Abstract
Post‐thrombotic syndrome (PTS) is a condition that can develop in about half of the patients with deep vein thrombosis (DVT) of lower limbs.
In the present study, we evaluated the expression of inflammatory biomarkers in the early phases of DVT and their correlation with the onset of PTS.
Patients were enrolled after the first episode of DVT and were followed up for 1, 4, 8, 12 and 18 months. At each visit, blood sample was collected to evaluate plasma levels of matrix metalloproteinase (MMP)‐1,‐2,‐3,‐7,‐8 and ‐9 MMP inhibitors, TIMP‐1,‐2, neutrophil gelatinase‐associated lipocalin (NGAL) and cytokines TNF‐α and IL‐6.
Analysis included 201 patients [86 males (42·79%) and 115 females (57·21%); average age 56 ± 7 years].
Of the 201 patients, 47 (23·38%; 21 males, 26 females) developed PTS during the follow‐up period.
The control group was made up of 60 individuals without DVT (22 males and 38 females).
High plasma levels of MMPs, NGAL and cytokines were recorded during the acute phase after DVT. Moreover, patients with PTS showed higher levels of MMP‐1 and MMP‐8 with respect to patients without PTS.
There is a close relationship between DVT, the individual risk of PTS and specific biomarkers such as MMPs and other related molecules, which may help guide prevention and therapy based on the patient's individual risk profile, and has to be studied in future.
Keywords: Cytokines, Deep vein thrombosis, Matrix metalloproteinases, Post‐thrombotic syndrome, Post‐thrombotic ulceration
Introduction
Post‐thrombotic syndrome (PTS) is a chronic complication of deep venous thrombosis (DVT) of the lower limbs arising in 20–50% of patients, within 2 years of onset of acute DVT 1, 2. Symptoms and signs of PTS consist of heaviness, pain, cramps, itching, tingling, oedema, venous ectasia, hyperpigmentation and venous ulcerations. These events may be related to inflammation that induces injury of venous valves and stiff, fibrotic vein wall degeneration, up to thrombosis 3. The most important risk factors for PTS development are: high body mass index (BMI), elderly age, proximal DVT, recurrent ipsilateral DVT and pre‐existent varicosities 4, 5, 6, 7. An increasing body of evidences shows that matrix metalloproteinases (MMPs) play a role in several vascular diseases 8, 9, 10, 11, 12 in which inflammation represents a key mechanism 13, 14. The activity of MMPs is controlled by tissue inhibitors of MMPs (TIMPs) and an imbalance between MMPs and TIMPs may contribute to the development of vascular diseases such as atherosclerosis, aneurysms and varicose veins 15, 16. MMPs are also involved in the pathophysiology of PTS, including tissue remodelling after acute venous thrombosis 17, endothelial dysfunctional activation 18, 19 and vessel recanalisation 20, 21. It has been reported that the expression of MMPs increases in the vein wall after DVT onset and its expression is related to the grade of inflammation 22, 23, 24. However, to date it is not easy to predict which patients will develop PTS and which will not 2.
In this context, in the present study we evaluated the expression of inflammatory biomarkers in the early phases of DVT and their correlation with the onset of PTS.
Specifically, we evaluated the expression of those MMPs for whom there was a well documented relationship with vascular disease, endothelial dysfunction and vascular remodelling, as well as with DVT, such as MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐8 and MMP‐9, their most studied inhibitors, TIMP‐1, TIMP‐2, neutrophil gelatinase‐associated lipocalin (NGAL), which has a known interplay with MMP‐9, 16, 25, 26 and two specific cytokines, tumour necrosis factor alpha (TNF‐α) and interleukin (IL)‐6, whose evidences in literature suggested for their linkage to MMP regulation 27.
Materials and methods
The study was a multicenter open‐label, prospective and parallel groups study performed in the Department of Medical and Surgical Sciences of University Magna Graecia of Catanzaro, the Department of Clinical Medicine and Surgery, Federico II University of Naples, the Department of Dentistry, Medical and Surgical Experimental Sciences of University of Messina and the Division of Vascular Surgery, S. Anna Hospital, Catanzaro, between February 2012 and February 2014. This study was approved by the Investigational Review Board, in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice. Before the beginning of the study, all participants were informed about the aim, procedures, risks and benefits of the study and written informed consent was obtained from them. The protocol was registered at the public trials registry, www.clinicaltrials.gov (trial identifier NCT02376764).
Population
Eligible patients were women and men, older than 18 years, with a clinical diagnosis of PTS performed using the Villalta score 28, 29 and confirmed by compression ultrasound (CUS) (Table 1).
Table 1.
Demographics
| Total cohort n =201 | PTS n = 47 (Group A) | No PTS n = 154 (Group B) | P‐value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 86 (42·79%) | 21 (44·68%) | 65 (42·20%) | 0·7642 |
| Female | 115 (57·21%) | 26 (55·32%) | 89 (57·79%) | 0·7642 |
| Age at diagnosis | ||||
| Age range | 48‐82 | 52‐82 | 48‐82 | |
| Median age | 65 | 66 | 65 | |
| Overweight | 42 (20·89%) | 12 (25·53%) | 30 (19·48%) | 0·3718 |
| Obesity | 33 (16·41%) | 13 (27·66%) | 20 (12·99%) | 0·0175 |
| Smoking | 39 (19·40%) | 10 (21·28%) | 29 (18·83%) | 0·7106 |
| Arterial hypertension | 27 (13·43%) | 8 (17·02%) | 19 (12·33%) | 0·4098 |
| Hyperlipidaemia | 32 (15·92%) | 9 (19·14%) | 23 (14·93%) | 0·4895 |
| Diabetes mellitus | 16 (12·56%) | 5 (10·63%) | 11 (7·14%) | 0·4384 |
| Known thrombofilia | 9 (4·48%) | 3 (6·38%) | 6 (3·90%) | 0·4705 |
| Surgery/trauma within past 2 months | 41 (20·40%) | 11 (23·40%) | 30 (19·48%) | 0·5590 |
| Positive family history for VTE | 13 (6·47%) | 4 (8·51%) | 9 (5·84%) | 0·5153 |
| Varicosities | 36 (17·91%) | 15 (31·91%) | 21 (13·64%) | 0·0042 |
| Location of DVT | ||||
| Distal | 24 (11·94%) | 5 (10·64%) | 19 (12·34%) | 0·7532 |
| Proximal | 177 (88·06%) | 42 (89·37%) | 135 (87·66%) | 0·7532 |
| Left | 97 (48·26%) | 21 (44·68%) | 76 (49·35%) | 0·5749 |
| Right | 104 (51·74%) | 26 (55·32%) | 78 (50·65%) | 0·5749 |
PTS, post‐thrombotic syndrome; DVT, Deep Vein Thrombosis; VTE, venous thromboembolism.
Clinical data were collected from the hospital electronic medical record system. Cardiovascular risk factors analysed included a history of smoking, diabetes mellitus, hypertension, hyperlipidaemia and obesity. Inclusion criteria were: acute DVT of lower limbs, no history of previous DVT and availability to make follow‐up appointments. Both patients with proximal DVT including the popliteal vein or above, and patients with distal DVT limited to the calf were included.
Exclusion criteria were: presence of cancer, hepatic failure, infectious or autoimmune diseases, arthritis, arterial aneurysms, hernias, chronic venous disease, nephritis, vascular ulcers, fibrosis and other diseases (e.g. chronic obstructive pulmonary diseases or ulcers) associated with increased levels of MMPs. Patients under treatment with corticosteroids or cytostatic drugs were also excluded.
Risk assessment criteria were based on preoperative medical history, full physical examination, biochemical and haematologic analyses, echocardiography, electrocardiography and lung function tests.
Patients were followed up for 18 months from the onset of acute DVT. At each visit, blood samples were collected from venipuncture in order to evaluate plasma levels of MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐8, MMP‐9, NGAL, TIMP‐1, TIMP‐2, TNF‐α and IL‐6.
Patients with DVT who developed PTS (Villalta score ≥ 5) during the follow‐up were defined as Group A; patients with DVT who did not develop PTS (Villalta score ≤ 4) during the follow‐up were defined as Group B.
All patients with DVT were treated with low‐molecular‐weight heparin (LMWH) for 1 week and then with warfarin for 3 months and were instructed to wear sized‐to‐fit elastic stockings (class III, 40 mmHg), thigh‐length in case of proximal DVT for the first 3 months followed by knee‐length stockings, or knee‐length stockings in case of distal DVT for 18 months.
Enzyme‐linked immunosorbent assay (ELISA)
ELISA test was performed as previously described 30, 31, 32. For the evaluation of plasma MMP, NGAL, TIMP, TNF‐α and IL‐6 levels blood samples collected at the time of the admission (first presentation, T0), and 1 (T1), 4 (T2), 8 (T3), 12 (T4) and 18 (T5) months later, and were frozen (−80 °C) for ELISA evaluation. The ratios of MMP‐1/TIMP‐1 and MMP‐8/TIMP‐1 were calculated in the peripheral blood. The results were evaluated with respect to individuals without DVT (control group).
Statistical analysis
All data are expressed as mean values ± standard error medium (SEM). Student's t‐test was used to analyse the difference between each group with their control. Analysis of variance (ANOVA) was used to evaluate the difference between the groups. Differences identified by ANOVA were further analysed by an unpaired Student's t‐test.
Adjusted analysis was performed using a proportional hazards Cox regression model.
A multivariable Cox regression analysis was performed to control for confounding. Data were further adjusted for other set of covariates such as traditional risk factors for clinical parameters (i.e. smoking, arterial hypertension, diabetes, total cholesterol, family history for VTE).
Pearson's test was used to evaluate the correlation between PTS, and plasma levels of MMPs, NGAL, TIMPs and ratio of MMP/TIMP. SPSS 21.0 software (IBM, Chicago, IL, USA) was used for statistical analysis. We defined this study as exploratory; therefore, we did not determine a power calculation. In this light, these results could only be labelled as exploratory.
Results
Patients
During the study period, 235 patients were enrolled and of these:
201 patients (85·6%) [86 males (42·79%) and 115 females (57·21%); average age 56 ± 7 years] completed a 18 months follow‐up and were included in our analysis (Table 1).
34 patients (14·6%) did not complete the study; therefore, were not enclosed in statistical analysis: 21 patients (61·8%) were lost to follow‐up, while 13 (38·2%) were considered non‐completers due to deviation from protocol.
47 of 201 patients (23·38%; 21 males, 26 females) developed PTS during the follow‐up. According to Villalta score 28, 29, PTS was defined as mild in 33 patients (70·21%), as moderate in 8 (17·02%) and as severe in 6 (12·76%). Among the six patients with severe PTS, five had active skin ulceration and one had severe skin induration.
PTS occurred, from the first DVT event, within a median time of 8 months (range 4–12).
We referred to DVT patients with PTS as Group A and DVT patients without PTS as Group B.
Finally 60 healthy blood donors without DVT were included in this study (22 males and 38 females) as control group (Group C).
ELISA test
At admission (T0), ELISA test revealed significantly higher levels of MMPs and NGAL in DVT patients (Group A and Group B) with respect to Group C (P < 0·01) (Figures 1, 2, 3). At this time, we recorded significantly higher levels of MMP‐1 and MMP‐8 in Group A with respect to Group B (P < 0·01) (Figures 1 and 3). In Group A and in Group B, pharmacological and non‐pharmacological treatment induced a significant decrease of symptoms and of plasma MMPs levels with a time‐related pattern (T1–T5) (P < 0·01) (Figures 1, 2, 3), as the decrease of MMP‐1 and MMP‐8 levels was less significant in Group A versus Group B (Figures 1 and 3).
Figure 1.
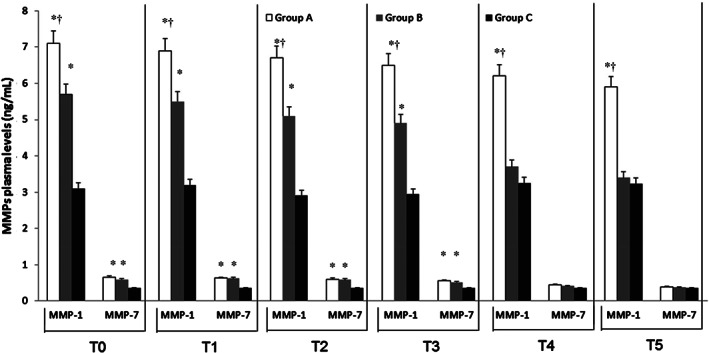
Time course of plasma matrix metalloproteinases (MMP) 1 and 7 at the time of admission (T0) and during the follow‐up [1 (Tl), 4 (T2), 8 (T3), 12 (T4) and 18 (T5) months after the admission] in deep vein thrombosis (DVT) patients with and without post‐thrombotic syndrome (Groups A and B) and in control non DVT patients (Group C). Data are expressed as ng/mL and are the mean ± standard error medium of three evaluations performed through ELISA test.*P < 0.01 versus Group C; † P < 0·01 versus Group B.
Figure 2.
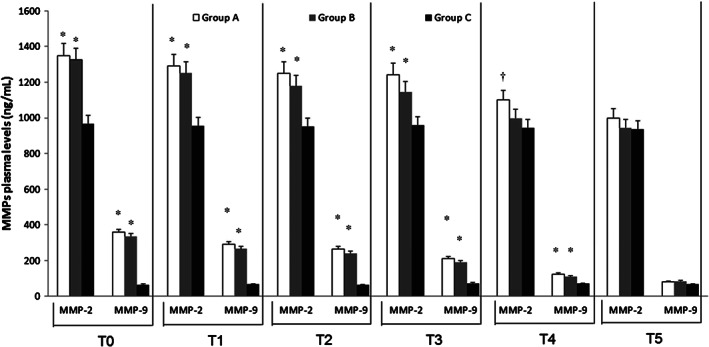
Time course of plasma matrix metalloproteinases (MMP) 2 and 9 at the time of admission (T0) and during the follow‐up [1 (Tl), 4 (T2), 8 (T3); 12 (T4) and 18 (T5) months after the admission] in deep vein thrombosis (DVT) patients with and without post‐thrombotic syndrome (Groups A and B) and in control non DVT patients (Group C). Data are expressed as ng/mL and are the mean ± standard error medium of three evaluations performed through ELISA test. *P < 0.01 versus Group C; † P < 0.05 versus Group C.
Figure 3.
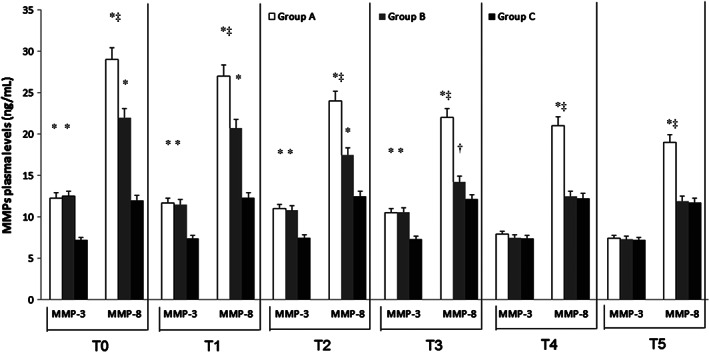
Time course of plasma matrix metalloproteinases (MMP) 3 and 8 at the time of admission (T0) and during the follow‐up [1 (Tl), 4 (T2), 8 (T3); 12 (T4) and 18 (T5) months after the admission] in deep vein thrombosis (DVT) patients with and without post‐thrombotic syndrome (Groups A and B) and in control non DVT patients (Group C). Data are expressed as ng/mL and are the mean ± standard error medium of three evaluations performed through ELISA test. *P < 0·01 versus Group C; † P < 0·05 versus Group C; ‡ P < 0.01 versus Group B.
At admission, the evaluation of plasma NGAL levels revealed significantly higher levels in Group A (283 ± 32 ng/mL) and in Group B (275 ± 29 ng/mL) with respect to control group (57 ± 15 ng/mL) (P < 0·01); plasma NGAL levels significantly reduced with a time‐related pattern (T5: Group A 67 ± 18 ng/mL; Group B 69 ± 16 ng/mL; control group: 59 ± 12 ng/mL).
Moreover, in Group A, patients with severe clinical manifestations showed higher plasma levels of MMP‐1, MMP‐8, MMP‐9 and NGAL with respect to patients with mild symptoms (data not shown).
ELISA test of TIMPs revealed a significant decrease of their plasma values in DVT patients (Groups A and B) with respect to Group C (P < 0·01; Figure 4).
Figure 4.
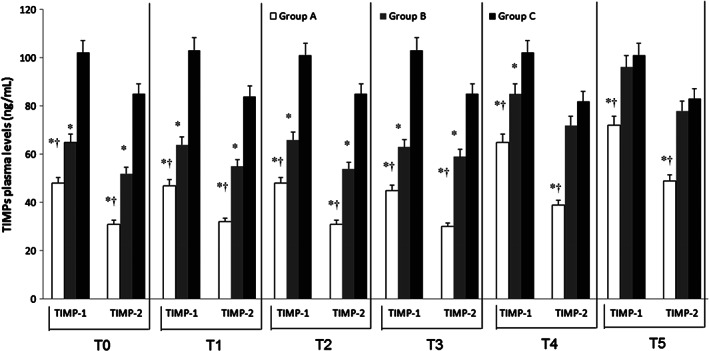
Time course of tissue inhibitors of matrix metalloproteinases (TIMP) 1 and 2 at the time of admission (T0) and during the follow‐up [1 (Tl), 4 (T2), 8 (T3), 12 (T4) and 18 (T5) months after the admission] in deep vein thrombosis (DVT) patients with and without post‐thrombotic syndrome (Groups A and B) and in control non DVT patients (Group C). Data are expressed as ng/mL and are the mean ± standard error medium of three evaluations performed through ELISA test. *P < 0.01 versus Group C; † P < 0.01 versus Group B.
We recorded that both pharmacological and non‐pharmacological treatments increased TIMP values by about 12 months (T4) after the beginning of these treatments as these levels were significantly lower (P < 0·01) with respect to Group C up to the end of this study (Figure 4).
The ratios MMP‐1/TIMP‐1 and MMP‐8/TIMP‐1 as well as MMP‐1/TIMP‐2 and MMP‐8/TIMP‐2 were significantly increased in Group A and Group B with respect to Group C (Tables 2 and 3). Both values were also significantly higher in Group A patients with severe symptoms with respect to those with mild ones (P < 0·01, data not shown).
Table 2.
Time course of MMP‐1/TIMP‐1 and MMP‐1/TIMP‐2 at the time of admission (T0) and during the follow‐up: 1 (T1), 4 (T2), 8 (T3), 12 (T4) and 18 (T5) months after the admission in DVT patients with and without PTS (Group A and Group B) and in control group (Group C)
| T0 | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| MMP‐1/TIMP‐1 | ||||||
| Group A | 0·148*,*** | 0·147*,*** | 0·140*,*** | 0·144*,*** | 0·095*,*** | 0·082*,*** |
| Group B | 0·088* | 0·086* | 0·077* | 0·078* | 0·044* | 0·035 |
| Group C | 0·030 | 0·031 | 0·029 | 0·029 | 0·032 | 0·032 |
| MMP‐1/TIMP‐2 | ||||||
| Group A | 0·229*,*** | 0·216*,*** | 0·216*,*** | 0·217*,*** | 0·159*,*** | 0·120*,*** |
| Group B | 0·110* | 0·100* | 0·094* | 0·083* | 0·051* | 0·044** |
| Group C | 0·036 | 0·038 | 0·034 | 0·035 | 0·040 | 0·039 |
DVT, deep vein thrombosis; MMP, matrix metalloproteinase; PTS, post‐thrombotic syndrome; TIMP, tissue inhibitors of MMPs.
P < 0·01 versus Group C;
P < 0·01 versus Group C;
P < 0·01 versus Group B.
Table 3.
Time course of MMP‐8/TIMP‐1 and MMP‐8/TIMP‐2 at the time of admission (T0) and during the follow‐up: 1 (T1), 4 (T2), 8 (T3), 12 (T4) and 18 (T5) months after the admission in DVT patients with and without PTS (Group A and Group B) and in the control group (Group C)
| T0 | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| MMP‐8/TIMP‐1 | ||||||
| Group A | 0·604*,*** | 0·574*,*** | 0·500*,*** | 0·489*,*** | 0·323*,*** | 0·264*,*** |
| Group B | 0·338* | 0·323* | 0·265* | 0·225* | 0·147** | 0·124 |
| Group C | 0·118 | 0·119 | 0·124 | 0·117 | 0·120 | 0·116 |
| MMP‐8/TIMP‐2 | ||||||
| Group A | 0·935*,*** | 0·844*,*** | 0·774*,*** | 0·733*,*** | 0·538*,*** | 0·388*,*** |
| Group B | 0·423* | 0·376* | 0·324* | 0·241* | 0·174** | 0·153 |
| Group C | 0·141 | 0·146 | 0·147 | 0·142 | 0·149 | 0·141 |
DVT, deep vein thrombosis; MMP, matrix metalloproteinase; PTS, post‐thrombotic syndrome; TIMP, tissue inhibitors of MMPs.
P < 0·01 versus Group C;
P < 0·01 versus Group C;
P < 0·01 versus Group B.
Finally, at baseline (T0), plasma levels of IL‐6 and TNF‐ α were higher in DVT patients (Groups A and B) compared to control group (P < 0·01). These values significantly reduced with a temporal pattern in both groups (Table 4).
Table 4.
ELISA evaluation of plasma interleukin 6 (IL‐6) and tumour necrosis factor alpha (TNF‐α) levels during the study in enrolled patients with DVT (Group A), with DVT + PTS (Group B) and in control group (Group C) at the time of admission (T0) and 1 (T1), 4 (T2), 8 (T3), 12 (T4) and 18 (T5) months later
| T0 | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| Group A | ||||||
| IL‐6 | 9·1 ± 3·2** | 8·7 ± 2·4** | 8·5 ± 2·9** | 7·5 ± 4·2* | 5·4 ± 3·5* | 4·6 ± 3·7 |
| TNF‐α | 7·8 ± 3·2** | 7·4 ± 2·8** | 6·8 ± 2·2** | 5·9 ± 3·1* | 4·2 ± 1·9 | 3·9 ± 1·8 |
| Group B | ||||||
| IL‐6 | 9·4 ± 3·5** | 8·8 ± 3·1** | 7·5 ± 3·6** | 7·2 ± 2·1* | 5·7 ± 2·3* | 3·8 ± 2·4 |
| TNF‐α | 7·6 ± 3·5** | 7·5 ± 2·9* | 6·7 ± 2·1** | 5·7 ± 2·2* | 3·9 ± 2·8 | 3·8 ± 2·5 |
| Group C | ||||||
| IL‐6 | 3·9 ± 2·1 | 3·7 ± 1·9 | 3·7 ± 2·0 | 3·8 ± 2·1 | 3·7 ± 2·3 | 3·8 ± 2·4 |
| TNF‐α | 3·8 ± 2·5 | 3·6 ± 2·2 | 3·9 ± 2·7 | 3·5 ± 2·3 | 3·7 ± 2·4 | 3·7 ± 2·3 |
Values are expressed in pg/mL as mean ± SEM.
DVT, deep vein thrombosis; ELISA, enzyme‐linked immunosorbent assay; PTS, post‐thrombotic syndrome.
P < 0·05 and
P < 0·01 versus Group C.
We recorded that obesity, hypertension and diabetes were independently associated with a higher risk of PTS development (relative risk: obesity 1·95; hypertension 1·32; diabetes: 1·38).
PTS was associated with high levels of MMPs and increased MMP/TIMP ratios in the multivariable model adjusted for traditional cardiovascular risk factors as well as for patients' (age and gender) associated characteristics (Table 5).
Table 5.
Cox regression analysis of PTS patients
| Variable | Unadjusted | Adjusted |
|---|---|---|
| MMP‐1 | 3·72 | 3·66 |
| MMP‐2 | 4·19 | 4·07 |
| MMP‐3 | 6·13 | 6·02 |
| MMP‐7 | 4·15 | 4·00 |
| MMP‐8 | 4·48 | 4·27 |
| MMP‐9 | 4·25 | 4·18 |
| TIMP‐1 | 2·87 | 2·74 |
| TIMP‐2 | 3·21 | 3·10 |
MMP, matrix metalloproteinase; PTS, post‐thrombotic syndrome.
Correlation
Using the Pearson's test we documented a negative correlation between age and plasma levels of MMPs, NGAL, TIMPs, TNF‐α and IL‐6 (−0·23, −0·28, −0·20, −0·24 and −0·21, respectively) and between symptoms and plasma levels of MMPs, NGAL, TIMPs, TNF‐α and IL‐6 (−0·36, −0·31, −0·38, −0·21 and −0·28, respectively).
Discussion
PTS is a chronic complication involving DVT of lower limbs, characterised by pain, itching, tingling, heaviness or swelling. These symptoms increase during standing or walking, and decrease with rest 33. Furthermore, multi‐segment DVT, recurrent DVT and early onset of symptoms predict long‐term PTS which has a significant influence on the quality of life and is related with high costs 34. Several papers have shown that inflammation plays a central role in all phases of pathophysiology of DVT and PTS 35, 36. In the first step, thrombus and venous wall are invaded by leukocytes that, secreting growth factors, proteases and cytokines, contribute to thrombus resolution 35, 36. In the second step, proteolytic enzymes and free radicals released by leukocytes damage venous valves and inactivate fibrinolysis, leading to residual thrombosis and progressive fibrosis 20, 37. In agreement with literature data 21, 23, 38, in our study we recorded, during the acute phase after DVT, high plasma levels of MMPs, NGAL and cytokines, suggesting that an inflammatory network was activated. Moreover, we also recorded higher levels of MMP‐1 and MMP‐8 in patients with PTS. MMP‐1 and MMP‐8 are pro‐fibrotic proteinases and their involvement in inflammatory diseases has been well reported 21, 38, 39, 40, 41.
Considering that PTS is a chronic inflammatory disease 42, 43 and MMP‐1 and MMP‐8 may represent a marker of fibrosis during an inflammatory process, we postulate that patients with DVT expressing high levels of these proteases may be at high risk of developing PTS.
After DVT onset, probably MMP‐1 and MMP‐8 act early on venous valve altering haemodynamic activity and predisposing to the onset of PTS (i.e. secondary varicose veins and non‐healing venous ulcers).
NGAL, a protein belonging to the lipocalin family and expressed by activated neutrophils, modulates the activity of MMP‐9, protecting MMP‐9 from proteolytic degradation. In this study, we documented high levels of NGAL at the time of admission, suggesting a role for NGAL in DVT.
Several papers have shown that drugs with anti‐protease activity are associated with faster and better outcomes in patients with vascular disease 9, 30, 44, 45, 46. In the present study, we documented that pharmacological treatment induced a decrease in MMP and NGAL levels as it has not been possible to reduce the plasma levels of MMP‐1 and MMP‐8. Therefore, we postulate that MMP‐1 and MMP‐8 could be markers for PTS (development, grade, response to therapy).
According to a recent study conducted on the arterial side in patients with critical limb ischaemia, MMP‐1 and MMP‐8 appear to be strictly related to chronic or irreversible complications of vascular disease 47.
TNF‐α is a pro‐inflammatory cytokine capable of inducing adhesion molecule and proteinase gene expression, and plays a role in the progression of several diseases. IL‐6 is a pro‐inflammatory cytokine with multiple functions, and is involved in inflammatory process as well as endothelial dysfunction 27, 31, 45, 46, 48, 49.
In this study, we recorded at the time of admission high levels of TNF‐α and IL‐6 that decreased during the study, confirming the role of the inflammation in the development of DVT.
Finally, we also showed that MMP, NGAL and cytokine levels are higher in DVT patients with respect to healthy subjects. MMP‐1 and MMP‐8 levels were increased during both early stage and acute phase of DVT in patients with PTS. Therefore, these could be considered as predictors for PTS.
However, this study has some limitations: the number of people enrolled is small; we did not evaluate other inflammatory markers (e.g. stress oxidative); we did not evaluate the role of food, job and environment in the development of DVT and PTS; we did not evaluate the role of other drugs (e.g. antibiotics or non‐steroidal anti‐inflammatory drugs, NSAIDs) in our patients during the study and after the enrolment. Furthermore, given the exclusion criteria, the study population is a highly selected patient cohort, precluding definitive conclusions and transfer of the study findings to everyday practice.
However, it is our opinion that this study could increase the knowledge related to a severe disease that can induce severe complication and could increase the opportunity for optimising preventive or therapeutic measures according to individual risk profiles.
Acknowledgements
The authors declare that they have no competing interests. This work received no funding.
References
- 1. Kahn SR. The post thrombotic syndrome. Thromb Res 2011;127(Suppl 3):S89–92. [DOI] [PubMed] [Google Scholar]
- 2. Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh ME, Weitz JI, American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing . The postthrombotic syndrome: evidence‐based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2014;130:1636–61. [DOI] [PubMed] [Google Scholar]
- 3. Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post‐thrombotic syndrome. J Thromb Haemost 2005;3:401–2. [DOI] [PubMed] [Google Scholar]
- 4. Baldwin MJ, Moore HM, Rudarakanchana N, Gohel M, Davies AH. Post‐thrombotic syndrome: a clinical review. J Thromb Haemost 2013;11:795–805. [DOI] [PubMed] [Google Scholar]
- 5. Kahn SR. The post‐thrombotic syndrome. Hematology Am Soc Hematol Educ Program 2010;2010:216–20. [DOI] [PubMed] [Google Scholar]
- 6. Lowe GD. Management of deep vein thrombosis to reduce the incidence of post‐thrombotic syndrome. Phlebology 2010;25(Suppl 1):9–13. [DOI] [PubMed] [Google Scholar]
- 7. Strijkers RH, Wittens CH, Kahn SR. Villalta scale: goals and limitations. Phlebology 2012;27(Suppl 1):130–5. [DOI] [PubMed] [Google Scholar]
- 8. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen 2013;21:395–401. [DOI] [PubMed] [Google Scholar]
- 9. Serra R, Gallelli L, Conti A, De Caridi G, Massara M, Spinelli F, Buffone G, Caliò FG, Amato B, Ceglia S, Spaziano G, Scaramuzzino L, Ferrarese AG, Grande R, de Franciscis S. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and ulcers of lower limbs. Drug Des Devel Ther 2014;8:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serra R, Grande R, Montemurro R, Butrico L, Caliò FG, Mastrangelo D, Scarcello E, Gallelli L, Buffone G, de Franciscis S. The role of matrix metalloproteinases and neutrophil gelatinase‐associated lipocalin in central and peripheral arterial aneurysms. Surgery 2015;157:155–62. [DOI] [PubMed] [Google Scholar]
- 11. Serra R, Grande R, Buffone G, Scarcello E, Tripodi F, Rende P, Gallelli L, de Franciscis S. Effects of glucocorticoids and TNF‐alfa inhibitors on both clinical and molecular parameters in patients with Takayasu Arteritis. J Pharmacol Pharmacother 2014;5:193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serra R, Volpentesta G, Gallelli L, Grande R, Buffone G, Lavano A, de Franciscis S. Metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin plasma and tissue levels evaluation in middle cerebral artery aneurysms. Br J Neurosurg 2014. DOI: 10.3109/02688697.2014.913777. [DOI] [PubMed] [Google Scholar]
- 13. Phillips LJ II, Sarkar R. Molecular characterization of post‐thrombotic syndrome. J Vasc Surg 2007;45(Suppl A):A116–22. [DOI] [PubMed] [Google Scholar]
- 14. Deroo S, Deatrick KB, Henke PK. The vessel wall: a forgotten player in post thrombotic syndrome. Thromb Haemost 2010;104:681–92. [DOI] [PubMed] [Google Scholar]
- 15. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827–39. [DOI] [PubMed] [Google Scholar]
- 16. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2008;75:346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alsaigh T, Pocock ES, Bergan JJ, Schmid‐Schönbein GW. Acute venous occlusion enhances matrix metalloprotease activity: Implications on endothelial dysfunction. Microvasc Res 2011;81:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chabasse C, Siefert SA, Chaudry M, Hoofnagle MH, Lal BK, Sarkar R. Recanalization and flow regulate venous thrombus resolution and matrix metalloproteinase expression in vivo . J Vasc Surg 2015;3:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deatrick KB, Luke CE, Elfline MA, Sood V, Baldwin J, Upchurch GR Jr, Jaffer FA, Wakefield TW, Henke PK. The effect of matrix metalloproteinase 2 and matrix metalloproteinase 2/9 deletion in experimental post‐thrombotic vein wall remodeling. J Vasc Surg 2013;58:1375–1384.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deatrick KB, Elfline M, Baker N, Luke CE, Blackburn S, Stabler C, Wakefield TW, Henke PK. Postthrombotic vein wall remodeling: preliminary observations. J Vasc Surg 2011;53:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roumen‐Klappe EM, Janssen MC, Van Rossum J, Holewijn S, Van Bokhoven MM, Kaasjager K, Wollersheim H, Den Heijer M. Inflammation in deep vein thrombosis and the development of post‐thrombotic syndrome: a prospective study. J Thromb Haemost 2009;7:582–7. [DOI] [PubMed] [Google Scholar]
- 22. Saedon M, Stansby G. Post‐thrombotic syndrome: prevention is better than cure. Phlebology 2010;25(Suppl 1):14–9. [DOI] [PubMed] [Google Scholar]
- 23. Bouman AC, Smits JJ, Ten Cate H, Ten Cate‐Hoek AJ. Markers of coagulation, fibrinolysis and inflammation in relation to post‐thrombotic syndrome. J Thromb Haemost 2012;10:1532–8. [DOI] [PubMed] [Google Scholar]
- 24. Bouman AC, Cheung YW, Spronk HM, Schalkwijk CG, ten Cate H, ten Wolde M, ten Cate‐Hoek AJ. Biomarkers for post thrombotic syndrome: a case–control study. Thromb Res 2014;134:369–75. [DOI] [PubMed] [Google Scholar]
- 25. Mosevoll KA, Lindås R, Tvedt TH, Bruserud Ø, Reikvam H. Altered plasma levels of cytokines, soluble adhesion molecules and matrix metalloproteases in venous thrombosis. Thromb Res 2015;136:30–9. [DOI] [PubMed] [Google Scholar]
- 26. de Franciscis S, Serra R. Matrix metalloproteinases and endothelial dysfunction: the search for new prognostic markers and for new therapeutic targets for vascular wall imbalance. Thromb Res 2015;136:5–6. [DOI] [PubMed] [Google Scholar]
- 27. Serra R, Grande R, Butrico L, Buffone G, Caliò FG, Squillace A, Rizzo BA, Massara M, Spinelli F, Ferrarese AG, De Caridi G, Gallelli L, de Franciscis S. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J 2014. DOI: 10.1111/iwj.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Wolf MA, Wittens CH, Kahn SR. Incidence and risk factors of the post‐thrombotic syndrome. Phlebology 2012;27(Suppl 1):85–94. [DOI] [PubMed] [Google Scholar]
- 29. Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post‐thrombotic syndrome. J Vasc Surg 2013;57:254–61. [DOI] [PubMed] [Google Scholar]
- 30. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2015;12:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J. 2014. doi: 10.1111/iwj.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allal‐Elasmi M, Zayani Y, Zidi W, Zaroui A, Feki M, Mourali S, Mechmeche R, Kaabachi N. The measurement of circulating matrix metalloproteinase‐8 and its tissue inhibitor and their association with inflammatory mediators in patients with acute coronary syndrome. Clin Lab 2014;60:951–6. [DOI] [PubMed] [Google Scholar]
- 33. Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698–707. [DOI] [PubMed] [Google Scholar]
- 34. Bäckman K, Carlsson P, Kentson M, Hansen S, Engquist L, Hallert C. Deep venous thrombosis: a new task for primary health care. A randomised economic study of outpatient and inpatient treatment. Scand J Prim Health Care 2004;22:44–9. [DOI] [PubMed] [Google Scholar]
- 35. Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008;28:387–91. [DOI] [PubMed] [Google Scholar]
- 36. Wakefield TW, Henke PK. The role of inflammation in early and late venous thrombosis: are there clinical implications? Semin Vasc Surg 2005;18:118–29. [DOI] [PubMed] [Google Scholar]
- 37. Henke PK, Varma MR, Deatrick KB, Dewyer NA, Lynch EM, Moore AJ, Dubay DA, Sukheepod P, Pearce CG, Upchurch GR Jr, Kunkel SL, Franz MG, Wakefield TW. Neutrophils modulate post‐thrombotic vein wall remodeling but not thrombus neovascularization. Thromb Haemost 2006;95:272–81. [DOI] [PubMed] [Google Scholar]
- 38. Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post‐thrombotic syndrome. Thromb Haemost 2009;101:505–12. [PubMed] [Google Scholar]
- 39. Capone F, Guerriero E, Sorice A, Maio P, Colonna G, Castello G, Costantini S. Characterization of metalloproteinases, oxidative status and inflammation levels in the different stages of fibrosis in HCV patients. Clin Biochem 2012;45:525–9. [DOI] [PubMed] [Google Scholar]
- 40. Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 2014;7:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, Grande R, Serra R, de Franciscis S. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J 2013. DOI: 10.1111/iwj.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jezovnik MK, Poredos P. Idiopathic venous thrombosis is related to systemic inflammatory response and to increased levels of circulating markers of endothelial dysfunction. Int Angiol 2010;29:226–31. [PubMed] [Google Scholar]
- 43. de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, Buffone G, Serra R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J 2015;12:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Act Phlebol 2013;14:99–107. [Google Scholar]
- 45. de Franciscis S, De Caridi G, Massara M, Spinelli F, Gallelli L, Buffone G, Caliò FG, Butrico L, Grande R, Serra R. Biomarkers in post‐reperfusion syndrome after acute lower limb ischaemia. Int Wound J 2014. DOI: 10.1111/iwj.12392. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siniscalchi A, Gallelli L, Malferrari G, Pirritano D, Serra R, Santangelo E, De Sarro G. Cerebral stroke injury: the role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol 2014;25:131–7. [DOI] [PubMed] [Google Scholar]
- 47. De Caridi G, Massara M, Spinelli F, David A, Gangemi S, Fugetto F, Grande R, Butrico L, Stefanelli R, Colosimo M, de Franciscis S, Serra R. Matrix metalloproteinases and risk stratification in patients undergoing surgical revascularisation for critical limb ischaemia. Int Wound J. 2015. doi: 10.1111/iwj.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gallelli L, Galasso O, Falcone D, Southworth S, Greco M, Ventura V, Romualdi P, Corigliano A, Terracciano R, Savino R, Gulletta E, Gasparini G, De Sarro G. The effects of nonsteroidal anti‐inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage 2013;21:1400–8. [DOI] [PubMed] [Google Scholar]
- 49. de Franciscis S, Butrico L, Settimio UF, Grande R, Serra R. The endothelial dysnfunction in chronic venous disease: a systematic review. Acta Phlebol 2015. [Google Scholar]


