Abstract
Advanced therapies such as bioengineered skin substitutes (BSS) and dehydrated human amnion/chorion membrane (dHACM) have been shown to promote healing of chronic diabetic ulcers. An interim analysis of data from 60 patients enrolled in a prospective, randomised, controlled, parallel group, multi‐centre clinical trial showed that dHACM (EpiFix®, MiMedx Group Inc., Marietta, GA) is superior to standard wound care (SWC) and BSS (Apligraf®, Organogenesis, Inc., Canton, MA) in achieving complete wound closure within 4–6 weeks. Rates and time to closure at a longer time interval and factors influencing outcomes remained unassessed; therefore, the study was continued in order to achieve at least 100 patients. With the larger cohort, we compare clinical outcomes at 12 weeks in 100 patients with chronic lower extremity diabetic ulcers treated with weekly applications of Apligraf (n = 33), EpiFix (n = 32) or SWC (n = 35) with collagen‐alginate dressing as controls. A Cox regression was performed to analyse the time to heal within 12 weeks, adjusting for all significant covariates. A Kaplan–Meier analysis was conducted to compare time‐to‐heal within 12 weeks for the three treatment groups. Clinical characteristics were well matched across study groups. The proportion of wounds achieving complete closure within the 12‐week study period were 73% (24/33), 97% (31/32), and 51% (18/35) for Apligraf, EpiFix and SWC, respectively (adjusted P = 0·00019). Subjects treated with EpiFix had a very significant higher probability of their wounds healing [hazard ratio (HR: 5·66; adjusted P: 1·3 x 10−7] compared to SWC alone. No difference in probability of healing was observed for the Apligraf and SWC groups. Patients treated with Apligraf were less likely to heal than those treated with EpiFix [HR: 0·30; 95% confidence interval (CI): 0·17–0·54; unadjusted P: 5·8 x 10−5]. Increased wound size and presence of hypertension were significant factors that influenced healing. Mean time‐to‐heal within 12 weeks was 47·9 days (95% CI: 38·2–57·7) with Apligraf, 23·6 days (95% CI: 17·0–30·2) with EpiFix group and 57·4 days (95%CI: 48·2–66·6) with the SWC alone group (adjusted P = 3·2 x 10−7). Median number of grafts used per healed wound were six (range 1–13) and 2·5 (range 1–12) for the Apligraf and EpiFix groups, respectively. Median graft cost was $8918 (range $1,486–19,323) per healed wound for the Apligraf group and $1,517 (range $434–25,710) per healed wound in the EpiFix group (P < 0·0001). These results provide further evidence of the clinical and resource utilisation superiority of EpiFix compared to Apligraf for the treatment of lower extremity diabetic wounds.
Keywords: Advanced wound care, Amniotic membrane, Comparative effectiveness, Cost‐effectiveness, Diabetic ulcers
Introduction
The growing economic and societal burden of diabetes mandates the identification of effective interventions that mitigate the complications associated with the condition 1. Lower extremity ulceration is a common complication for patients with diabetes with a lifetime risk as high as 25% 2. Diabetic foot ulcers lead to some form of amputation in 20% of patients and are associated with higher morbidity and mortality 3. The presence of peripheral vascular disease, neuropathy and poor blood glucose control contribute to the development of lower extremity wounds, their slow rate of healing and their propensity to recur 4.
The primary clinical goal in treating lower extremity wounds is to achieve rapid and complete healing. Evidence‐based guidelines for the management of lower extremity diabetic ulcers include moist dressings, debridement, wound offloading, infection control and implementation of advanced wound therapies if the ulcer does not decrease in size by 40% or more after 4 weeks of standard therapy 5, 6. Although the use of advanced wound care products may increase short‐term expenditures, overall cost savings can be achieved through increased healing rates, faster time‐to‐heal and reduced incidence of infection and amputation 7.
Concomitant comorbidities can complicate chronic wound management, rendering it unlikely that a single wound intervention can be established as superior for all patients in all clinical situations 8. Factors that contribute to impaired wound healing include ischaemia, infection, advanced age, malnutrition, diabetes, renal disease, ill‐fitting shoes and poor clinical management 9, 10.
Clinicians in practice consider multiple factors while planning wound management. The efficacy and direct and indirect costs associated with using a wound intervention and convenience of use for the clinician, facility and patient are all part of the equation. The purpose of comparative effectiveness research (CER) is to assist consumers, clinicians, purchasers and policy makers in making informed decisions that will improve health care on both the individual and population levels. CER guides clinical practice and is increasingly used to guide policy based on evidence‐based care pathways 11.
Previous clinical studies have established that bioengineered skin substitutes (BSS) such as Apligraf® (Organogenesis, Inc., Canton, MA) and dehydrated human amnion/chorion membrane (dHACM) allografts (EpiFix®, MiMedx Group Inc., Marietta, GA) promote healing, resulting in more rapid and frequent complete healing of diabetic lower extremity ulcers compared with standard wound therapy 12, 13, 14. A retrospective comparative review of published data suggested that dHACM is superior to BSS in clinical and cost effectiveness for the treatment of chronic lower extremity ulcers 15. In addition, an interim analysis of a prospective, randomised, controlled, parallel group, multi‐centre comparative trial enrolling 60 patients demonstrated that dHACM is superior to BSS in achieving complete wound closure within 4–6 weeks 16. Because well‐designed prospective clinical trials are superior to retrospective analyses in determining the efficacy of interventions, and the foundation for true CER, based on recommendations from the Centers for Medical Technology Policy 17, we extended the study to 12 weeks with the expansion of clinical study sites to include more geographical variety and increased enrollment to 100 patients. Increasing enrollment also allowed for a stronger statistical examination of clinical factors within the context of the study that may have affected rates of wound healing among the study groups.
We report the final results of the CER study of dHACM versus BSS with the primary objectives of comparing time‐to‐heal between study groups, rates of complete healing, costs of advanced wound therapies and other clinical factors associated with more rapid healing.
Methods
A prospective, randomised, controlled, parallel group, multi‐centre clinical trial was conducted to compare healing outcomes in patients with chronic lower extremity diabetic ulcers, receiving one of three treatments: BSS, dHACM or standard wound care (SWC) with collagen‐alginate dressings. The study was conducted at four outpatient wound care centres in the United States; three in the State of Virginia and one in the State of Oklahoma. The study protocol and subject consent form were reviewed and approved by an independent Investigational Review Board (IRB). Written consent was obtained from all subjects prior to any study‐related procedures. The trial was pre‐registered in ClinicalTrials.gov (NCT01921491), conducted in compliance with applicable regulatory requirements, in accordance with the provisions of the Declaration of Helsinki and in adherence to Good Clinical Practice (GCP). Confidentiality was maintained with all patient records.
Patient screening and eligibility
Patients with Type 1 or Type 2 diabetes presenting to the clinic for care of a lower extremity ulcer were screened for study eligibility based on inclusion and exclusion criteria listed in Table 1. Those patients meeting the criteria who were willing to participate in the clinical study with the weekly visits and follow‐up regimen entered the 2‐week study run‐in period prior to study enrollment and randomisation. Only patients with indolent, hard‐to‐heal ulcers were enrolled in the treatment phase, so a run‐in period was designed to eliminate patients whose ulcer responded to the pre‐defined standard of care for this trial. Daily dressing changes were performed by the patients using collagen‐alginate dressings and gauze, and wounds were offloaded with an offloading cast walker (Royce Medical Active Offloading Walker, Royce Medical, Inc., Camarillo, CA). As our goal was to provide a high level of standard of care in accordance with contemporary guidelines, and we were comparing advanced wound care modalities, collagen‐alginate dressings were chosen over simple wet‐to‐dry dressings as the standard of care. Patients were seen weekly in the clinic during the run‐in period for wound assessment, sharp debridement and wound measurements. After the 2‐week run‐in period, patients remained eligible for randomisation if their wound had not healed by >20% and if they continued to meet study inclusion and exclusion criteria.
Table 1.
Major inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Treatment phase of the study
Patients were randomised to one of three study groups (BSS, dHACM or SWC with collagen‐alginate dressings) in a 1:1:1 ratio. In three of four study sites, concealment of randomisation was ensured using opaque envelopes prepared by the study sponsor containing a slip of paper designating the study group. The envelopes were randomly shuffled and labeled sequentially. When a patient was scheduled for randomisation, the appropriate envelope was delivered to the study site and opened in the presence of the patient who then signed the envelope and paper slip inside, acknowledging their group assignment. Due to the remote location of one study site, an electronic randomisation tool was utilised in lieu of the envelope method. Group assignment was not blinded to the treating physician or patient due to the different handling characteristics of the products used. However, study adjudicators and validators were blinded about group assignment when examining photographic images of the entire study population to confirm the appropriateness of wounds enrolled and confirmation of healing on completion of the study.
Patients were seen at least once every 7 days (±3 days) by the investigator at the study site for up to 12 weeks or until 1 week after complete healing, whichever occurred first. Per study protocol, any patient whose wound failed to heal by ≥ 50% within the first 6 weeks of study enrollment was exited from the study to seek alternative treatment. Procedures conducted at each study visit included ulcer debridement and cleansing with normal sterile saline solution, ulcer measurement and photography, assessment for adverse events and wound dressing. Wound surface area was calculated by width x length, and an acetate tracing of the wound was also performed. All measurements, tracings and photographs were conducted after debridement. Grafts were applied weekly, after debridement, in the manner outlined in the respective product insert for subjects enrolled in the BSS or dHACM groups. A non‐adherent dressing (Adaptic Touch, Systagenix, San Antonio, TX or equivalent), a moisture‐retentive dressing (NuGel, Systagenix, San Antonio, TX or equivalent) and a compressive dressing was then applied. Dressings were changed weekly at the study site for patients in the BSS and dHACM groups. Patients that were randomised to the SWC control group had their wounds debrided weekly and were instructed to change their wound dressing daily using the provided collagen‐alginate and gauze dressing supplies. Wounds were offloaded using a diabetic cam walker during both the run‐in and study periods in all study groups.
Wounds with complete (100%) re‐epithelialisation without drainage or need for dressing were defined as healed. One week after 100% re‐epithelialization occurred, a follow‐up visit was conducted to confirm that the wound remained closed prior to study exit. Further validation of healing was conducted by three independent physicians specialising in wound care, including one vascular surgeon, one plastic surgeon and one expert in angiogenesis. These adjudicators who were blinded to group assignment reviewed wound photos for appropriateness of enrollment and validated the healing status of each patient at the time of study completion.
Study outcomes
The primary objective of this comparative effectiveness study was to compare healing characteristics between groups treated weekly with BSS or dHACM and SWC during the 12‐week study period. The secondary objectives of this study were to compare the direct costs of these advanced wound therapies and to examine clinical factors associated with more rapid healing at 12 weeks. Because the actual cost of BSS and dHACM are variable as a result of contractual prices, cost estimates were based on allowable charges for each product from the Centers for Medicare and Medicaid Services (CMS) product reimbursement schedule 18.
Data analysis
An intent‐to‐treat analysis was used, which included all patients as originally allocated after randomisation and those who received at least one treatment. For missing observations, the last known value was carried forward. Study variables were summarised as means and standard deviations (SDs) for continuous variables unless the data were non‐normal, as determined by the Shapiro–Wilk test, in which case medians were also reported, and proportions or percentages for categorical variables. Parametric and non‐parametric tests were used as appropriate. Analysis of variance (ANOVA) or the Kruskal–Wallis test was used to test for differences in continuous variables. For categorical variables, χ 2 or Fisher exact tests were performed to test for statistical differences. Statistical testing between groups at baseline was not undertaken as per CONSORT guidelines 19. A Kaplan–Meier analysis was conducted to compare time to heal within 12 weeks for the three treatment groups. A Cox regression to analyse time to heal within 12 weeks, adjusting for all covariates known to be significant on wound healing, was also carried out. Proportional hazard assumptions for each covariate in the model were verified by examining the slope of the Schoenfeld residuals and adding additional time‐dependent covariates if these were significant. To adjust for the family‐wise error rate (FWER), P‐values were reported using the Hochberg step‐up procedure. Adjusted two‐sided P‐values < 0·05 were considered significant. PASW 19 (IBM, Chicago, IL) was used to perform the statistical testing.
Although sample size calculations (PASS 11) showed that group sample sizes of 23 in group one and 23 in group two could achieve 81% power to detect a difference between the group proportions of 0·4 (proportion healed), study enrollment continued until a total of 100 patients who met the inclusion and exclusion criteria were recruited. The proportion in group one (dHACM) was assumed to be 0·3 under the null hypothesis and 0·7 under the alternative hypothesis. The proportion in group two (BSS) was 0·3. The test statistic used is the two‐sided Z test with pooled variance. The significance level of the test was targeted at 0·05; the significance level actually achieved by this design is 0·0497. Patients receiving standard care only were included as a reference group.
Results
As indicated in the CONSORT flow diagram (Figure 1), a total of 126 subjects were screened and entered the study for the 2‐week run‐in period between September 2013 and August 2015. On the conclusion of the run‐in period, 22 patients were no longer eligible for randomisation. One hundred and four subjects were then randomised into the three treatment groups: 34 received BSS, 35 received dHACM and 35 received SWC. One subject in the dHACM group was withdrawn from the study as a result of an adverse event requiring hospitalisation prior to administration of the study intervention and did not receive treatment. Additionally, three randomised subjects were excluded from analysis, two in the dHACM group and one in the BSS group, because of absolute protocol deviations (did not meet the study inclusion criteria): one subject's wound decreased by >20% during the run‐in period, one subject's wound was >52 weeks in duration and one subject's ulcer was <1 cm2 at randomisation. After these exclusions, a total of 100 subjects were included for analysis. Thirty‐three received BSS, 32 received dHACM and 35 received SWC.
Figure 1.

Consort flow diagram. BSS, bioengineered skin substitutes; dHACM, dehydrated human amnion/chorion membrane; SWC, standard wound care.
Study population
In the final study population (N = 100), the majority (92·0%) of subjects were Caucasian. Forty‐six percent (46%) were age 65 or older. Over half (55%) were male, and 63% were obese [body mass index (BMI) ≥30]. Eighty‐three percent (83%) of the study wounds were located on the plantar surface of the foot. Demographics and wound characteristics of the study population are presented in Table 2. The three study groups were well matched for clinical factors, including presence of comorbidities, blood glucose control as well as location, duration and size of the study ulcer.
Table 2.
Demographics and wound characteristics at study enrollment
| BSS (n = 33) | dHACM (n = 32) | SWC (n = 35) | |
|---|---|---|---|
| Age (years) | 63·8 (11·86) | 63·3 (12·25) | 60·6 (11·55) |
| Age ≥ 65 years | 17 (16·8%) | 17 (16·8%) | 12 (11·9%) |
| Gender: male | 14 (13·9%) | 19 (18·8%) | 22 (21·8%) |
| Race | |||
| Caucasian | 30 (29·7%) | 31 (30·7%) | 31 (30·7%) |
| African‐American | 3 (3·0%) | 2 (2·0%) | 3 (3·0%) |
| Smoker | 6 (18·2%) | 9 (28·1%) | 12 (34·3%) |
| Hx Hypertension | 24 (72·7%) | 22 (68·8%) | 26 (74·3%) |
| Hx CAD | 5 (15·2%) | 6 (18·8%) | 10 (28·6%) |
| Hx CHF | 5 (15·2%) | 2 (6·3%) | 3 (8·6%) |
| Hx Prior DFU | 23 (69·7%) | 20 (62·5%) | 20 (57·1%) |
| Plantar Ulcer | 26 (78·8%) | 25 (78·1%) | 32 (91·4%) |
| Ulcer Location | |||
| Toe | 14 (42·4%) | 9 (28·1%) | 11 (31·4%) |
| Forefoot | 11 (33·3%) | 9 (28·1%) | 12 (34·3%) |
| Midfoot | 4 (12·1%) | 8 (25·0%) | 6 (17·1%) |
| Hindfoot/ankle | 4 (12·1%) | 6 (18·8%) | 6 (17·1%) |
| Body mass index |
33·3 (8·92) 31·0 (17·72, 58·91) |
33·9 (6·99) 34·1 (23·09, 48·42) |
34·7 (9·35) 33·4 (20·12, 64·01) |
| Body mass index ≥ 30 | 20 (19·8%) | 20 (19·8%) | 23 (22·8%) |
| HbA1c |
7·9 (1·79) 7·5 (5·4, 11·9) |
7·5 (1·51) 7·4 (5·5, 11·2) |
8·2 (1·78) 8·3 (5·5, 11·8) |
| HbA1c ≥ 9% | 8 (7·9%) | 5 (4·9%) | 11 (10·9%) |
| Hx of index ulcer (weeks) |
19·0 (14·78) 16 (4, 52) |
17·3 (15·3) 12 (3·52) |
14·1 (12·9) 8 (2, 50) |
| Baseline wound size (cm2) |
2·7 (2·75) 1·7 (1·0, 14·7) |
2·6 (2·97) 1·7 (1·0, 16·9) |
3·1 (3·17) 1·8 (1·0, 15·5) |
Data presented as mean (standard deviation), median (minimum, maximum) or number (%) as indicated.
BSS, bioengineered skin substitutes; CAD, coronary artery disease; CHF, congestive heart failure; dHACM, dehydrated human amnion/chorion membrane; DFU, diabetic foot ulcer; SWC, standard wound care.
Overall healing rates and time to heal
Within the 12‐week study period, complete healing occurred in 73% of subjects treated with BSS (24/33), 97% of subjects treated with dHACM (31/32) and 51% of subjects receiving SWC alone (18/35) (adjusted P = 0·00019). Mean time‐to‐heal within 12 weeks were 47·9 days (95% CI: 38·2–57·7) for the BSS group, 23·6 days (95% CI: 17·0–30·2) for the dHACM group and 57·4 days (95% CI: 48·2–66·6) for the SWC group (adjusted P = 3·2 x 10−7).
Cox regression modelling
Four continuous covariates had to be transformed into ordinal factors for Cox regression as the variables were non‐normal: HbA1C: 1 (<6·5), 2 (6·5–9·0), 3 (>9·0); BMI: 1 (<30, normal weight or overweight), 2 (30–39·99, obese), 3 (>40, morbidly obese); initial wound area: 1 (<1·2 cm2), 2 (1·2–2·5 cm2), 3 (>2·5 cm2); wound age: 1 <42 days), 2 (42·1–112 days) 3 (>112 days). The following covariates were entered as a block into the initial model: group, patient age (continuous variable), race, gender, prior ulcer, coronary artery disease (CAD), congestive heart failure (CHF), hypertension, smoker, wound location, plantar surface, HbA1c (factor), BMI (factor), wound age (factor) and initial area (factor). Model refinement was initially carried out by eliminating stepwise covariates with the highest (non‐significant) P value. The final model had a −2 log likelihood of 560·8 compared to the intercept only value of 607·6 (Model 1). Location was retained in the model as one of the levels was just outside of statistical significance and would be useful in defining clinical factors associated in healing. Initial wound area failed the proportional hazards assumption, and an additional model (Model 2) was built in which location was dropped and a time‐based covariate (T*initial wound area factor) was added. The −2 log likelihood of Model 2 was 552·6, indicating a better fit of the data compared to Model 1, although the difference is small.
Table 3 shows the corresponding hazard ratios (HRs) for the covariates in Model 1, while Table 4 shows the corresponding HRs for the covariates in Model 2 (time‐dependent covariate not shown). Model 2 represents the definitive Cox regression results.
Table 3.
Hazard ratios (HRs) and associated 95% confidence intervals (CIs) for covariates included in Model 1. Location was retained in the model to identify any trends as the covariate was close to statistical significance
| Covariate | P | HR | 95% CI for HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Group* | ||||
| dHACM | 5·1 x 10−8 | 5·88 | 3·12 | 11·11 |
| BSS | 0·167 | 1·55 | 0·83 | 2·90 |
| Hypertension† | 0·037 | 1·82 | 1·04 | 3·18 |
| Location‡ | ||||
| Forefoot | 0·055 | 1·79 | 0·99 | 3·23 |
| Midfoot | 0·656 | 1·16 | 0·60 | 2·27 |
| Rearfoot/ankle | 0·686 | 1·17 | 0·55 | 2·51 |
| Initial wound area (cm2)§ | ||||
| 1·2–2·5 | 0·076 | 0·60 | 0·34 | 1·06 |
| >2·5 | 5·1 x 10−5 | 0·26 | 0·14 | 0·50 |
P values not adjusted for multiplicity of testing in regard to other statistical tests conducted in our study.
BSS, bioengineered skin substitutes; dHACM, dehydrated human amnion/chorion membrane.
Standard of care.
No hypertension.
Toes.
Area <1·2 cm2.
Table 4.
Hazard ratios (HRs) and associated 95% confidence intervals (CIs) for covariates in Model 2
| Covariate | P | HR | 95% CI for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Group* | ||||
| dHACM | 5·5 x 10−8 | 5·66 | 3·03 | 10·57 |
| BSS | 0·091 | 1·70 | 0·92 | 3·15 |
| Hypertension† | 0·024 | 1·91 | 1·09 | 3·34 |
| Initial wound area (cm2)‡ | ||||
| 1·2–2·5 | 0·02 | 0·30 | 0·11 | 0·83 |
| >2·5 | 1·3 x 10−5 | 0·032 | 0·007 | 0·15 |
P values not adjusted for multiplicity of testing.
BSS, bioengineered skin substitutes; dHACM, dehydrated human amnion/chorion membrane.
Standard of care.
No hypertension.
Area <1·2 cm2.
In terms of treatment group, subjects treated with dHACM had a significantly higher probability of healing their wounds (HR: 5·66; adjusted P: 1·3 x 10−7) compared to SWC alone, whereas subjects treated with BSS did not differ significantly compared to SWC alone. Using the same model 2 in which the reference for group was dHACM instead of SWC, the HR for BSS was significantly reduced (HR: 0·30; 95% CI: 0·17–0·54; unadjusted P: 5·8 x 10−5), indicating superiority of dHACM.
After assessing the type of treatment received, increased wound area is the most significant factor in wound healing as larger wounds have a lower probability of healing. Compared to small wounds of area <1·2 cm2, wounds with an area of >2·5 cm2 had a reduced HR of 0·032. Hypertension also had a lesser but significant effect, increasing the HR in regard to healing, compared to no hypertension. Location of the diabetic foot ulcer (DFU) trended towards significance in Model 1, with a DFU located on the forefoot having the best chance for improved probability of healing.
Kaplan–Meier plot of time‐to‐heal
A Kaplan‐Meier plot of time‐to‐heal within 12 weeks by the study group, after adjusting for significant covariates, demonstrated a superior wound‐healing trajectory for dHACM compared to BSS or SWC (Figure 2).
Figure 2.
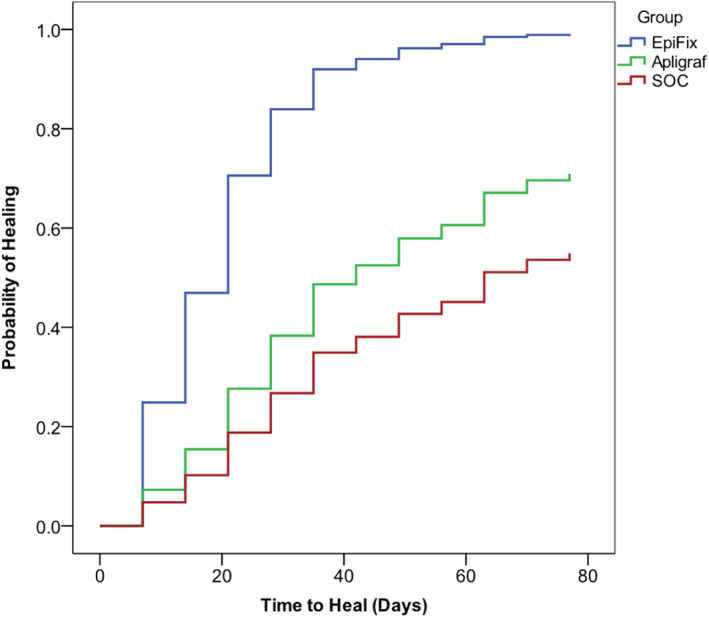
A Kaplan–Meier plot of time‐to‐heal within 12 weeks by the study group, after adjusting for significant covariates, demonstrated a superior wound‐healing trajectory for dehydrated human amnion/chorion membrane compared to bioengineered skin substitutes or standard change from standard wound care to standard of care (SOC).
Cost comparison
A comparison of cost based on the amount of graft material used in the BSS and dHACM groups is presented in Table 5. Subjects receiving dHACM received an average of 58% fewer grafts than those receiving BSS and used 94·4% less graft material than those receiving BSS. Median cost of graft material in the dHACM group was 83% less than the median cost of graft material in the BSS group. Absolute risk reduction for healing within 12 weeks with dHACM versus SWC was 45·4% (95% CI 27·8–63·1%). Number needed to treat (NNT) is a metric that identifies how many patients have to be treated with one treatment in comparison to another in order for one patient to benefit. Compared with SWC, the NNT was 2·2 (95% CI 1·59–3·60) with dHACM, meaning that approximately 1 out of every 2 patients will benefit from treatment. The NNT for BSS versus SWC was 4·7 (95% CI 2·29–84·09). A confidence interval that crosses zero implies there is no statistically significant difference between the groups, and it is not possible to conclude if treatment with BSS is helpful, has no effect or is harmful compared with SWC.
Table 5.
Graft utilisation and associated costs
| BSS (n = 33) | dHACM (n = 32) | Adjusted P‐value | |
|---|---|---|---|
| Grafts used during study period |
5·9 (3·6) 6 (1, 13) |
3·4 (2·9) 2·5 (1, 12) |
0·003 |
| Overall cm2 graft material |
261·4 (156·6) 264 (44, 572) |
14·6 (21·9) 8 (2, 118·6) |
3·9 x 10−11 |
| Wasted graft cm2 |
253·4 (153·7) 259·6 (42·8, 555·8) |
7·7 (9·9) 4·3 (0·3, 47·8) |
3·9 x10−11 |
| Cost of graft material per pt ($) |
8828 (5293) 8918 (1486·19323) |
2798 (4528) 1517 (434, 25710) |
3·8 x 10−7 |
Data presented as mean (SD), median (minimum, maximum).
Study completion
Per study protocol, a total of four subjects in the BSS group and 13 subjects in the SWC group discontinued their assigned intervention and exited the study when <50% wound closure was achieved after 6 weeks. One additional subject discontinued intervention at 10 weeks to seek an alternative when their index wound grew in size. Five SWC subjects discontinued the assigned intervention and withdrew consent to seek an alternative treatment between weeks 3, 4, 5 and 11.
Adverse events
A total of 10 adverse events were reported during the course of the study. Seven of the reported adverse events were wound/foot infections with two of these resulting in hospitalisation of the subject. Two of these infections resulted in the withdrawal of the subject from the study. One of the adverse events was a urinary tract infection (UTI) that necessitated admission of the subject to the hospital for treatment. Two of the remaining adverse events were injuries: one was sustained in a car accident that resulted in hospitalisation of the subject and one was local trauma of the study foot with bruising.
Four of these adverse events were considered serious because they resulted in hospitalisation of the subject. Two subjects were hospitalised for wound infections (both were in the SWC treatment group) with one subject diagnosed with osteomyelitis, requiring withdrawal from the study. As mentioned above, the remaining two subjects were hospitalised for a UTI (BSS group) and a car accident (dHACM group). None of the reported adverse events were considered product related.
Discussion
Every patient presenting with a chronic wound is different in some aspect. Patients present to the clinician with different circumstances, unique medical histories and laboratory values as well as different clinical findings, including an array of wound types. Patients also exhibit different levels of compliance with clinician recommendations. CER is an important tool that can be used to assist the clinician and patient to better understand various treatment modalities. CER offers an opportunity for improved clinical outcomes and quality by providing more and better information on how a product or treatment plan performs, which in turn may reduce health care costs. Conducting a comparative effectiveness analysis within a randomised controlled study design, the gold standard in defining evidence‐based wound research, reduces bias and gives each treatment regimen an equal opportunity to achieve healing for those patients that meet the inclusion and exclusion criteria.
In the present randomised controlled comparative effectiveness trial, we evaluated clinical outcomes related to the use of BSS, dHACM and SWC for the treatment of chronic lower extremity ulcers in patients with diabetes. We found that chronic lower extremity ulcers treated with dHACM were more likely to heal, and healed more rapidly, than ulcers treated with either BSS or SWC. These data add to the growing body of evidence supporting the clinical effectiveness of dHACM as an advantageous treatment for chronic lower extremity ulcers.
The overall closure rate within 12 weeks was used to compare treatment modalities. Rates of complete healing reported in the current randomised controlled trial are consistent with a previously published retrospective review comparing outcomes of advanced wound care products including BSS and dHACM 15. In that analysis, after 12 weeks of treatment, patients treated with weekly or biweekly application of dHACM (n = 64) had a 92% rate of complete healing compared to a rate of 56% for patients treated with up to five weekly applications of BSS (n = 112). Compared with the retrospective review 15, the complete healing rate with weekly application of dHACM in the current 12‐week study was 97% and 73% for patients treated with BSS. The differences in the previously reported healing rate with BSS of 56% and the 73% healing rate in the current study may be because of sample size, differences in inclusion criteria and comorbidities within study populations as well as improvement in contemporary clinical management of chronic wounds, including aggressive debridement and offloading. The pivotal trial for BSS approval by the U.S. Food and Drug Administration was limited to five applications 13. We recognise that the safety and effectiveness of greater than five applications of the device were not established by that trial. However, to reduce the bias of unequal applications of graft material, patients in the current study received weekly applications of BSS during the 12‐week study period and were not limited to only five applications.
In the current study, after controlling for factors known to influence healing, the wounds of patients receiving dHACM were almost six times more likely to heal than the SWC controls (HR: 5·66; adjusted P: 1·3 x 10–7). No significant differences were observed between the healing rates of BSS versus SWC controls. The prospective study used to obtain FDA approval for BSS reported a HR of 1·59 (95% CI 1·26–2·00) 13. It should be noted that in that trial, saline‐moistened gauze was used as the SWC control, offloading was not standardised and all patients who had not healed by 5 weeks were treated with saline‐moistened gauze with twice daily dressing changes between weeks 5 and 12. We believe that the more advanced standard of care used in the current study contributes to the improved healing rates that we report for both SWC and BSS compared with the study published by Veves in 2001 13. Comparison of healing speed is valuable because rapid complete closure of a wound results in reduced risk of infection and costs associated with medical care and treatment. In the present study, the mean healing time for wounds treated with dHACM at 23·6 days (95% CI: 17·0–30·2) was half that of wounds treated with BSS at 47·9 days (95% CI: 38·2–57·7). The mean time‐to‐heal at 23·6 days (95% CI: 17·0–30·2) for dHACM patients was also consistent with previous studies that reported mean days to complete healing of 17·5 days, 29·4 days and 22·4 days, respectively 14, 20, 21.
While overall healing rates and time to healing have remained relatively consistent in randomised studies of dHACM, these results differ from those of a retrospective evaluation of data derived from an electronic medical record 22. In that retrospective study, healing rates at 12 weeks were 48% versus 28%, and median healing times were 93·1 days versus 182 days for patients receiving BSS and dHACM, respectively 22. There are many possible explanations for their findings. The authors acknowledge that treatment patterns were not uniform for all patients and between treatment groups across the 99 centres in 29 states entering data into the database, and specific information regarding methods and techniques for offloading and debridement were not available. They acknowledged that the database from which the information was obtained was not designed specifically for research purposes. The reasons for treatment selection were not disclosed, and treated wounds were larger than in the current randomised controlled trial. Healing results reported in that study 22 were poorer for both BSS and dHACM‐treated groups when compared with results obtained in the current and past randomised studies.
It is not unusual for randomised studies and observational studies addressing the same question to have differing or even conflicting results. This is because of many factors, but it is believed that confounding is the greatest bias in an observational study compared to a randomized controlled trial (RCT) 23. Many confounding factors contribute to the failure of wound healing, and these factors must be taken into consideration by clinicians in determining an individual treatment plan. Although the guidelines and experts are clear as to what constitutes good standard wound care, a gap often exists between what the evidence supports and what is performed in clinical practice 24, 25, 26, 27. The primary treatment plan for the management of lower extremity ulcers includes moist dressings, debridement, wound offloading and infection control 5, 6. When evaluating and comparing clinical outcomes and studies of advanced wound care treatments, it is important to evaluate what components of good standard wound care were implemented and adhered to as these advanced therapies must be used in addition to, and not in lieu of, standard wound care. Although considered standard care in high‐quality wound centres, the inability to report consistency in the delivery of these basic wound care standards is a major confounder in retrospective studies, such as the one conducted by Kirsner et al. 22. As with most medical therapies, it is unlikely that ‘one size fits all’ or that one advanced treatment modality will promote healing in all patients, all of the time. Clinicians depend upon the weight of the evidence collected in well‐designed randomised controlled trials to advance clinical practice. Ongoing data synthesis and retrospective analysis may help the clinician determine which evidence‐based treatments are the best for each particular patient circumstances and select the right treatment for the right patient and at the right time. When widely divergent results are observed between data collected under controlled versus uncontrolled situations, it is vital to examine possible reasons this has occurred. Reasons may include bias in study design, significant confounding and poor data quality. Biomedical databases constitute a potentially invaluable research resource, but researchers, analysts and other stakeholders must appreciate that these datasets often contain errors, are incomplete or suffer from other shortcomings 28.
In multiple randomised controlled trials, the gold standard for determining treatment efficacy, dHACM has been shown to be superior compared to SWC, with complete healing rates within 6 weeks of >90% 14, 16, 21. Since the publication of the initial results 14, additional studies have been conducted to examine the efficacy and effectiveness of dHACM in a broader patient population 16, 20, 21. One would expect that as these additional studies are completed and as more wounds are treated in patients outside of a strictly controlled study setting, there will be some regression to the mean for treatment outcomes 29. Although we anticipate that healing rates observed in a broader patient population may be less dramatic than those observed in the strictly controlled prospective studies, the magnitude of difference observed between the results of the retrospective study by Kirsner et al. 22 and those of the randomised trials of dHACM 14, 16, 21 appear excessive, particularly in light of the results of the current study that includes more patients over a longer study duration.
CER is valuable for examination of both clinical and cost‐effectiveness. Cost‐effectiveness of an advanced wound care product is driven by the efficacy of the product to rapidly and completely heal the wound, thereby reducing the rates of complications, hospitalisation and amputation 30. In the present study, fewer grafts were required to achieve complete closure in the dHACM group, resulting in an average reduction in the cost of graft material of 68·3% compared with the BSS group. Patients treated with dHACM had a superior rate of wound healing and a more rapid rate of wound closure than those treated with BSS while utilising an average of 42·7% fewer grafts and 94·4% fewer square centimeters of graft material. Including NNT alongside other cost data allows clinicians to better understand and apply results of cost‐effectiveness analyses 31. In the current study, the NNT for treatment with dHACM compared to SWC was 2·2 (95% CI 1·59–3·60), indicating that dHACM is a very effective treatment for chronic lower extremity ulcers.
Direct and indirect costs associated with product storage and handling characteristics as well as convenience for the clinician, facility and patient also impact overall cost‐effectiveness but are more difficult to objectively measure. dHACM has a 5‐year shelf life at ambient temperatures, requires minimal storage space and is easy to apply to the wound bed, whereas BSS requires storage in a nutrient medium at 68–73°F with a shelf life of only 14 days.
Limitations exist within the randomised, multi‐centre study presented. Patients were followed for only 1 week after complete healing, and wound recidivism was not recorded. Additional studies will evaluate the recurrence rate over time. The cost data were obtained from a CMS reimbursement schedule, and these do not reflect the actual cost of material across all clinical settings. We did not examine ancillary costs related to differences in product handling, storage and application procedures, which may have further impacted costs.
Data from a variety of study designs that suggest that amniotic membrane therapy can facilitate rapid healing in the subset of patients who fail to respond to standard wound care continue to accumulate 32, 33. The high healing rates in all three study groups illustrate the importance of consistent evidence‐based wound care and substantiates the basic science data supporting that amniotic tissue stimulates chronic wound healing 34, 35, 36.
In conclusion, patients treated with dHACM exhibited the highest rates of complete healing, and their wounds healed significantly faster than those treated with BSS or SWC with collagen‐alginate dressings. Wounds larger than 2·5 cm2 were less likely to heal during the 12‐week study period. Wound location did not influence healing rates. The dHACM was more cost‐effective than BSS in this study because of the fewer number of grafts required to achieve complete healing and the ability to use a graft that is closer in size to the wound being treated, leading to less wastage of graft material. The results of this comparative effectiveness study are useful in providing guidance to clinicians regarding expectations of clinical outcomes with the use of dHACM or BSS in addition to evidence‐based standard of care for diabetic patients with chronic lower extremity wounds. These data are also important for administrators and payers in their evaluation of the cost‐effectiveness of advanced wound care products.
References
- 1. Herman WH. The economic costs of diabetes: is it time for a new treatment paradigm? Diabetes Care 2013;36:775–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL Sr, Mueller MJ, Sheehan P, Wukich DK, American Diabetes Association , American Association of Clinical Endocrinologists . Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the ADA, with endorsement by the AACE. Diabetes Care 2008;8:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu S, Armstrong DG. Risk assessment of the diabetic foot and wound. Int Wound J 2005;2:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snyder R. Wound percent area reduction and making decisions about utilizing advanced therapies. Podiatry Manage 2010;29:197–201. [Google Scholar]
- 6. Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma‐Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 2006;14:680–92. [DOI] [PubMed] [Google Scholar]
- 7. Albert S. Cost‐effective management of recalcitrant diabetic foot ulcers. Clin Podiatr Med Surg 2002;19:483–91. [DOI] [PubMed] [Google Scholar]
- 8. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care 2007;20(9 Pt 1):493–508; quiz 509–10. [DOI] [PubMed] [Google Scholar]
- 9. Harding KG, Morris HL, Patel GK. Healing chronic wounds. Br Med J 2002;324:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361:1545–51. [DOI] [PubMed] [Google Scholar]
- 11. Pearson S. From better evidence to better care: using comparative effectiveness research to guide practice and policy. In: Implementing comparative effectiveness research: priorities, methods and impact. Washington, DC: Brookings Institution, 2009:55–82. [Google Scholar]
- 12. Ho C, Tran K, Hux M, Sibbald G, Campbell K. Artificial skin grafts in chronic wound care: a meta‐analysis of clinical efficacy and a review of cost‐effectiveness [Technology report no 52]. Ottawa: Canadian Coordinating Office for Health Technology Assessment, 2005.
- 13. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer Study . Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 14. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Manag Care 2014;23:31–8. [PubMed] [Google Scholar]
- 16. Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi‐centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 2015;12:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonnad SS, Goldsack JC, Mohr P, Tunis S. Methodological recommendations for comparative research on the treatment of chronic wounds. J Wound Care 2013;22:470–80. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Medicare and Medicaid Services . Medicare Part B Drug Average Sales Price, July 2014, ASP Drug Pricing Files 09/03/14. URL https://www.cms.gov/apps/ama/license.asp?file=/McrPartBDrugAvgSalesPrice/downloads/Jul‐2014‐ASP‐Pricing‐File.zip [accessed on 9 October 2015].
- 19. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG; Consolidated Standards of Reporting Trials Group. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;340:e1–37. [Google Scholar]
- 20. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care 2013;22:347–8, 350–351. [DOI] [PubMed] [Google Scholar]
- 21. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 2014;11:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirsner RS, Sabolinski ML, Parsons NB, Skornicki M, Marston WA. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen 2015;23:737–44. [DOI] [PubMed] [Google Scholar]
- 23. Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials (Review). The Cochrane Library, 2014, Issue 4. URL http://www.thecochranelibrary.com [accessed on 10 November 2015] [DOI] [PMC free article] [PubMed]
- 24. Snyder RJ, Frykberg RG, Rogers LC, Applewhite AJ, Bell D, Bohn G, Fife CE, Jensen J, Wilcox J. The management of diabetic foot ulcers through optimal off‐loading: building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc 2014;104:555–67. [DOI] [PubMed] [Google Scholar]
- 25. Karavan M, Olerud J, Bouldin E, Taylor L, Reiber GE. Evidence‐based chronic ulcer care and lower limb outcomes among Pacific Northwest Veterans. Wound Repair Regen 2015;23:745–52. [DOI] [PubMed] [Google Scholar]
- 26. Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence‐based management strategies for treatment of chronic wounds. Eplasty 2009;9:e19. [PMC free article] [PubMed] [Google Scholar]
- 27. Fife CE, Carter MJ, Walker D, Thomson B, Eckert KA. Diabetic foot ulcer off‐loading: the gap between evidence and practice. Data from the US Wound Registry. Adv Skin Wound Care 2014;27:310–6. [DOI] [PubMed] [Google Scholar]
- 28. Hoffman S, Podgurski A. The use and misuse of biomedical data: is bigger really better? Am J Law Med 2013;39:497–538. [DOI] [PubMed] [Google Scholar]
- 29. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it [Review]. Int J Epidemiol 2005;34:215–20. [DOI] [PubMed] [Google Scholar]
- 30. Langer A, Rogowski W. Systematic review of economic evaluations of human cell‐derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res 2009;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garg V, Shen X, Cheng Y, Nawarskas JJ, Raisch DW. Use of number needed to treat in cost‐effectiveness analyses. Ann Pharmacother 2013;47:380–7. [DOI] [PubMed] [Google Scholar]
- 32. Lintzeris D, Yarrow K, Johnson L, White A, Hampton A, Strickland A, Albert K, Cook A. Use of a dehydrated amniotic membrane allograft on lower extremity ulcers in patients with challenging wounds: a retrospective case series. Ostomy Wound Manage 2015;61:30–6. [PubMed] [Google Scholar]
- 33. Mrugala A, Sui A, Plummer M, Altman I, Papineau E, Frandsen D, Hill D, Ennis WJ. Amniotic membrane is a potential regenerative option for chronic non‐healing wounds: a report of five cases receiving dehydrated human amnion/chorion membrane allograft. Int Wound J 2015. DOI: 10.1111/iwj.12458. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013;10:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353–62. [DOI] [PubMed] [Google Scholar]
- 36. Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, Li WW. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]


