Abstract
Detection of subcutaneous tissue damage before it is visible can trigger early intervention and decrease hospital‐acquired pressure ulcer (HAPU) rates. The objective of this two‐phase study was to evaluate the clinical utility of the Sub‐Epidermal Moisture (SEM) Scanner (Bruin Biometrics (BBI), LLC), a hand‐held device that assesses increases in interstitial fluid or subepidermal moisture, indicating early tissue damage. Phase 1: Patients were provided standard‐of‐care risk assessment and interventions and were scanned with the SEM Scanner, but the resulting SEM scores were not used to determine interventions. This gave a baseline pressure ulcer incidence rate. Phase 2: This phase is the same as Phase 1 except the resulting SEM scores were used in conjunction with risk assessment scores to determine appropriate interventions and care planning. In Phase 1, 12 of the 89 subjects or 13.5% developed visible pressure ulcers—4 Stage I's, 6 Stage II's, 1 Stage III, and 1 deep tissue injury. In Phase 2, 2 of the 195 subjects or 1.0% developed visible pressure ulcers—1 Stage I and 1 Stage II. Patients in Phase 2 were more incontinent, less mobile, and had longer lengths of stay than those in Phase 1. Use of the Scanner resulted in a 93% decrease in HAPU. No deep injuries developed in Phase 2.
Keywords: patient safety, pressure ulcer, prevention, SEM scanner, subepidermal moisture
1. INTRODUCTION
Pressure ulcers (PUs)—sometimes called pressure injuries—have been regarded as a significant health issue in primary and secondary care settings for decades. On a human scale, they may cause intense pain, severely curtail quality of life, and contribute to increased morbidity and mortality rates.1, 2 On a larger scale, PUs contribute to increased treatment costs and extended hospital stays for health care systems.1, 3 PUs, especially those that develop during a patient's hospitalisation, are high on clinical and policy agendas as they are recognised as indicators of poor quality of care.4 PUs are diagnosed and classified, or staged, based on the depth of tissue damage and wound characteristics as described by the International Pressure Ulcer Guidelines.4
In Canada, PUs are 1 of the 50 measures of patient safety and in‐hospital care that are reported by every hospital and made available to the general public in a report card system.5 Report cards also allow hospitals to compare themselves with their peers and to identify the most effective places to invest resources to improve care. In the United States, Centers for Medicare and Medicaid Services publishes Hospital Compare, a website rating hospitals with a star system summarising 57 measures of quality of care, including PUs.6 Typically, the frequency of PUs in hospitals is measured and reported in prevalence (the number of all PUs present in a given facility at a given point in time) and incidence (the proportion of new PUs occurring per set number of patients entering a facility). Incidence is also known as the hospital acquired pressure ulcer (HAPU) rate. 4 In both Canada and the United States, PUs are considered in a hospital's accreditation, which is critical to continuing operations.
Incidence is reported in hospitals across the world as a key measure of quality. In 2013, Bergquist‐Beringer and colleagues reported a 3.6% HAPU rate among all surveyed inpatients and 7.9% among those at risk of PUs in a study of 1419 hospitals and 710 626 patients in US acute care hospitals.7 Drosler et al. reported on HAPU in the United States, United Kingdom, Sweden, Spain, Germany, Canada, and Australia.8 Of particular application to this study is a comprehensive examination by Woo et al. of HAPU in all health care settings in Ontario, Canada, that showed a 4.5% incidence rate and a 10.2% prevalence rate in acute care hospitals between 2010 and 2013. In addition, 28% of PUs in long‐term care settings in Ontario developed within 1 week after discharge from acute care. The authors of that study theorise that some of these may have begun developing during the acute care hospitalisation and only became visible much later.9
Deep tissues are more susceptible to pressure and shear than the visible skin, so damage happens below the skin before its effects show on the skin.10 All soft tissues, especially subcutaneous tissues, respond to pressure, shear, and deformation with inflammation and oedema, which lead to ischaemia, nutrient and oxygen depletion, oxidative stress, and the accumulation of toxic metabolites in these sensitive tissues.11 These changes progress to cellular damage, then localised tissue damage in the deep tissues, and extend into the more superficial tissues. These changes are invisible to the eye (Figure 1). The damage can be happening days before it is visible on the epidermis.11
Figure 1.
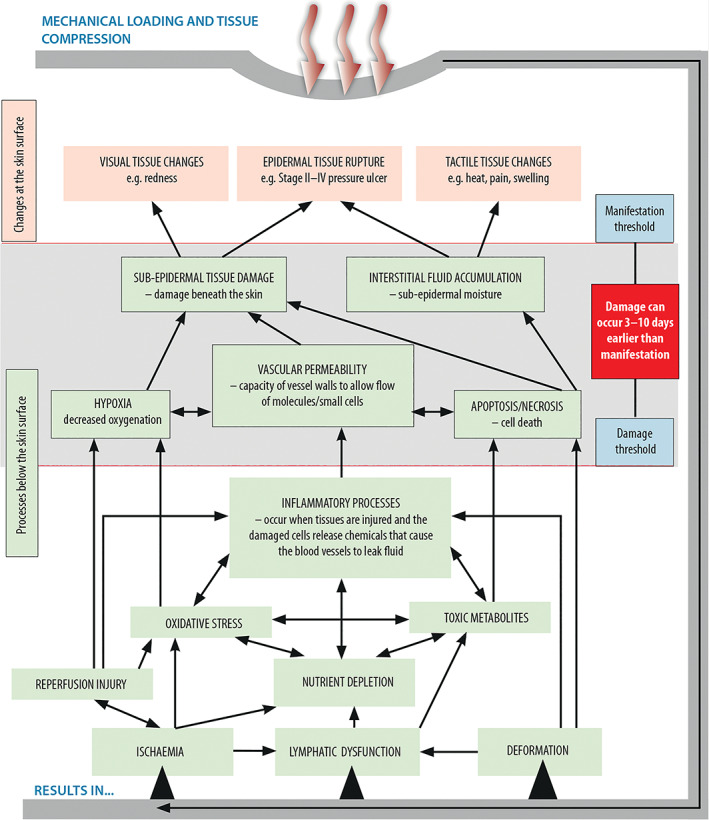
Biological processes that lead to tissue damage (adapted from Moore et al, 201718 [Colour figure can be viewed at wileyonlinelibrary.com]
The most important intervention to prevent or to treat PUs is to reduce or remove pressure.12 It is widely believed that most PUs can be prevented with appropriate use of these interventions prophylactically. The following bedside steps are frequently recommended to prevent HAPU in acute care hospitals: dedicated wound specialist, staff education, skin and pressure risk assessment on admission and at regular intervals during stay, risk assessment linked to interventions, pressure redistribution surfaces, frequent repositioning, nutritional support, and moisture management.4
1.1. Site
Scarborough Health Network (SHN), formerly Rouge Valley Hospital System (RVHS) and The Scarborough Hospital (TSH) in Toronto, Ontario, Canada, is comprised of three hospitals and five satellite sites.
SHN has systematically tracked hospital‐wide PU prevalence and incidence on a monthly basis between 2010 and 2016 (Figure 2) and annually as part of the International Pressure Ulcer Prevalence Survey conducted by Hill‐Rom (Batesville, Indiana). Reported incidence is calculated through a visual inspection of the skin of all inpatients on a specific day by the Save Our Skin (SOS) Team, comprised of specially trained registered practical nurses (RPNs, equivalent to licensed practical nurses in the United States) whose responsibilities include PU monitoring, implementing PU prevention initiatives, bedside staff education, wound care planning, and allocation of support surfaces.
Figure 2.
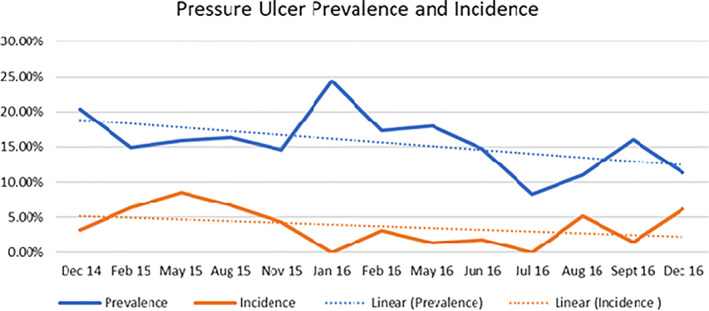
Monthly prevalence and incidence rates for SHN Dec 2014 to Dec 2016. Abbreviations: SHN, Scarborough Health Network [Colour figure can be viewed at wileyonlinelibrary.com]
In 2010, hospital management and staff began to implement a variety of strategies to address high PU rates, compared with those reported in the literature and with the peer hospitals, and began to implement changes to successfully prevent and manage PUs. These strategies began with the employment of a Nurse Specialised in Wound, Ostomy and Continence (NSWOC) or Enterostomal Therapy (ET) nurse to oversee the programme. Figure 3 illustrates some of the strategies or interventions employed by the ET nurse and SRH. These interventions are based on a Cochrane review by Joyce et al., which identified categories of appropriate organisational interventions that must be undertaken to successfully prevent and treat PUs.13 An algorithm was developed to direct staff to implement various measures for pressure management depending on the individual patient's risk for developing a pressure ulcer. An abbreviated version of this algorithm is provided in Figure 4.
Figure 3.
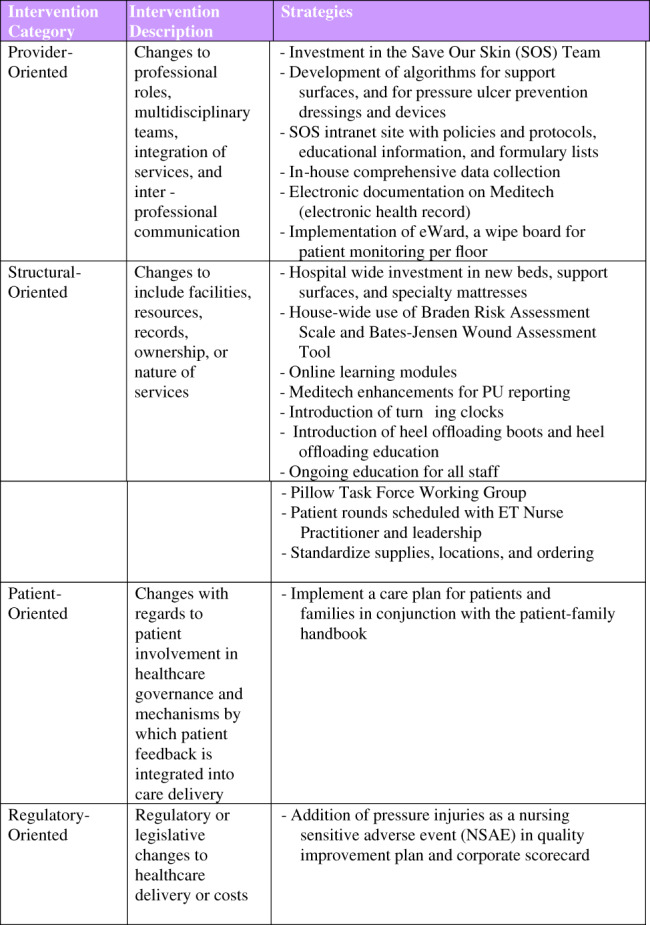
Strategies or interventions employed by SHN, beginning in 2010, to reduce HAPU.13 Abbreviations: HAPU, hospital‐acquired pressure ulcer [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
Abbreviated pressure management algorithm used at SHN to illustrate general preventive interventions used in phases 1 and 2
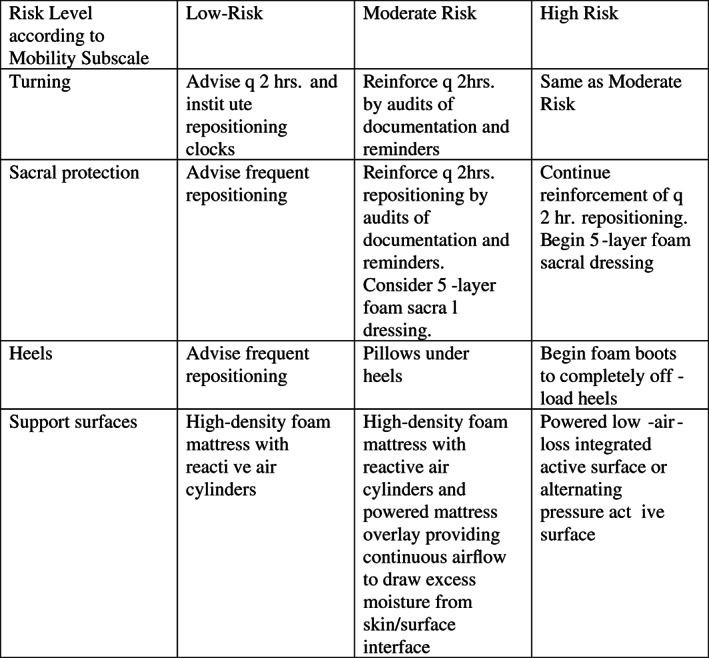
Support surfaces are an important intervention in HAPU prevention in hospitals. This hospital has a protocol for assigning various levels of support surfaces, including foam, foam and air cylinder, alternating pressure, low air loss, and powered air surfaces, depending on the Braden Scale Total Score and the Mobility Subscale of the Braden. A Mobility Score of 3 or lower qualifies a patient for a more advanced support surface.
These efforts resulted in a meaningful reduction in hospital‐wide PU incidence to 3%, which is lower than the national average. However, hospital leadership was not satisfied and set a goal to eliminate all avoidable PUs. Despite implementation of additional initiatives, HAPU rates plateaued.
In 2016, nursing management was introduced to a hand‐held tissue assessment device called the SEM Scanner (BBI, LLC, Los Angeles, CA). (See Figure 5) The scanner was of interest because it is a non‐invasive device that is reportedly being used in the United Kingdom, Ireland, and Spain to reduce the incidence of PUs by detecting tissue damage below the skin an average of 3.9 days before that damage is visible to standard nursing assessment. 14, 15
Figure 5.
SEM Scanner™ 200 [Colour figure can be viewed at wileyonlinelibrary.com]
1.2. Device
The SEM Scanner is a non‐invasive, portable wound assessment device designed to be used as an adjunct to clinical judgement in the detection of early pressure‐induced tissue damage (PUs or deep tissue injury) as part of PU prevention programmes. The SEM Scanner offers an objective method for the detection of early tissue damage before the damage becomes visible to the unaided eye.16, 17, 18, 19
The SEM Scanner assesses changes in sub‐epidermal moisture (SEM), a biophysical indicator of localised oedema. In the earliest stages of PU development, apoptosis, necrosis, and the inflammatory process lead to leakage from vasculature and other changes that modify the underlying structure of the damaged tissue. This results in an increase in the interstitial fluid or SEM, which is assessed by the scanner.11, 19 These changes have been shown to occur 3–10 days before visually identifiable skin breakdown. 20, 21, 22, 23, 24
The SEM Scanner detects these changes in SEM using an integrated electrode sensor to measure, using capacitance, the relative quantity of skin, and tissue water, when in the presence of an electrical force.25, 26, 27 The scanner provides a numerical score, providing an objective way for staff and patients to interpret and “see” the invisible changes.14, 16, 17, 28 A resulting score, called the SEM delta value, of 0.6 or greater may indicate that a patient may have underlying tissue damage.
The scanner is placed on an anatomical site, usually over a bony prominence, and multiple readings are taken (recommended six at the sacrum and four at the heel) so that the delta can be calculated. The delta is derived from the difference between the highest and the lowest reading for each anatomical site A delta of 0.6 or above indicates very localised differences in the SEM or oedema, thus indicating non‐visible early‐stage damage that may manifest at the skin surface as a pressure injury. The delta score is recorded and can be used to help determine interventions. Complete instructions for use can be found at www.bruinbiometrics.com.
The scanner is designed to be used over intact skin. It is not used on open wounds.
A series of papers have explored the successful use of SEM in the early detection of PU development in persons with spinal cord injury, in acute care settings, and in nursing homes.20, 25, 26, 29 The scanner has been shown to detect PUs up to 10 days ahead of visual assessment.20, 28, 30, 31 Data from use in clinical practice scanning 632 patients across nine hospitals in the United Kingdom, Canada, and Spain reported results that five (56%) hospitals achieved 100% reduction in HAPUs and two (22%) hospitals achieved 87 to 90% reduction in HAPU in the high‐risk patient populations that were scanned. It is reported that the two that did not achieve these reductions lacked leadership to implement necessary changes to practice.30
In April 2016, a two‐phase evaluation was undertaken at the 504‐bed SHN community hospital, Centenary Hospital, to assess this new technology to assist clinicians in reducing HAPU incidence.
2. PURPOSE
The objective is to evaluate the clinical utility of incorporating the SEM Scanner into clinical workflow and of associating interventions informed by the SEM Scanner with decreases in PU incidence. This product evaluation compared outcomes from using a standard prevention and intervention hospital protocol with interventions supplemented by information from the scanner. Clinical utility is measured by:
Clinical impact as measured by reductions in HAPU and
Nurse experience as provided verbally from users of the scanner
Hypothesis: If damage was detected subcutaneously by the presence of SEM at the inflammation stage before visible manifestation, and PU prevention interventions were based on both standard protocol for risk and on scanner‐detected damage, then:
All or most of HAPUs could be prevented.
Equipment and staff time could be targeted most effectively to those who need it most.
Care plans could be customised to the individual patient
The effectiveness of interventions could be objectively measured.
3. MATERIALS & METHODOLOGY
3.1. Evaluation methodology
Centenary Hospital is a large community hospital consisting of medical; complex continuing care; rehabilitation; cardiac; critical care; surgery; women's health; children's health; and mental health units.
The evaluation was structured in two phases. In Phase 1, patients were provided standard of care for risk assessment and interventions and were scanned with the SEM Scanner, but the resulting SEM scores were not used to determine interventions. In Phase 2, patients were provided standard of care for risk assessment and were scanned with the SEM Scanner, and the resulting SEM scores were used in conjunction with risk assessment scores to guide appropriate interventions and care planning. The two phases were designed to assess if potential improvements in PU incidence in Phase 2 could be attributed to the Hawthorne effect—a change in outcomes because of a change in behaviour of the participants as a result of being involved in a study and being observed rather than the object of study.
3.1.1. Phase 1
Phase 1 was conducted from April 4 to May 4, 2016. The purpose of this phase was two‐fold: (1) to establish a baseline PU incidence and (2) to determine if a reduction in PU incidence might be seen by using standard protocols reinforced by daily follow up by the SOS team as they would be scanning the patient daily. During this phase, patients were assessed and monitored for their risk for PU development or for the existence of a PU as usual, and they were scanned with the scanner. The SEM delta value was recorded, but interventions were based only on the site's standardised protocols. The SEM delta value could not be used to determine interventions because the SOS staff were not instructed in the meaning of the scanner results nor provided guidelines on how to act on those results. Thus, the SEM delta value could not have influenced care.
Patient subjects
This cohort included patients (N = 89) newly admitted to the medical/stroke unit during the study period. This unit was chosen because of the high incidence of PU and the willingness of the nurse manager to participate. This is a high‐capacity 32‐bed unit. Basic demographics of the unit and the subject cohort are the same: gender: females 55%: males 45%; weight: 45 to 68 kg—>50%, 68 to 90 kg—20 to 35%; age: majority >60 years old, minority >80 years old. More than 60% were incontinent and required briefs. More than 60% had a Braden score of 14 or less. More than 60% had length of stay of 4 to 7 days. Patients with Braden Mobility Subscale scores of 3 or less were targeted because this sub‐score is the first trigger to increase interventions according to the standard protocol described earlier. There is a strong association between activity and mobility limitations and the development of PUs.4 Patients with open wounds on either the sacrum or heels were excluded as this is a contraindication for use with the scanner. Patients who were unable to be positioned comfortably to conduct regular scanning were excluded.
Method
Patients were assessed and scanned by the trained examiners five times per week for 1 month or to the end of their length of stay. At each assessment, readings were taken at each high‐risk body site—sacrum, right heel, and left heel—as per manufacturer instructions.
3.1.2. Phase 2
Phase 2 was conducted from May 4–September 30, 2016. The purpose of this phase was to measure and assess incidence when patients are scanned by the examiners, and interventions are based on both the scanner reading and standardised assessment. In Phase 2, the SOS team was told the meaning of the SEM delta values and given instruction on how to use those values to supplement the Braden risk assessment scales in designing customised plans of care for patients, with the goal of preventing nosocomial PUs.
Patient subjects
Unfortunately, internal logistics prevented the use of the medical/stroke unit for Phase 2. In order to most closely match the patient populations of the two phases, patients for Phase 2 were identified from a pool of newly admitted patients to the alternative care unit (ACU) (N = 29) and from a pool of patients admitted to any unit in the hospital from the emergency room (N = 166) who had a score of 3 or lower on the Braden Mobility sub‐score. Alternative care serves patients who are awaiting placement for long‐term care or are too stable to stay in hospital but are too complex to be admitted for long‐term care, for example, care requires IV therapies. While recruited from different wards, patients with comparable characteristics and risk profiles were recruited. Demographics of this entire cohort were similar to those of the Phase 1 cohort, except that more than 90% were incontinent and required the use of briefs, had a total Braden score of 14 or less, and had an average length of stay of 14 to 90 days. In short, as a group, they were at higher risk for PU development than the Phase 1 group.
Patients with open wounds on either the sacrum or heels were excluded as this is contraindicated for use with the scanner. Patients who refused or were unable to be moved to conduct scanning at regular intervals were also excluded.
Method
As in Phase 1, patients were assessed and scanned by the trained examiners. Patients in the ACU were scanned three to five times per week for up to 14 days. Emergency room patients were scanned in the ER and then three times during the first 7 days of stay. The differences in time frames are because of the lengths of stay for each of the two groups. ACU patients averaged 30‐day stays, so tracking was carried out for up to 14 days. Patients who came from the ER averaged only 10‐day stays, so tracking was carried out for a shorter time period than the ACU patients. At each assessment, readings were taken at the sacrum, right heel, and left heel to determine the SEM delta value.
In Phase 2 of the study, examiners used SEM delta values of 0.6 or greater as indicators of high risk or tissue damage, even if the Braden score and subscales indicated low risk. These SEM values triggered increased interventions such as more advanced support surfaces, increased turning and repositioning schedules, more frequent full‐body assessment by the SOS team member, heel boots or positioning devices, and a special sacral dressing. The subscales of the Braden and the SEM value at the individual body site directed targeted interventions.
3.2. Participants and training
This evaluation was led by the ET wound specialist nurse practitioner. There was no need for ethics review board approval as this was undertaken as a quality improvement project. Clinicians selected to participate as examiners were chosen by the lead clinician and consisted of nine nurses on the SOS team, as described in Figure 3.
Examiners completed a series of training activities provided by the manufacturer‘s representative. Examiner preparation and training consisted of:
Clinical orientation—a didactic review and education of the role of sub‐epidermal moisture in pressure‐induced tissue damage.
Product demonstration—detailed didactics and hands‐on demonstration with tips on proper positioning of device on skin (eg, what is good versus better placement position). Included in demonstration was a review on device operations, cleaning, placement locations, and clinical interpretation of SEM delta value of 0.6 and above indicating possible tissue damage. Examiners were asked to demonstrate comprehension on the use of the device by completing a reverse hands‐on exercise by demonstrating on the participants and/or each other.
Skill Checks: The trainer performed one‐on‐one skill checks 2 weeks after initial training to ensure accuracy and consistency of use of the device and data collection.
The trainer was available to the examiners throughout the course of the evaluation. The lead clinician instructed the examiners on filling out the data collection form provided by the manufacturer (Figure 6), did skill checks, and monitored nurse experience and feedback.
Figure 6.
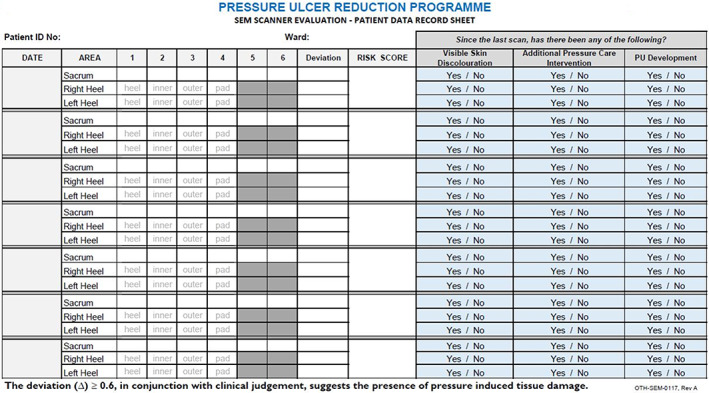
Data collection tool [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Data collection
Daily assessments included SEM assessment, risk assessment, and visual assessment (See Table 1).
Table 1.
Assessments and data collected
| Assessment | Data collected |
|---|---|
| SEM assessment | SEM Delta value (as displayed on the SEM scanner device) |
| Risk assessment |
Braden sub‐scores Braden total risk score |
| Visual assessment |
Observations of skin discolouration Observations of erythema Observations of PU development |
PU, Pressure ulcers.
These data were recorded in the data collection tool. This tool was used in evaluations at many of the study sites referenced above. The tool was customised to fit this facility's protocols. In addition, all pressure care interventions that were deemed necessary as part of patient care planning were also recorded in the data collection tool after each patient assessment session.
In Phase 1, all patient assessments were recorded on one page for the whole unit for each day. A new page was used each day so that intervention decisions for an individual patient were not biased by previous scanner readings but were based solely on standard assessment that day.
In Phase 2, each individual patient's assessments and delta scores were recorded consecutively on a single page throughout their participation, making comparisons with assessments and interventions from previous days easy. This ensured consistency in interventions and cued the examiner to add interventions if risk increased, as indicated by increasing or consistently high scanner readings or decreasing Braden scores for the individual patient. Data were used to justify the need for increased interventions on a continuous basis and to provide all data for final analysis.
Nurse feedback was collected informally in conversations between the examining clinicians and the lead clinician. This feedback was monitored throughout both phases of the evaluation and at the end of Phase 2. Comments made were recorded by the lead clinician in personal memos.
3.4. Analysis
Data from the individual data collection sheets were collated onto single spreadsheets by the medical unit using Microsoft Excel (Microsoft, Bellevue, WA, USA). Descriptive statistics were used to determine incidence rates in each phase (calculated as the number of PU that developed divided by the entire number of subjects in each phase). Using Phase 1 incidence as a baseline, we were able to compare the incidence of PUs when SEM delta values were incorporated into patient assessments and care planning. The lead clinician was also able to assess interventions used and compare, at a glance, the Braden scores with the SEM delta values.
4. RESULTS
4.1. Clinical impact
4.1.1. Phase 1
Of the 89 patients scanned in Phase 1, 12 developed PUs despite standard protocol for risk assessment and interventions. The distribution of PUs is displayed in Table 2.
Table 2.
Phase 1 distribution of pressure ulcer stage and occurrence
| Pressure ulcer classification | Number (%) |
|---|---|
| Stage I | 4 (33%) |
| Stage II | 6 (50%) |
| Stage III | 1 (8%) |
| Stage IV | 0 (0%) |
| Unstageable/deep tissue injury | 1 (8%) |
| Total | 12 (100%) |
The incidence rate for Phase 1 was 13.5%.
4.1.2. Phase 2
Twenty‐nine patients from the AC unit and 166 patients from the ER admissions group were followed. Of the total 195 patients scanned, 2 developed PUs, 1 from each group. The distribution of PUs is displayed in Table 3.
Table 3.
Phase 2 distribution of pressure ulcer stages and occurrence
| Pressure ulcer classification | Number (%) |
|---|---|
| Stage I | 1 (50%) |
| Stage II | 1 (50%) |
| Stage III | 0 (0%) |
| Stage IV | 0 (0%) |
| Unstageable/deep tissue injury | 0 (0%) |
| Total | 2 (100%) |
One PU was a Stage I and healed before patient discharge from the hospital, and the other was a Stage II and was still present on patient discharge, who was then lost to follow up.
The incidence rate for Phase 2 was 1.0%.
There was a significant difference in the observed PU incidence between Phase 1 and Phase 2 (see Table 4). This would suggest a 93% reduction in PU incidence when patient assessments and care planning incorporated SEM delta values.
Table 4.
Comparison of incidence between phase 1 and phase 2
| Phase | Dept | N | PU | Phase incidence |
|---|---|---|---|---|
| 1 | Med/stroke | 89 | 12 | |
| Total phase 1 | 89 | 12 | 13.5% | |
| 2 | ER | 166 | 1 | |
| 2 | ALC | 29 | 1 | |
| Total phase 2 | 195 | 2 | 1.0% |
PU, Pressure ulcers.
4.2. Nurse experience
Nurse experience and feedback on the device improved with experience. Initial training and follow‐up skills checks were important in ensuring consistency and accuracy. Examiners gained confidence in their skills and in the results of the implementation of the scanner as their experience increased and data results were shared with them.
5. DISCUSSION
PUs are believed to largely be preventable, but in order to make the best use of both human and physical resources, and maintain patient comfort and dignity, it is important to identify the people most at risk and deliver timely preventative care.32 Many PU assessment tools have been developed to help to identify those who are most at risk. International PU guidelines and most facilities call for the use of risk assessment scales as part of the standard of care for PU prevention and management. Common PU risk assessment tools include Waterlow, Braden, and Norton assessment scales. These scales are tools in looking for common risk factors, including skin moisture, age, haematological measures, mobility, and nutrition.
In a systematic review by Chou et al.,2 the Braden Scale had a sensitivity of 74%; in other words, 26% of the patients at risk will be missed. According to Vanderwee et al, there is no sound evidence base that supports the superior clinical effectiveness of using risk assessment scales.33 In this study that examined the use of the Braden Scale versus clinical judgement alone, while double the proportion of patients received preventative interventions, the resulting incidence was the same in both populations. This could be because of several factors including: low sensitivity and specificity of risk assessment tools, low inter‐rater reliability, infrequent or incomplete assessment, clinicians not matching risk assessment to intervention, and using tools designed for specific populations across all patient groups (non‐targeted care).32
At SHN, system‐wide, the standard of care for PU prevention included the use of the Total Braden Scale Score and the Mobility Subscale Score. Because of the limitations of the risk assessment tools cited above, and specifically low sensitivity, the Scale may miss a sizeable portion of patients at risk for PUs.2, 32 This helped to explain, in part, why incidence rates were still above the desired levels and supported the need for new tools, such as the SEM Scanner, that are able to provide incremental information to what can be clinical or visibly observed.
The incidence rate of 13.5% in Phase 1 was much higher than the reported hospital‐wide incidence rates seen in Figure 2. There are several reasons for this difference. The medical/stroke unit normally has a high incidence rate as a large number of patients admitted to this ward are at high risk because of low mobility scores. The hospital‐wide incidence rate is calculated from a 1‐day snapshot of all patients in the hospital, while the incidence rate for this study was conducted longitudinally over 30 days. Finally, the hospital‐wide snapshot includes all patients, without regard for their mobility or risk level; therefore, the number of high risk patients becomes diluted by the large number of low‐risk patients, and the incidence rate is affected.
Nosocomial PU rates dropped significantly between Phase 1 and Phase 2 when the scanner was incorporated into initial and ongoing patient assessment. The 93% reduction in HAPU mirrors reported aggregate results reported by similar hospitals in which 56% of hospitals that implemented the SEM achieved a reduction in HAPU of 100%.30, 34, 35 In this context, working towards a goal of zero was not only ethical but also seemed reachable.19
Direct cost savings were not calculated but are easy to infer from a 93% reduction in the number of HAPUs between the two study periods and the severity of the HAPUs in Phase 1. Physical resources of support surfaces, positioners, and extra dressings were cost‐effectively managed to address the most at‐risk patients and those with not‐yet‐visible tissue damage in order to affect this substantial decrease. Each increase in intervention involves a higher investment of staff time, resources, and more complex equipment. Providing resources to those truly at high risk of PU development is the most cost‐effective for any health care system. Using the metrics employed by regulators in forming national health policy and guidelines, Deloitte Health Analytics, an analytical services company in the United Kingdom, calculated that a 210‐bed acute care hospital can realise a savings of GBP 680000 £ (USD $911200) in the first year of implementation of the SEM scanner or GBP 56£ (USD $75.04) per admitted patient.36
Theoretically, staff may be able to obtain more cooperation from patients to adhere to turning or off‐weighting protocols if the patients are shown visual, numerical confirmation of a problem that is not yet visible at a high‐risk site such as the sacrum or the heel.
Phase 1 of the study was undertaken to see if the Hawthorne effect of simply using this device as an extra step in risk assessment would call extra attention to skin assessment and result in a change in practice and therefore a decrease in nosocomial rates. This phase also helped to achieve buy‐in from sceptical participating staff and floor nurses who believed that the scanner was not necessary because they believed that PU reduction could be achieved by following protocols appropriately in a consistent manner.
In fact, nurses did not change their practice in prevention strategies in Phase 1, and nosocomial rates did not decrease in Phase 1. Interventions continued to be implemented based on standard protocols based mainly on Total Braden Score, Mobility Subscale, and clinical judgement. The extra step of scanning did not significantly impact assessment time and interventions followed standard protocols from risk assessment and visual inspection. This phase did allow examiners to become more skilled with the scanner and helped them to learn how to incorporate it naturally into normal assessment.
The decrease in PU incidence was attributed to the use of the Scanner to guide interventions.
6. LIMITATIONS
Logistics necessitated that Phase 2 could not be carried out in the same unit as Phase 1. This limitation was addressed by relatively high numbers of patients in each phase, the same protocol interventions, and continuing the requirement of making the sensitive Mobility Braden sub‐score the criteria for inclusion in Phase 2 as in Phase 1. Of the six Braden subscales, the Mobility subscale most closely associates with PU development.4 Indeed, the patients in Phase 2 were at higher risk because of the increased proportion of patients with incontinence, Braden of 14 or less, and longer lengths of stay. Yet, the incidence decreased.
There was no trending baseline for direct comparison of nosocomial rates achieved with the scanner because of the diverse locations of the mobility impaired subjects in Phase 2. The available baseline data were tracked at the “all‐hospital” level and therefore could not be used as a comparison with the results of the evaluation.
General baseline data were available as the hospital had tracked PU rates regularly since 2010 to assess the effectiveness of a multifactorial programme as it was being developed and implemented. Both incidence and prevalence rates showed a steady decline from 2010 to 2014 and then increased slightly in 2015. SHN learned that comparable Canadian hospitals had also experienced a slight increase in prevalence and incidence in 2015. As this current evaluation did not include all patients in the hospital during the study period, this baseline data could not be used for comparison with the results of the evaluation, which was limited to a small subset of patients at one hospital.
7. CONCLUSION
Current practice providing pressure interventions based on risk assessment alone is limited by the invisible development of PUs in subcutaneous tissues. By the time the damage is visually evident, significant tissue damage has already occurred, and the opportunity for prevention has been missed.
The SEM Scanner made the non‐visible damage detectable by providing a numerical readout, alerting clinicians to implement stronger prevention measures. This change in practice resulted in a decrease in HAPUs by 93% between the two evaluation periods. Clinicians were able to target interventions, lower incidence, affect earlier recovery, save considerable pain, and lower costs of care.
We showed that there was no Hawthorne effect simply with the use of the Scanner. PU incidence only decreased when the use of the Scanner was used to influence clinical interventions.
As a result of this study, wound care and nursing management decided to incorporate the Scanner into standard practice throughout the hospital.
Declaration of interest
There is no conflict of interest by the authors. SEM Scanner technology units were provided by Bruin Biometrics at no cost.
Funding
Ms. Rappl was paid by BBI for manuscript preparation and submission.
Raizman R, MacNeil M, Rappl L. Utility of a sensor‐based technology to assist in the prevention of pressure ulcers: A clinical comparison. Int Wound J. 2018;15:1033–1044. 10.1111/iwj.12974
Funding information BBI
REFERENCES
- 1. Hauck KD, Wang S, Vincent C, Smith PC. Healthy life‐years lost and excess bed‐days due to 6 patient safety incidents: empirical evidence from English hospitals. Med Care. 2017;55(2):125‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chou R, Dana T, Bougatsos C, et al. Pressure ulcer risk assessment and prevention: a systematic comparative effectiveness review. Ann Intern Med. 2013;159(1):28‐38. [DOI] [PubMed] [Google Scholar]
- 3. Demarre L, Van Lancker A, Van Hecke A, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud. 2015;52(11):1754‐1774. [DOI] [PubMed] [Google Scholar]
- 4. National Pressure Ulcer Advisory Panel . In: Haesler E, ed. European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Perth, Australia: Cambridge Media; 2014. [Google Scholar]
- 5.The Fraser Institute Hospital Report Card: Ontario [June 26, 2017]. http://www.hospitalreportcards.ca/on.html.
- 6. Medicare.gov. Hospital Compare [July 6, 2017]. https://www.medicare.gov/hospitalcompare/search.html?
- 7. Bergquist‐Beringer S, Dong L, He J, Dunton N. Pressure ulcers and prevention among acute care hospitals in the United States. Jt Comm J Qual Patient Saf. 2013;39(9):404‐414. [DOI] [PubMed] [Google Scholar]
- 8. Drosler SE, Klazinga NS, Romano PS, et al. Application of patient safety indicators internationally: a pilot study among seven countries. International J Qual Health Care. 2009;21(4):272‐278. [DOI] [PubMed] [Google Scholar]
- 9. Woo KY, Sears K, Almost J, Wilson R, Whitehead M, VanDenKerkhof EG. Exploration of pressure ulcer and related skin problems across the spectrum of health care settings in Ontario using administrative data. Int Wound J. 2017;14(1):24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agam L, Gefen A. Toward real‐time detection of deep tissue injury risk in wheelchair users using hertz contact theory. J Rehabil Res Dev. 2008;45(4):537‐550, 8 p following 50. [DOI] [PubMed] [Google Scholar]
- 11. Gefen A. Etiology of pressure ulcers, and need to assess under the skin. Wound Care: From Innovations to Clinical Trials; June 20–21, 2017; Manchester, United Kingdom.
- 12. Registered Nurses' Association of Ontario . Risk Assessment and Prevention of Pressure Ulcers (Revised). Toronto, Canada: Registered Nurses' Association of Ontario; 2005. [Google Scholar]
- 13. Joyce P, Moore Z, Christie J, Dumville J. Organisation of health services for preventing and treating pressure ulcers (protocol). Cochrane Database Syst Rev. 2016;3:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore Z, Malloy S, O'Connor R, O'Brien G. Advancing pressure ulcer prevention with SEM scanner. Wounds UK. 2016;12(1):70‐73. [Google Scholar]
- 15. O'Brien G, Moore Z, Patton D, O'Connor T, eds. The Relationship Between Nurses' Assessment of Early Pressure Ulcer Damage and Subepidermal Moisture Measurement: A Prospective Explorative Study. Wounds UK 2017; Harrowgate, UK; 2017. [DOI] [PubMed]
- 16. Gershon S, Okonkwo H, Rhodes S, Burns M. SEM Scanner readings to assess pressure induced tissue damage. Paper presented at: 17th Annual European Pressure Ulcer Advisory Panel (EPUP) Meeting; Stockholm, Sweden; 2014.
- 17. Clendenin M, Jaradeh K, Shamirian A, Rhodes SL. Inter‐operator and inter‐device agreement and reliability of the SEM scanner. J Tissue Viability. 2015;24(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 18. Oliveria AL, Moore Z, O Connor T, Patton D. Accuracy of ultrasound, thermography and subepidermal moisture in predicting pressure ulcers: a systematic review. J Wound Care. 2017;26(5):199‐215. [DOI] [PubMed] [Google Scholar]
- 19. Moore Z, Patton D, Rhodes SL, O'Connor T. Subepidermal moisture (SEM) and bioimpedance: a literature review of a novel method for early detection of pressure‐induced tissue damage (pressure ulcers). Int Wound J. 2017;14(2):331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bates‐Jensen BM, McCreath HE, Pongquan V, Apeles NC. Subepidermal moisture differentiates erythema and stage I pressure ulcers in nursing home residents. Wound Repair Regen. 2008;16(2):189‐197. [DOI] [PubMed] [Google Scholar]
- 21. Bates‐Jensen BM, Guihan M, Garber SL, Chin AS, Burns SP. Characteristics of recurrent pressure ulcers in veterans with spinal cord injury. J Spinal Cord Med. 2009;32(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bates‐Jensen BM, McCreath HE, Patlan A. Subepidermal moisture detection of pressure induced tissue damage on the trunk: the pressure ulcer detection study outcomes. Wound Repair Regen. 2017;25:502‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guihan M, Bates‐Jensen BM, Chun S, Parachuri R, Chin AS, McCreather H. Assessing the feasibility of subepidermal moisture to predict erythema and stage 1 pressure ulcers in persons with spinal cord injury: a pilot study. J Spinal Cord Med. 2012;35(1):46‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bates‐Jensen BM, McCreath HE, Kono A, Apeles NC, Alessi C. Subepidermal moisture predicts erythema and stage 1 pressure ulcers in nursing home residents: a pilot study. J Am Geriatr Soc. 2007;55(8):1199‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrow JJ, Mayrovitz HN. Subepidermal moisture surrounding pressure ulcers in persons with a spinal cord injury: a pilot study. J Spinal Cord Med. 2014;37(6):719‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swisher SL, Lin MC, Liao A, et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat Commun. 2015;6:6575. [DOI] [PubMed] [Google Scholar]
- 27. Zhang L, Liu P, Dong X, Zhou D, Shi X. A method for in vivo detection of abnormal subepidermal tissues based on dielectric properties. Biomed Mater Eng. 2014;24(6):3455‐3462. [DOI] [PubMed] [Google Scholar]
- 28. Moore Z, Gershon S, Fletcher J. SEM scanner made easy. Wounds International; 2016.
- 29. Guihan M, Bombardier CH. Potentially modifiable risk factors among veterans with spinal cord injury hospitalized for severe pressure ulcers: a descriptive study. J Spinal Cord Med. 2012;35(4):240‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore Z. Using SEM Measurement for Timely Detection and Prevention of Pressure Ulcers. Wound Care: From Innovations to Clinical Trials; June 20–21, 2017; Manchester, United Kingdom.
- 31. O'Brien G. An investigation of the accuracy of early pressure ulcer damage assessment using subepidermal moisture measurement versus nurses’ visual skin assessment. RCSI; Dublin, Ireland 2015. Also presented by Moore Z and O'Connor T at Wounds UK, Harrogate, United Kingdom; 2015.
- 32. Fletcher J. An overview of pressure ulcer risk assessment tools. Wounds UK. 2017;13(1):18‐26. [Google Scholar]
- 33. Vanderwee K, Grypdonck M, Defloor T. Non‐blanchable erythema as an indicator for the need for pressure ulcer prevention: a randomized‐controlled trial. J Clin Nurs. 2007;16(2):325‐335. [DOI] [PubMed] [Google Scholar]
- 34. Smith G. Improved Patient Safety with Use of the SEM Scanner (A Pilot Study). Wounds UK; Harrowgate, UK; 2016.
- 35. Lester R. Using Real‐world to Evaluate the Clinical Benefit of Incorporating Early Detection Technology into PU Prevention Programs. Amsterdam, Netherlands: European Wound Management Association; 2017. [Google Scholar]
- 36. Tsang K. SEM scanner ‐ an initial overview of a QALY: reporting the impact of the SEM scanner in PU prevention. Wound Care From Innovation to Clinical Trials; June 13–14, 2018; Edinburgh, United Kingdom.


