing until the burn wounds were closed |
WHT: 9·9 ± 2·5 versus 12·4 ± 2·7 days (IIa burns, bFGF versus control, P < 0·01); 17·0 ± 4·6 versus 21·2 ± 4·9 days (IIb burns, bFGF versus control, P < 0·01) WHR on the 12th, 15th, 21st day: 56·0%, 78·4%, 95·6% versus 67·0%, 89·2%, 99·2%, respectively (IIb burns, aFGF versus control) No adverse effects were seen locally or systemically with bFGF |
|
Ma 2007 |
China |
39/39 |
NA |
37 |
39/39 |
IIb |
T: 3·81 ± 0·43 C: 3·73 ± 0·41 |
Beginning: within 5 days of burn injury Concentration: 100 AU (175 IU) per cm2 area Frequency: daily Continuing until the burn wounds were closed |
WHT: 17·23 ± 0·53 versus 18·92 ± 0·49 days (aFGF versus control, P < 0·01) No significant adverse drug events or adverse drug reactions in this study |
|
EGF‐treated versus control | |||||||||
|
Guo 2010 |
China |
40/40 |
42/38 |
70 |
38/34 |
IIa and IIb |
Comparison I T: 13·9 ± 4·7 C: 12·3 ± 5·5 Comparison II T: 13·9 ± 5·74 C: 14 ± 6·36 |
Beginning: within 1 day of burn injury Concentration: 40 IU per cm2 area Frequency: daily Continuing until complete wound healing occurred |
WHT: 14·30 ± 1·26 versus 16·22 ± 1·40 days (IIa burns, EGF versus control, P < 0·05); 26·11 ± 2·97 versus 29·13 ± 4·99 days (IIb burns, EGF versus control, P < 0·05) No other side reactions were observed in treatment group except for flash stabbing pain (four cases) and pruritus (two cases) |
|
Liao 2003 |
China |
60/60* |
41/19 |
32 |
60/60* |
IIa and IIb |
NA |
Beginning: within 2 days of burn injury Concentration: 40 IU per cm2 area Frequency: daily Continuing until complete wound healing occurred |
WHT: 10·20 ± 2·20 versus 13·20 ± 2·40 days (IIa burns, EGF versus control, P < 0·05); 17·2 ± 3·12 versus 21·1 ± 3·40 days (IIb burns, EGF versus control, P < 0·01) Wound‐edge inflammatory reaction was observed in nine control cases with second‐degree burns |
|
Wang 2002 |
China |
105/105 |
NA |
33·2 |
105/105 |
IIa and IIb |
Comparison I T: 6·85 ± 3·19 C: 6·37 ± 2·92 Comparison II T: 7·66 ± 2·87 C: 7·34 ± 4·01 |
Beginning: immediately after thorough debridement Concentration: 0·8 µg (400 IU) per cm2 area Frequency: daily Continuing until complete wound healing occurred |
WHT: 8·39 ± 2·25 versus 9·52 ± 2·56 days (IIa burns, EGF versus control, P < 0·05); 16·80 ± 2·99 versus 18·27 ± 3·17 days (IIb burns, EGF versus control, P < 0·01) WHR on the 5th, 7th, 9th days: 46·0%, 76·1%, 94·2% versus 38·0%, 64·2%, 84·3%, respectively (IIa burns, EGF versus control) WHR on 12th, 15th, 18th days: 57·9%, 81·3%, 93·9% versus 43·4%, 69·7%, 88·1%, respectively (IIb burns, EGF versus control) |
|
Wang 2003 |
China |
37/37* |
23/14 |
33 |
37/37* |
IIb |
9·33 ± 9·54 |
Beginning: within 1 day of burn injury Concentration: 600 IU per cm2 area Frequency: daily Continuing until complete wound healing occurred |
WHT: 7·19 ± 1·67 versus 8·92 ± 1·78 days (EGF versus control, P < 0·01) The VSS scores evaluated 1 year and 4 years after wound healing are significantly lower in EGF‐treated group than that in the control group |
|
GM‐CSF‐treated versus control | |||||||||
|
Liu 2011 |
China |
58/58* |
36/22 |
32·4 |
58/58* |
IIb |
5·2 ± 2·1 |
Beginning: NA Concentration: NA Frequency: every other day Continuing until complete wound healing occurred |
WHT: 18·41 ± 2·47 versus 23·58 ± 3·35 days (GM‐CSF versus control, P < 0·01) |
|
Wang 2008 |
China |
214/107 |
NA |
NA |
200/102 |
IIb |
<50 |
Beginning: immediately after thorough debridement Concentration: 1 µg per cm2 area Frequency: every other day Continuing for 4 weeks |
WHR on the 8th, 14th, 20th, 28th days: 24·5%, 70·5%, 95·3%, 99·6% versus 15·1%, 51·4%, 84·6%, 97·1%, respectively (GM‐CSF versus control) No side effect was observed |
|
Yan 2012 |
China |
32/33 |
41/24 |
20·6 |
45/8 |
IIb |
<5 |
Beginning: NA Concentration: NA Frequency: daily Continuing until complete wound healing occurred |
WHT: 12·2 ± 5·0 versus 15·5 ± 4·7 days (rhGM‐CSF versus control, P < 0·01) Transient wound pain was reported in three cases of the GM‐CSF group and five cases of the control group WHR on the 7th, 14th day: 27·7%, 85·9% versus 31·8%, 97·5%, respectively (GM‐CSF versus control) No other side reaction was observed during the study |
|
Zhang 2009 |
China |
60/30 |
75/15 |
33·7 |
56/27 |
IIb |
T: 12·4 ± 10·7 C: 16·2 ± 12·2 |
Beginning: NA Concentration: 1 µg per cm2 area Frequency: every other day Continuing for 4 weeks |
WHT: 14·71 ± 0·48 versus 20·48 ± 1·09 days (GM‐CSF versus control, P < 0·01) WHR on the 14th, 20th, 28th days: 84·6%, 97·6% 100·0%, versus 44·4%, 80·2%, 96·7%, respectively (GM‐CSF versus control) There were no significant differences in adverse reactions found between the experimental and the control group |
EGF, epidermal growth factor; FGF, fibroblast growth factor; GM‐CSF, granulocyte macrophage‐colony stimulating factor; ITT, intention‐to‐treat; NA, data not available; T, treated group; C, control group; M, male; F, female; TBSA, total body surface area; VSS, Vancouver scar scale; IU, international unit; WHT: wound healing time; WHR: wound healing rate.
The reference studies are self‐controlled trials.
Quality assessment
According to the Jadad Scoring System, the included RCTs were of moderate to high quality with a mean Jadad score of 3·0 (range, 2·0–5·0). Risk of attrition bias was not present across the studies included. Ten trials did not provide adequate description of allocation concealment, introducing an unclear risk of selection bias. Mild performance bias was present for inadequate description of double blinding in four trials. Unclear risk of detection bias was present for inadequate description of blinded outcome assessment in eight trials. Mild reporting bias was present for missing reporting on endpoint outcomes in three trials (Figure 2). In addition, age was introduced as a participant characteristic for the inclusion criteria of two trials 31, 35, which might be a potential source of bias for the analysis.
Figure 2.
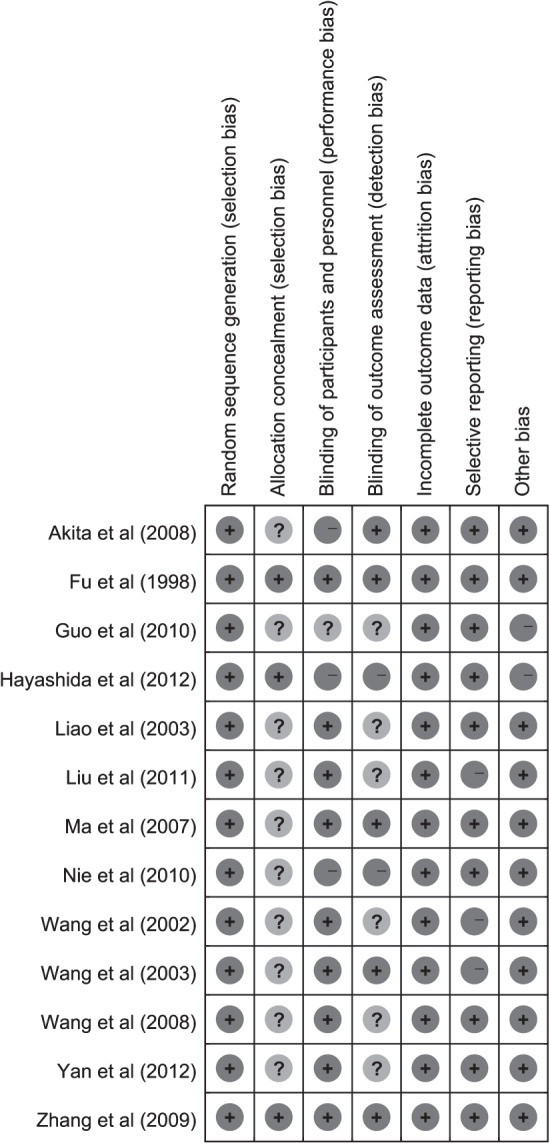
Overall risk of bias assessment.
Fibroblast growth factor
Add‐on therapy with FGF has been examined by five RCTs comprising 885 participants with 1060 wounds (532 FGF versus 528 controls). All the five trials showed significant difference between the arms receiving FGF and control. The study by Fu et al. 33 further reported favourable effect of locally administered bFGF on both superficial and deep partial‐thickness burns (P = 0·0008 and P = 0·0003, respectively). Three of the five trials documented the scarring at half to 1 year of follow‐up using VSS. The studies by Akita et al. 34 and Hayashida et al. 35 favoured FGF therapy over standard wound care for less scarring (P < 0·01 and P < 0·01, respectively), whereas in the study by Nie et al 36, only a favourable trend with marginal statistical significance was presented.
A meta‐analysis under the REM showed significantly shortened healing time for add‐on therapy with FGF as compared with standard wound care used alone (1060 wounds, WMD = −5·02; 95% CI, −7·42 to −2·62, P < 0·01) (Figure 3); significant improvement of scarring with FGF therapy was evident in terms of pigmentation (388 wounds, WMD = −0·85; 95% CI, −1·01 to −0·7, P < 0·01), pliability (388 wounds, WMD = −1·09; 95% CI, −1·63 to −0·54, P < 0·01), height (388 wounds, WMD = −0·83; 95% CI, −1·11 to −0·54, P < 0·01) and vascularity (388 wounds, WMD = −1·01; 95% CI, −1·38 to −0·65, P < 0·01) (Figure 4).
Figure 3.
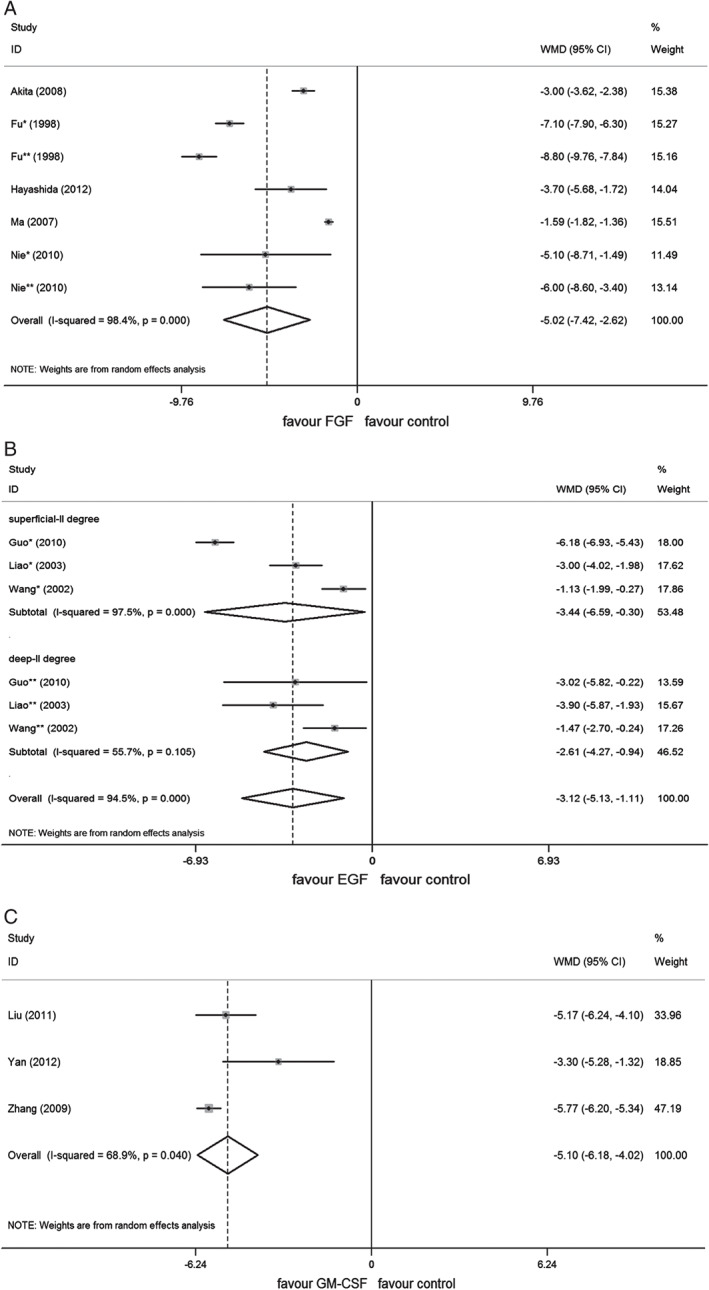
Forest plot depicting the meta‐analysis of wound healing time between growth factors (GFs) versus control group. (A) Fibroblast growth factor (FGF), (B) epidermal growth factor (EGF), (C) granulocyte macrophage‐colony stimulating factor (GM‐CSF). CI, confidence interval; WMD, weighted mean difference; *, comparison of patients with IIa burns; **, comparison of patients with IIb burns.
Figure 4.
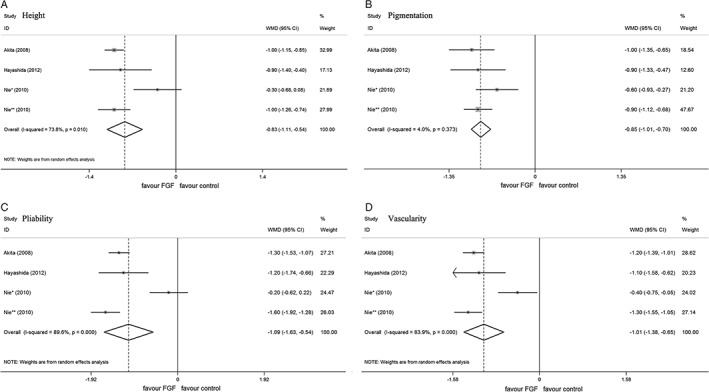
Forest plot depicting the meta‐analysis of Vancouver Scar Scale (VSS) scores between EGF versus control group. (A) Height, (B) pigmentation, (C) pliability, (D) vascularity. CI, confidence interval; WMD, weighted mean difference; *, comparisons of patients with IIa burns; **, comparison of patients with IIb burns.
Epidermal growth factor
Add‐on therapy with EGF has been examined by four RCTs comprising 387 participants with 476 wounds (240 EGF‐treated versus 136 controls). Three of the four trials reported on the complete wound healing time, and all showed significant difference between the arms receiving EGF and control. Wang et al. 30 advocated that the healing acceleration following topical EGF use exhibited a dose‐dependent manner (0·5 µg/g, 12·2 ± 1·5 days versus 10 µg/g, 9·6 ± 2·1 days versus 50 µg/g, 8·4 ± 2·3 days), and recommended 10 µg/g (400 IU/cm2) as an optimal dose regimen with respect to cost‐effectiveness and potential adverse reaction. Similar healing acceleration was achieved in the trials by Liao 32 and Guo 31 with EGF hydrogel of 40 IU/cm2 for both superficial and deep partial‐thickness burns. Study by Wang et al. 37 documented the scar appearance at 1 to 4 years of follow‐up using VSS, and showed significant clinical benefit with the EGF treatment compared with standard wound care used alone (P < 0·01).
A meta‐analysis under the REM showed significantly shortened healing time for add‐on therapy with EGF as compared with standard wound care used alone (402 wounds, WMD = −3·12; 95% CI, −5·13 to −1·11, P < 0·01); Favourable effect of EGF was further observed in the subgroups of both superficial and deep partial‐thickness burns (IIa: 238 wounds, WMD = −3·44; 95% CI, −6·59 to −0·3, P = 0·032; IIb: 172 wounds, WMD = −2·61; 95% CI, −4·27 to −0·94, P < 0·01) (Figure 3).
Granulocyte macrophage‐colony stimulating factor
Add‐on therapy with GM‐CSF has been examined by four randomised, double‐blind, controlled trials comprising 592 participants with 594 wounds (359 GM‐CSF‐treated versus 235 controls). Three of the four trials reported on the complete wound healing time, and all showed significant difference between the arms receiving GM‐CSF and the control. Zhang et al. 41 found significantly higher reduction in wound size for GM‐CSF group (94·64%) compared with that for the control group (51·85%) at the 20th day after therapy (P < 0·01). Similarly, the study of Liu et al. 42 displayed higher reduction in wound size with GM‐CSF treatment (98·36%) compared with standard wound care (68·88%) at the 10th day after therapy (P < 0·05). Moreover, Liu et al. demonstrated that healing with GM‐CSF was characterised by a more rapid growth of granulation tissue, generally observed in the wound bed at the 6th day, whereas in the control group that was not observed until day 10 42.
A meta‐analysis under the REM showed significantly shortened healing time for add‐on therapy with GM‐CSF as compared with standard wound care used alone (292 wounds, WMD = −5·1; 95% CI, −6·18 to −4·02, P < 0·01) (Figure 3).
Safety
AEs with GF use were evaluated in seven trials, generally, minor to mild. The incidence and profile of AEs were similar between the arm receiving GFs and control (4·6% GFs versus 4·8% Control). Non‐infectious wound‐edge reaction and transient local pain were the two most common AEs seen in both arms, the majority of which were relieved without further medical intervention. No severe allergic reaction, toxic side effect or systematic reaction was reported.
A meta‐analysis under the FEM failed to show significant difference between the two arms on AEs (OR 0·80, 95% CI 0·46–1·38, P = 0·427) (Figure 5). Exclusion of any of the comparisons did not change the results.
Figure 5.
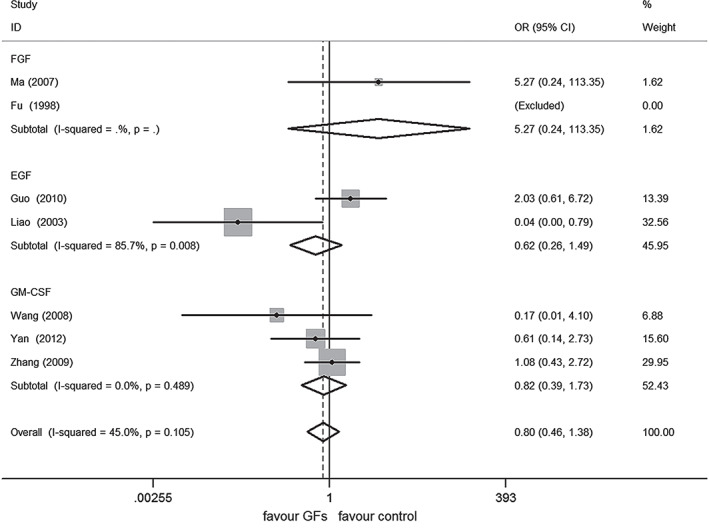
Forest plot depicting the meta‐analysis of adverse events (AEs) between growth factors GFs versus control group. CI, confidence interval; OR, odds ratio.
Discussion
Analysis of the available data illustrates that GF therapy as an add‐on to standard wound care is associated with consistent and significant clinical benefit for partial‐thickness burn injuries. This association scarcely varied by study design, by year of publication or by subclassification of grade II burns. With respect to the primary outcome, add‐on therapy with FGF, EGF and GM‐CSF significantly enhances wound healing, reducing average healing time by 5·02 days (N = 1060, 95% CI, 2·62 to 7·42, P < 0·01), 3·12 days (N = 402, 95% CI, 1·11 to 5·13, P < 0·01) and 5·1 days (N = 292, 95% CI, 4·02 to 6·18, P < 0·01), respectively, as compared with standard treatment alone. With regard to the secondary outcomes, the result of the pooled analysis showed significant improvement of scarring with FGF therapy. Similar scar lightening effect was achieved in sporadic trials on EGF.
Previous reviews have demonstrated that the effect of GFs on tissue regeneration may vary by the mode of delivery. Fernandez 17 advocated that intralesional injection of EGF achieves faster wound healing and lower amputation rates than topical application in the management of severe chronic diabetic ulcer (Wagner grade III to IV), by providing better diffusion and bioavailability of the active agent to the deep layers of the wounds, and the related adverse reaction remains mild to moderate. Still, Lee 43 demonstrated that either local injection or topical application of GFs may lead to side effects owing to the extremely high initial concentration, and conversely may not allow a wide enough time‐frame for sufficient levels of the factors to be sensed by the target tissue, owing to GF's rapid degradation and clearance. However, in this systematic review, our finding suggests that FGF, EGF and GM‐CSF are effective and safe for topical use.
The consistent clinical efficacy of GFs presented by this review may be explained by the biological characteristics of the GFs we assessed and the nature of the burn wound. Depth of partial‐thickness burn is limited to the papillary dermis for grade IIa burns and to the reticular dermis for grade IIb burns, with the majority of hypodermal structures such as subcutaneous adipose tissue, connective tissue and blood vessels spared underneath the wound bed. FGF and EGF are direct mitogens for endothelial cells and dermal fibroblasts, and have been shown to accelerate reepithelialisation, increase proliferation and tensile strength of healed dermis 44, 45, 46, 47, 48. GM‐CSF works directly on the keratinocyte and endothelial cell, and indirectly by mediating the production and release of other cytokines such as interleukin‐6, interleukin‐2 and interferon‐γ 49, 50. Wound bed of partial‐thickness burns provides better nourishment and waste removal for the residual and regenerative cellular constituents of the skin than burns of higher grade or other deep wounds such as chronic diabetic ulcers and deep pressure ulcer, maintaining better cellular sensitivity to these GFs 51. Additionally, the high‐frequency strategy for local GF application may likewise assist against the impairment of biological activity of the agents.
In the overview for safety, our findings failed to identify inferiority of GF therapy to the control arms. The results were identical with most previous studies 52, 53, 54. However, lack of long‐term AEs is highlighted in most trials, that the maximum follow‐up duration of 4 weeks falls short to achieve definitive safety conclusions for GFs use. Considering mechanisms whereby most GFs stimulate tissue repair are similar to ones involved in tumours development 55, further high‐quality trials of large‐scale and long‐term follow‐up would be important to confirm and strengthen our findings.
Our findings showed methodological flaws in study design of the current trials. As a common phenomenon, the efficacy of GFs was examined at various concentrations varying across the trials. A better understanding of dosage regimen for clinician is of great importance to achieve maximum patient's benefit, cost‐effectiveness and minimum risk of adverse reaction. Regretfully, there were no studies specifically designed to address the issue of optimal dosage for GFs use, except for one study on EGF 30, which recommended that dosage regimen was nevertheless inadequately powered for lack of supporting evidence from other trials. There is methodological variation for other aspects of treatment that may influence wound healing. In terms of wound management, different types of wound dressing were alternately used among trials, antibiotics therapies are likewise inconsistently used (varying in administration route, timing and duration). In addition, the reporting of outcomes, especially AEs, has been variable in definition and surveillance. Although the treatment benefit seen is not in question with these variations, for the same criteria were applied to both arms of the trials, the variations may still be potential source of bias, posing challenges to drawing clear conclusions from the observations made here.
Of note, there are three RCTs 30, 38, 39 declaring conflict of interest due to affiliation with biologics manufacturers. Researches with such ties are more likely to contribute to conditions that are conducive to the relatively successful outcomes of patients receiving GFs products.
Conclusion
Results of the present systematic review and meta‐analysis suggest that GF therapy could be an effective and safe add‐on to standard wound care for partial‐thickness burns. Noticing that current evidence is not powerful enough with limitations, future work should focus on well‐designed prospective studies, consistent with therapeutic regimen and reporting outcomes. In addition, financial support from the medical industry should be avoided, and longer follow‐up data should be presented before any definitive conclusions to be established.
Supporting information
Figure S1. Begg's funnel plot of enrolled studies evaluating wound healing time with pseudo 95% confidence limits. (A) FGF, (B) EGF, (C) GM‐CSF. WMD, weighted mean difference.
Figure S2. Begg's funnel plot of enrolled studies evaluating adverse events (AEs) (A) and total body surface area (TBSA) (B) with pseudo 95% confidence limits. WMD, weighted mean difference; OR, odds ratio.
Figure S3. Begg's funnel plot of enrolled studies evaluating Vancouver Scar Scale (VSS) scores with pseudo 95% confidence limits. (A) Height, (B) pigmentation, (C) pliability, (D) vascularity. WMD, weighted mean difference.
Figure S4. Forest plot depicting the meta‐analysis of total body surface area (TBSA) between GF patients versus control group. CI, confidence interval; WMD, weighted mean difference; *, comparison of patients with IIa burns; **, comparison of patients with IIb burns.
Acknowledgements
The authors thank Dr Chuanchang Dai and Dr Lian Zhu for research comment, Dr Hua Xu and Dr Jiao Wei for assistance with the preparation of the article. None of these persons received compensation for the work performed. The authors have declared no conflicts of interest and sources of funding.
References
- 1. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns 2012;38:630–7. [DOI] [PubMed] [Google Scholar]
- 2. Johnson RM, Richard R. Partial‐thickness burns: identification and management. Adv Skin Wound Care 2003;16:178–87 quiz 88–9. [DOI] [PubMed] [Google Scholar]
- 3. Bombaro KM, Engrav LH, Carrougher GJ, Wiechman SA, Faucher L, Costa BA, Heimbach DM, Rivara FP, Honari S. What is the prevalence of hypertrophic scarring following burns? Burns 2003;29:299–302. [DOI] [PubMed] [Google Scholar]
- 4. Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–73. [DOI] [PubMed] [Google Scholar]
- 5. Lawrence WT. Physiology of the acute wound. Clin Plast Surg 1998;25:321–40. [PubMed] [Google Scholar]
- 6. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 7. Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol 2012;26:812–20. [DOI] [PubMed] [Google Scholar]
- 8. Demidova‐Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care 2012;25:349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Travis TE, Mauskar NA, Mino MJ, Prindeze N, Moffatt LT, Fidler PE, Jordan MH, Shupp JW. Commercially available topical platelet‐derived growth factor as a novel agent to accelerate burn‐related wound healing. J Burn Care Res 2014. In press. DOI: 10.1097/BCR.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 10. Brown GL, Curtsinger L 3rd, Brightwell JR, Ackerman DM, Tobin GR, Polk HC Jr, George‐Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med 1986;163:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danilenko DM, Ring BD, Tarpley JE, Morris B, Van GY, Morawiecki A, Callahan W, Goldenberg M, Hershenson S, Pierce GF. Growth factors in porcine full and partial thickness burn repair. Differing targets and effects of keratinocyte growth factor, platelet‐derived growth factor‐BB, epidermal growth factor, and neu differentiation factor. Am J Pathol 1995;147:1261–77. [PMC free article] [PubMed] [Google Scholar]
- 12. Galeano M, Deodato B, Altavilla D, Squadrito G, Seminara P, Marini H, Stagno d'Alcontres F, Colonna M, Calo M, Lo Cascio P, Torre V, Giacca M, Venuti FS, Squadrito F. Effect of recombinant adeno‐associated virus vector‐mediated vascular endothelial growth factor gene transfer on wound healing after burn injury. Crit Care Med 2003;31:1017–25. [DOI] [PubMed] [Google Scholar]
- 13. Xie JL, Bian HN, Qi SH, Chen HD, Li HD, Xu YB, Li TZ, Liu XS, Liang HZ, Xin BR, Huan Y. Basic fibroblast growth factor (bFGF) alleviates the scar of the rabbit ear model in wound healing. Wound Repair Regen 2008;16:576–81. [DOI] [PubMed] [Google Scholar]
- 14. Robson MC, Phillips TJ, Falanga V, Odenheimer DJ, Parish LC, Jensen JL, Steed DL. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor‐2) to accelerate wound healing in venous ulcers. Wound Repair Regen 2001;9:347–52. [DOI] [PubMed] [Google Scholar]
- 15. Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, Andia I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater 2008;84:415–21. [DOI] [PubMed] [Google Scholar]
- 16. Upton Z, Wallace HJ, Shooter GK, van Lonkhuyzen DR, Yeoh‐Ellerton S, Rayment EA, Fleming JM, Broszczak D, Queen D, Sibbald RG, Leavesley DI, Stacey MC. Human pilot studies reveal the potential of a vitronectin: growth factor complex as a treatment for chronic wounds. Int Wound J 2011;8:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez‐Montequin JI, Valenzuela‐Silva CM, Diaz OG, Savigne W, Sancho‐Soutelo N, Rivero‐Fernandez F, Sanchez‐Penton P, Morejon‐Vega L, Artaza‐Sanz H, Garcia‐Herrera A, Gonzalez‐Benavides C, Hernandez‐Canete CM, Vazquez‐Proenza A, Berlanga‐Acosta J, Lopez‐Saura PA. Intra‐lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo‐controlled, double‐blind study. Int Wound J 2009;6:432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, Tamaki K, Hirakata H, Ohura T, Furue M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 2009;19:461–8. [DOI] [PubMed] [Google Scholar]
- 19. Margolis DJ, Bartus C, Hoffstad O, Malay S, Berlin JA. Effectiveness of recombinant human platelet‐derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005;13:531–6. [DOI] [PubMed] [Google Scholar]
- 20. Tuyet HL, Nguyen Quynh TT, Vo Hoang Minh H, Thi Bich DN, Do Dinh T, Le Tan D, Van HL, Le Huy T, Doan Huu H, Tran Trong TN. The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results. Int Wound J 2009;6:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, Carbonin PU, Bernabei R. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med 2003;139:635–41. [DOI] [PubMed] [Google Scholar]
- 22. Payne WG, Ochs DE, Meltzer DD, Hill DP, Mannari RJ, Robson LE, Robson MC. Long‐term outcome study of growth factor‐treated pressure ulcers. Am J Surg 2001;181:81–6. [DOI] [PubMed] [Google Scholar]
- 23. Ching YH, Sutton TL, Pierpont YN, Robson MC, Payne WG. The use of growth factors and other humoral agents to accelerate and enhance burn wound healing. Eplasty 2011;11:e41. [PMC free article] [PubMed] [Google Scholar]
- 24. Rys P, Wladysiuk M, Skrzekowska‐Baran I, Malecki MT. Review articles, systematic reviews and meta‐analyses: which can be trusted? Pol Arch Med Wewn 2009;119:148–56. [PubMed] [Google Scholar]
- 25. Sullivan T, Smith J, Kermode J, McIver E, Courtemanche DJ. Rating the burn scar. J Burn Care Rehabil 1990;11:256–60. [DOI] [PubMed] [Google Scholar]
- 26. Jadad AR, McQuay HJ. Meta‐analyses to evaluate analgesic interventions: a systematic qualitative review of their methodology. J Clin Epidemiol 1996;49:235–43. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 30. Wang SL, Ma JL, Chai JK. Acceleration of burn wound healing with topical application of recombinant human epidermal growth factor ointments. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2002;16:173–6. [PubMed] [Google Scholar]
- 31. Guo X, Tan M, Guo L, Xiong A, Li Y, He X. Clinical study on repair of burn wounds of degree II with recombinant human epidermal growth factor in elderly patients. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2010;24:462–4. [PubMed] [Google Scholar]
- 32. Liao Y, Guo L, Ding EY. A comparative study on burn wound healing treated by different methods of recombinant human epidermal growth factor. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2003;17:301–2. [PubMed] [Google Scholar]
- 33. Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z. Randomised placebo‐controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second‐degree burns. Lancet 1998;352:1661–4. [DOI] [PubMed] [Google Scholar]
- 34. Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second‐degree burn wound healing. Wound Repair Regen 2008;16:635–41. [DOI] [PubMed] [Google Scholar]
- 35. Hayashida K, Akita S. Quality of pediatric second‐degree burn wound scars following the application of basic fibroblast growth factor: results of a randomized, controlled pilot study. Ostomy Wound Manage 2012;58:32–6. [PubMed] [Google Scholar]
- 36. Nie K, Li P, Zeng X, Sun G, Jin W, Wei Z, Wang B, Qi J, Wang Y, Wang D. Clinical observation of basic fibroblast growth factor combined with topical oxygen therapy in enhancing burn wound healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2010;24:643–6. [PubMed] [Google Scholar]
- 37. Wang GY, Xia ZF, Zhu SH, Tang HT, Huan JN, Chen YL, Ge SD. Clinical observation of the long‐term effects of rhEGF on deep partial‐thickness burn wounds. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2003;19:167–8. [PubMed] [Google Scholar]
- 38. Ma B, Cheng DS, Xia ZF, Ben DF, Lu W, Cao ZF, Wang Q, He J, Chai JK, Shen CA, Sun YH, Zhang GA, Hu XH. Randomized, multicenter, double‐blind, and placebo‐controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial‐thickness burns and skin graft donor site. Wound Repair Regen 2007;15:795–9. [DOI] [PubMed] [Google Scholar]
- 39. Wang ZY, Zhang Q, Liao ZJ, Han CM, Lv GZ, Luo CQ, Chen J, Yang SX, Yang XD, Liu Q. Effect of recombinant human granulocyte‐macrophage colony stimulating factor on wound healing in patients with deep partial thickness burn. Zhonghua Shao Shang Za Zhi 2008;24:107–10. [PubMed] [Google Scholar]
- 40. Yan H, Chen J, Peng X. Recombinant human granulocyte‐macrophage colony‐stimulating factor hydrogel promotes healing of deep partial thickness burn wounds. Burns 2012;38:877–81. [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Chen J, Han C. A multicenter clinical trial of recombinant human GM‐CSF hydrogel for the treatment of deep second‐degree burns. Wound Repair Regen 2009;17:685–9. [DOI] [PubMed] [Google Scholar]
- 42. Liu L, Fang Y, Yao M, Yu W, Li X. Effect of recombinant human granulocyte‐macrophage colony‐stimulating factor on wound debridement and healing of deep II thickness burn. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2011;25:1059–62. [PubMed] [Google Scholar]
- 43. Lee K, Silva EA, Mooney DJ. Growth factor delivery‐based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011;8:153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 45. Sun L, Xu L, Chang H, Henry FA, Miller RM, Harmon JM, Nielsen TB. Transfection with aFGF cDNA improves wound healing. J Invest Dermatol 1997;108:313–8. [DOI] [PubMed] [Google Scholar]
- 46. Alemdaroglu C, Degim Z, Celebi N, Sengezer M, Alomeroglu M, Nacar A. Investigation of epidermal growth factor containing liposome formulation effects on burn wound healing. J Biomed Mater Res A 2008;85:271–83. [DOI] [PubMed] [Google Scholar]
- 47. Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006;32:319–27. [DOI] [PubMed] [Google Scholar]
- 48. Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, Curtsinger LJ 3rd, Holtzin L, Schultz GS, Jurkiewicz MJ, Lynch JB. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med 1989;321:76–9. [DOI] [PubMed] [Google Scholar]
- 49. Mann A, Breuhahn K, Schirmacher P, Blessing M. Keratinocyte‐derived granulocyte‐macrophage colony stimulating factor accelerates wound healing: stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol 2001;117:1382–90. [DOI] [PubMed] [Google Scholar]
- 50. Molloy RG, Holzheimer R, Nestor M, Collins K, Mannick JA, Rodrick ML. Granulocyte‐macrophage colony‐stimulating factor modulates immune function and improves survival after experimental thermal injury. Br J Surg 1995;82:770–6. [DOI] [PubMed] [Google Scholar]
- 51. Ousey K, McIntosh C. Understanding wound bed preparation and wound debridement. Br J Community Nurs 2010;15 S22, S4, S6, passim. [DOI] [PubMed] [Google Scholar]
- 52. Berlanga‐Acosta J, Gavilondo‐Cowley J, Lopez‐Saura P, Gonzalez‐Lopez T, Castro‐Santana MD, Lopez‐Mola E, Guillen‐Nieto G, Herrera‐Martinez L. Epidermal growth factor in clinical practice – a review of its biological actions, clinical indications and safety implications. Int Wound J 2009;6:331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, Matsushita T, Nakamura K. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo‐controlled trial. J Bone Miner Res 2010;25:2735–43. [DOI] [PubMed] [Google Scholar]
- 54. Baldelli CM, Ruella M, Scuderi S, Monni M, Passera R, Omede P, Tarella C. A short course of granulocyte‐colony‐stimulating factor to accelerate wound repair in patients undergoing surgery for sacrococcygeal pilonidal cyst: proof of concept. Cytotherapy 2012;14:1101–9. [DOI] [PubMed] [Google Scholar]
- 55. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Begg's funnel plot of enrolled studies evaluating wound healing time with pseudo 95% confidence limits. (A) FGF, (B) EGF, (C) GM‐CSF. WMD, weighted mean difference.
Figure S2. Begg's funnel plot of enrolled studies evaluating adverse events (AEs) (A) and total body surface area (TBSA) (B) with pseudo 95% confidence limits. WMD, weighted mean difference; OR, odds ratio.
Figure S3. Begg's funnel plot of enrolled studies evaluating Vancouver Scar Scale (VSS) scores with pseudo 95% confidence limits. (A) Height, (B) pigmentation, (C) pliability, (D) vascularity. WMD, weighted mean difference.
Figure S4. Forest plot depicting the meta‐analysis of total body surface area (TBSA) between GF patients versus control group. CI, confidence interval; WMD, weighted mean difference; *, comparison of patients with IIa burns; **, comparison of patients with IIb burns.


