Abstract
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, recurrent inflammatory disease affecting skin that bears apocrine glands. It is characterised by the presence of tender subcutaneous nodules that may rupture, resulting in deep dermal abscesses, fibrosis with dermal contractures and induration of the skin. The management of HS is a challenge for physicians as the pathogenesis is not clearly defined and prevents the use and development of directed therapies. Treatment options are oral agents (antibiotics, immunomodulators) and surgical excision. Historically, surgical management has been complicated by difficult closure and high recurrent rates. In the last 10 years, negative pressure wound therapy (NPWT) has proven to be a great adjunct for wound management as it provides the adequate conditions for wound healing, promotes granulation and helps to control infection. Here, we report a case of severe perineal HS treated with radical excision and NPWT as an adjunct. The patient only had a recurrence 3 years after primary treatment and was surgically treated for cosmetic reasons without any complications. Finally, we present a review of the relevant literature.
Keywords: Acne inversa, Hidradenitis suppurativa, Negative pressure wound therapy, Split‐thickness skin grafting
Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, recurrent inflammatory disease affecting the skin that bears the apocrine glands 1, 2. It is characterised by the presence of tender subcutaneous nodules that may rupture, resulting in deep dermal abscesses 3. These lesions are accompanied by symptoms such as burning pruritus, local warmth, hyperhidrosis, pain and suppuration 4. Abscesses may heal, producing skin fibrosis with dermal contractures and induration of the skin. Flares may last from 7 to 10 days 2, 4, 5.
The pathogenesis of HS is not clearly defined, but it is believed to include factors such as luminal plugging of apocrine glands, infection and inflammation 6. A genetic factor has been considered as an autosomal dominant heritance has been indentified in one‐third of HS patients. 5. Smoking and obesity are both well‐known factors related to its development and severity 7, 8. Studies have shown that this disease has a negative impact on the quality of life when compared with other chronic skin conditions. There is a high absence from work and self‐reported poor health in this group of patients 9, 10.
People in their second and third decade of life are most commonly affected 11, 12. European studies show a prevalence of 1% in persons older than 15 years and up to 4% in young adults (18–33 years old) 12, 13. Women are more commonly affected than men (3:1) 12. HS lesions may occur in the intertriginous apocrine glands at the axillary, inguinal, perianal, perineal, mammary, inframammary, buttock, pubic, chest, scalp, retroauricular and eyelid areas 14. Several studies show that axillary involvement occurs in around 72% of cases followed by perianal (32%), groin (24%) and mammary involvement (8%) 15, 16, 17. Evidence in other studies shows that perianal involvement is more common in males and genitofemoral lesions are more common in females 4, 12.
The diagnosis of HS is made clinically, and biopsies are seldom required. HS may be categorised into acute and chronic forms. Acute HS is characterised by multiple nodules coalescing into cord‐like bluish and red structures 4, 18; chronic HS is characterised by multiple abscesses, scars, ulcerations and infections that may extend into the fascia 18, 19. HS is usually classified into two staging systems: Hurley and Sartorius. Hurley's system is simple, relies on the presence and extent of cicatrisation and sinuses 20 and is divided into three stages: Stage I, characterised by isolated abscesses with no sinus tracts; Stage II, characterised by recurrent abscesses with sinus tract and scarring; and Stage III, characterised by multiple interconnected sinus tracts and abscesses across an entire region 20. The Sartorius staging system is based on anatomic regions, number and types of lesions and presence of tracts and is more sophisticated than Hurley's 21.
The management of HS is a challenge for physicians as the pathogenesis is not clearly defined and prevents the use and development of directed therapies 22. Medical treatment (antibiotics, immunosuppressants and biological agents) is non‐specific, and as a result, it has limited success in some mild and moderate cases (Hurley I and II), and it has high recurrence rates 23, 24, 25. For recurrent cases of moderate disease (Hurley II), surgical treatment may be required. Local excision and primary closure may control the disease, but it is prone to recurrences. Hence, wide excision is the recommended treatment nowadays 26.
For severe cases, where medical treatment has shown not to have any impact, wide excision with secondary closure (if it is a large contaminated lesion) or skin grafting and patch closure is the best option for treatment and prevention of recurrences 22, 27. Secondary closure would classically involve multiple dressing changes for a prolonged time. However, negative pressure wound therapy (NPWT) has been developed recently, and is commonly used in management of open and contaminated wounds nowadays 28. NPWT consists of a vacuum‐type system that creates a symmetrical physical pressure layer over the lesion. A number of studies have shown that it reduces wound contamination and promotes granular tissue synthesis and angiogenesis 29. As it is a potentially beneficial treatment for HS, we present the case of a female patient with severe, chronic perineal HS treated with NPWT and also review current literature.
Case report
A 34‐year‐old Caucasian female with previous history of HS consulted for persistent lesions associated with symptoms of suppuration, haemorrhage and pain. She was diagnosed 5 years ago, and since then, she has undergone different therapies including clindamycin and ampicillin/sulbactam. Past medical history included diabetes treated with oral agents which helped to bring blood glucose level under control. Clinical examination revealed multiple draining sinus tracts with active haemorrhages in the crural and perineal regions. Scars and skin retraction were identified in the axillary area.
Blood tests and swab cultures were ordered, and antibiotic management with vancomycin and piperacillin/tazobactam was initiated. Insulin therapy was started for her diabetes control. Blood test results returned to normal. Ultrasonography of the soft tissue identified collection of echogenic fluid of a laminar disposition. No other collections were identified. Skin cultures showed Staphylococcus hominis and Staphylococcus haemolyticus, both receptive to antibiotics. On the fourth day of hospitalisation, she was taken to surgery for wide skin debridement of compromised tissue.
An incision was made bilaterally in the right pubic spine region along the inguinal area to the ischial tuberosity, leaving a 1 cm margin of skin in the labia majora and the anal orifice (Figure 1). Haemostasis was achieved, and the wound was covered with polyurethane dressings and connected to a negative pressure system with a pressure of 150 mmHg. The procedure was done without any complications. Post‐operative analgesia was achieved with a morphine Patient controlled analgesia (PCA) pump and acetaminophen.
Figure 1.
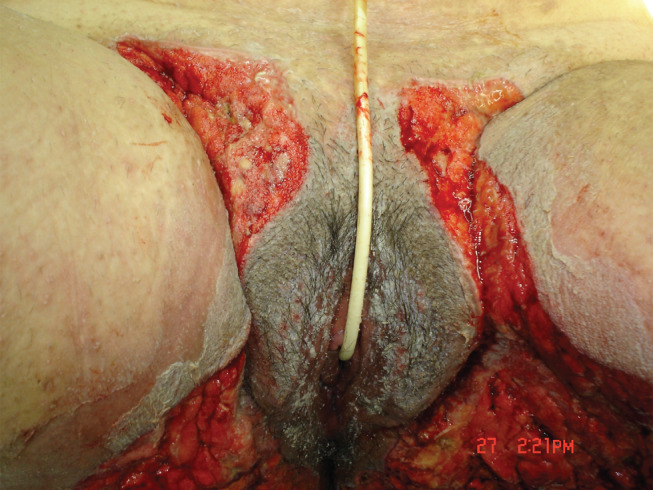
Inmediate postoperative period without the negative pressure wound therapy dressings. A wide resection of the affected regions in the perineal area was done.
The patient had adequate post‐operative recovery with a mean drainage of 50 cc for the first 2 days. On the second day, a post‐operative diet was initiated, Patient controlled analgesia pump was removed and the patient continued acetaminophen/hydrocodone treatment for analgesia. The negative pressure system was changed on the third and sixth days, post‐operatively, without any complications or significant findings. The patient continued to recover stably for the next few days (Figure 2).
Figure 2.
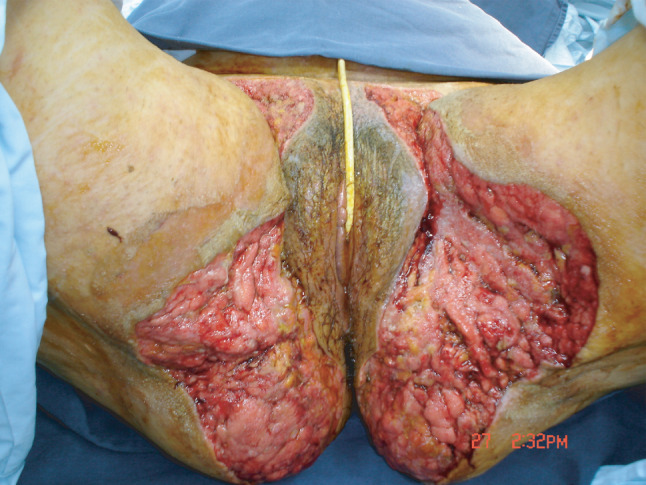
8Th day postoperatively before the reconstructive procedure.
She was taken to the OR again on the ninth day, post‐operatively, for a primary delayed closure. First, pubic split‐thickness grafts were advanced into the inguinal crease and were sutured with polyglactin‐0 and polypropylene‐0. Then, two fasciocutaneous gluteal flaps were advanced to the midline and sutured with polyglactin‐0 and polypropylene‐0. Finally, in the medial aspect of the thighs, local flaps were advanced and sutured in the same fashion. Redundant tissue was removed, and haemostasis was achieved (Figure 3). A Hemovac drain was left for drainage. There were no complications; post‐operative pain was managed in the same manner as in the previous procedure.
Figure 3.
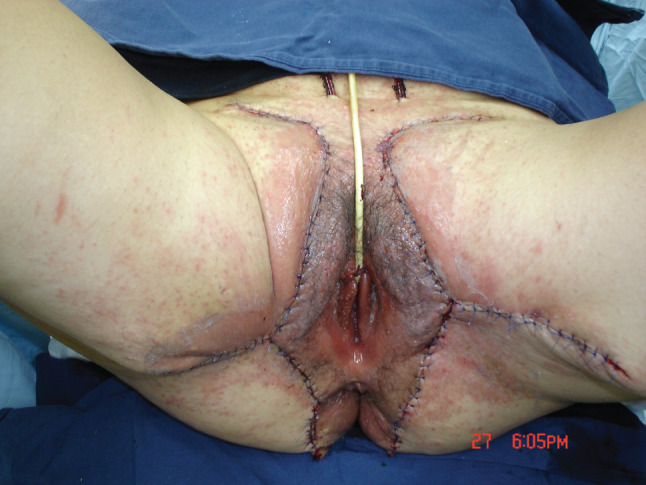
Reconstruction of the vulvar area with pubic split‐thickness grafts and two fasciocutaneous gluteal flaps
She had an adequate post‐operative recovery. Antibiotics were suspended after completing 14 days of treatment. The Hemovac drain had a mean drainage of 75 cc in 24 hours. It was removed on the third day, post‐operatively. She was discharged from the hospital on the fourth day, post‐operatively, without any symptoms. Six months after surgery, the tissue had already scarred (Figure 4).
Figure 4.
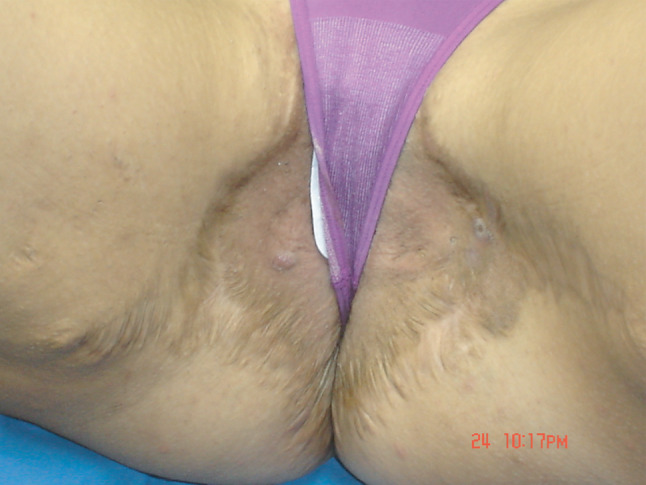
6th month after discharge. Tissue is scared and no signs of relapse are observed.
Three years later, she was hospitalised again for residual lesions in the perineal and gluteal area, which were asymptomatic. They were resected in the OR, and a primary closure was done. A course of intravenous antibiotics (vancomycin and ertapenem) was initiated. Cultures showed a Staphylococcus epidermidis infection that was receptive to antibiotics. After the fourth day, post‐operatively, the patient was discharged with ambulatory antibiotic treatment (ampicilin/sulbactam). She has not had any relapses after a 6‐year follow‐up.
Discussion
We present a case of severe HS in which we used NPWT as a useful adjunct to provide a sterile environment to a wound that was left open after wide skin debridement. Following our clinic protocols, we changed the dressings every 3 days without any complications. The patient complained of mild to moderate pain that was managed with opioid and Non steroidal anti‐inflammatory drugos (NSAID) analgesia. Because of our experience, we believe that NPWT, besides controlling infection, helped in the healing process and provided a better result after skin grafting was performed. We did not face any complications, and the second intervention 3 years later was carried out for cosmetic reasons as residual lesions and skin contraction were present.
NPWT in HS has been used more frequently in severe cases that comprise large defects primarily in the axillary, buttock and groin regions 22, 26, 30, 31, 32. It has been suggested that this system improves wound healing by increasing blood flow and granulation tissue formation, reducing bacterial load and thereby reducing the size and complexity of the wound 22, 28, 33, 34. Ricci et al. report four cases of severe vulvar HS. Closure was achieved in three of the cases using NPWT solely as an adjunct for secondary closure for 3 months. The fourth patient presented with very large defects and was treated successfully with NPWT plus a split‐thickness skin graft 32.
When NPWT is used concomitantly with skin grafts as a method of delayed closure, studies have shown that it promotes angiogenesis of the underlying fat and helps to secure the grafts so that they will not be prone to shearing forces 30, 35, 36, 37, 38. Chen and Friedman report a series of 11 cases and 24 lesions of HS managed with wide excisions, Vacuum assisted closure (VAC) therapy and skin grafting. VAC therapy showed promising results when used with split‐thickness skin grafts (graft take in 75% and need for re‐grafting in 21%); however, this study does not comment on the definitive healing of lesions 26. It comments that this technique can provide a long‐term benefit in the treatment of HS despite an increased hospital stay and immobilisation time as angiogenesis needs to be stimulated in order to achieve better graft patency. Elwood and Bolitho reported two cases of axillary HS successfully treated with NPWT and grafts, and Hynes et al. reported the successful treatment of five cases of severe axillary HS treated with NPWT and grafting 30, 31. All of these cases report healing without compromise in the shoulder motion.
Additionally, Ludolph et al. report successful treatment of penile HS and Fournier's gangrene with an acellular collagen matrix plus NPWT followed by a split‐thickness skin graft 39. Jianbing et al. report a successful case of buttock HS treated with NPWT coupled with a split‐thickness graft 40. Rhode et al. report a series of five cases of severe vulvar HS treated with split‐thickness skin grafts with an effectiveness of 100% in four cases. The fifth patient was treated successfully through secondary intervention but developed a skin stricture that required surgical management 41. In contrast, before NPWT was developed, a series of 10 patients with HS reported a success rate of only 60%, and another series of 16 HS patients showed incomplete graft take in 6 patients and contractures in 5 patients 42, 43.
In cases of severe HS, bilateral dermal regeneration templates (BDRT) used concurrently with NPWT have also been successful. Gonzaga et al. report a series of four cases of severe axillary HS treated successfully with BDRT and without recurrences in follow‐up ranging from 6 to 38 months 44. The advantage of BDRT is that the excision can be done more freely and can improve cosmetic results; however, it increases hospital costs and stay that have not proven to be cost‐effective 44.
Apart from the described method of NPWT, an internal vacuum‐assisted closure method was developed as it is a potentially better way of management 22. It is different from the classical NPWT as it involves closing the defect by using a sponge 22. Studies have shown that it potentiates wound healing before closure, enhances patient comfort and minimises anxiety and narcotic use 45. Chen et al. report 60 cases of severe HS from 27 patients in whom internal vacuum‐assisted closure was done followed by simple closure. The study showed that this system can be potentially beneficial as a bridging therapy when large defects are encountered 22. They suggest that the internal vacuum‐assisted closure diminishes the time between excision and closure 22. As yet, there are no studies comparing classical NPWT with internal vacuum‐assisted closure.
In an attempt to involve NPWT in the management of HS, Chen et al. developed an algorithm of treatment for HS based on Hurley's staging system 20, 22. Stage I involves pharmacological treatment. For Stage II, a trial of immunomodulators should be done first. In case of refractory Stage II or Stage III disease, wide local excision must be performed keeping two premises in mind: if the wound is clean and the lesion is <70 cm2 in size, a primary closure can be attempted, but if the wound is infected or the lesion is > 70 cm2 in size , NPWT should be used followed by a delayed closure that can be performed with one of the techniques previously described.
Conclusion
HS is a dermatological condition of varying degrees that can be chronic, severe, relapsing and debilitating. As its pathophysiology is not well defined, treatment options tend to be limited as the severity increases. For large wounds and severe cases, skin debridement should be considered with NPWT as an adjunct. This formula potentially improves the wound conditions, accelerates healing and increases the effectiveness of posterior surgical closure with aids (such as grafts or synthetic matrix) or with a primary delayed closure.
When using NPWT, it is important to consider the following key points 40: (i) the wound debridement should be performed carefully because it has a critical impact on NPWT and other delayed closure techniques; (ii) as necrotic tissue can block the NPWT tube, the use of saline irrigation to prevent blockage is recommended; (iii) to avoid sponge hardening and bacterial infection, we recommend changing the dressing every 72 hours; and (iv) all concomitant diseases that may impair healing, such as diabetes mellitus or adrenal insufficiency, should be aggressively treated.
Acknowledgements
The authors declare that they have no conflict of interest.
References
- 1. Jemec GB. Hidradenitis suppurativa. J Cutan Med Surg 2003;7:47–56. [DOI] [PubMed] [Google Scholar]
- 2. von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2000;14:389–92. [DOI] [PubMed] [Google Scholar]
- 3. Slade DE, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg 2003;56:451–61. [DOI] [PubMed] [Google Scholar]
- 4. Anderson MJ Jr, Dockerty MB. Perianal hidradenitis suppurativa; a clinical and pathologic study. Dis Colon Rectum 1958;1:23–31. [DOI] [PubMed] [Google Scholar]
- 5. Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012;366:158–64. [DOI] [PubMed] [Google Scholar]
- 6. Buimer MG, Wobbes T, Klinkenbijl JH. Hidradenitis suppurativa. Br J Surg 2009;96:350–60. [DOI] [PubMed] [Google Scholar]
- 7. Konig A, Lehmann C, Rompel R, Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology 1999;198:261–4. [DOI] [PubMed] [Google Scholar]
- 8. Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009;161:831–9. [DOI] [PubMed] [Google Scholar]
- 9. Jemec GB, Heidenheim M, Nielsen NH. A case–control study of hidradenitis suppurativa in an STD population. Acta Derm Venereol 1996;76:482–3. [DOI] [PubMed] [Google Scholar]
- 10. Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J. Quality of life group of the French Society of D. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol 2007;56:621–3. [DOI] [PubMed] [Google Scholar]
- 11. Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet 1985;22:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Revuz JE, Canoui‐Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, Poli F, Faye O, Roujeau JC, Bonnelye G, Grob JJ, Bastuji‐Garin S. Prevalence and factors associated with hidradenitis suppurativa: results from two case–control studies. J Am Acad Dermatol 2008;59:596–601. [DOI] [PubMed] [Google Scholar]
- 13. Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996;35(2 Pt 1):191–4. [DOI] [PubMed] [Google Scholar]
- 14. Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009;60:539–61; quiz 62–3. [DOI] [PubMed] [Google Scholar]
- 15. Barth JH, Layton AM, Cunliffe WJ. Endocrine factors in pre‐ and postmenopausal women with hidradenitis suppurativa. Br J Dermatol 1996;134:1057–9. [PubMed] [Google Scholar]
- 16. Jackman RJ. Hidradenitis suppurativa: diagnosis and surgical management of perianal manifestations. Proc R Soc Med 1959;52(Suppl):110–2. [PMC free article] [PubMed] [Google Scholar]
- 17. Jackman RJ, Mc QH. Hidradenitis suppurativa; its confusion with pilonidal disease and anal fistula. Am J Surg 1949;77:349–51. [DOI] [PubMed] [Google Scholar]
- 18. Ching CC, Stahlgren LH. Clinical review of hidradenitis suppurativa: management of cases with severe perianal involvement. Dis Colon Rectum 1965;8:349–52. [DOI] [PubMed] [Google Scholar]
- 19. Ramasastry SS, Conklin WT, Granick MS, Futrell JW. Surgical management of massive perianal hidradenitis suppurativa. Ann Plast Surg 1985;15:218–23. [DOI] [PubMed] [Google Scholar]
- 20. Hurley H. Dermatological surgery, principles and practice. New York: Marcel Dekker, 1989. [Google Scholar]
- 21. Sartorius K, Lapins J, Emtestam L, Jemec GB. Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa. Br J Dermatol 2003;149:211–3. [DOI] [PubMed] [Google Scholar]
- 22. Chen YE, Gerstle T, Verma K, Treiser MD, Kimball AB, Orgill DP. Management of hidradenitis suppurativa wounds with an internal vacuum‐assisted closure device. Plast Reconstr Surg 2014;133:370e–7. [DOI] [PubMed] [Google Scholar]
- 23. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983;22:325–8. [DOI] [PubMed] [Google Scholar]
- 24. Gener G, Canoui‐Poitrine F, Revuz JE, Faye O, Poli F, Gabison G, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology 2009;219:148–54. [DOI] [PubMed] [Google Scholar]
- 25. van der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology 2009;219:143–7. [DOI] [PubMed] [Google Scholar]
- 26. Chen E, Friedman HI. Management of regional hidradenitis suppurativa with vacuum‐assisted closure and split thickness skin grafts. Ann Plast Surg 2011;67:397–401. [DOI] [PubMed] [Google Scholar]
- 27. Lipshutz H. Closure of axillary hidradenitis defects with local triangular flaps. Plast Reconstr Surg 1974;53:677–9. [DOI] [PubMed] [Google Scholar]
- 28. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76; discussion 77. [PubMed] [Google Scholar]
- 29. Cro C, George KJ, Donnelly J, Irwin ST, Gardiner KR. Vacuum assisted closure system in the management of enterocutaneous fistulae. Postgrad Med J 2002;78:364–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elwood ET, Bolitho DG. Negative‐pressure dressings in the treatment of hidradenitis suppurativa. Ann Plast Surg 2001;46:49–51. [DOI] [PubMed] [Google Scholar]
- 31. Hynes PJ, Earley MJ, Lawlor D. Split‐thickness skin grafts and negative‐pressure dressings in the treatment of axillary hidradenitis suppurativa. Br J Plast Surg 2002;55:507–9. [DOI] [PubMed] [Google Scholar]
- 32. Ricci EK, Brace JA, Rosenblum NG, Chatterjee S. The use of the negative pressure wound therapy in vulvar wounds. J Pelvic Med Surg 2010;16:S38. [Google Scholar]
- 33. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 34. Moues CM, Heule F, Hovius SE. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg 2011;201:544–56. [DOI] [PubMed] [Google Scholar]
- 35. Blackburn JH 2nd, Boemi L, Hall WW, Jeffords K, Hauck RM, Banducci DR, et al. Negative‐pressure dressings as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 36. Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum‐assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28:211–7. [DOI] [PubMed] [Google Scholar]
- 37. Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, et al. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–22. [DOI] [PubMed] [Google Scholar]
- 38. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96; discussion 97–8. [DOI] [PubMed] [Google Scholar]
- 39. Ludolph I, Titel T, Beier JP, Dragu A, Schmitz M, Wullich B, Horch RE. Penile reconstruction with dermal template and vacuum therapy in severe skin and soft tissue defects caused by Fournier's gangrene and hidradenitis suppurativa. Int Wound J 2014. DOI: 10.1111/iwj.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jianbing T, Biao C, Qin L, Yanhong W. Topical negative pressure coupled with split‐thickness skin grafting for the treatment of hidradenitis suppurativa: a case report. Int Wound J 2015;12:334–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rhode JM, Burke WM, Cederna PS, Haefner HK. Outcomes of surgical management of stage III vulvar hidradenitis suppurativa. J Reprod Med 2008;53:420–8. [PubMed] [Google Scholar]
- 42. Morgan WP, Harding KG, Hughes LE. A comparison of skin grafting and healing by granulation, following axillary excision for hidradenitis suppurativa. Ann R Coll Surg Engl 1983;65:235–6. [PMC free article] [PubMed] [Google Scholar]
- 43. Soldin MG, Tulley P, Kaplan H, Hudson DA, Grobbelaar AO. Chronic axillary hidradenitis‐‐the efficacy of wide excision and flap coverage. Br J Plast Surg 2000;53:434–6. [DOI] [PubMed] [Google Scholar]
- 44. Gonzaga TA, Endorf FW, Mohr WJ, Ahrenholz DH. Novel surgical approach for axillary hidradenitis suppurativa using a bilayer dermal regeneration template: a retrospective case study. J Burn Care Res 2013;34:51–7. [DOI] [PubMed] [Google Scholar]
- 45. Chariker ME, Gerstle TL, Morrison CS. An algorithmic approach to the use of gauze‐based negative‐pressure wound therapy as a bridge to closure in pediatric extremity trauma. Plast Reconstr Surg 2009;123:1510–20. [DOI] [PubMed] [Google Scholar]


