Abstract
Biological alternatives to synthetic meshes are increasingly utilised in complex abdominal wall reconstruction. There is a lack of evidence demonstrating that non‐cross‐linked porcine acellular dermal matrix vascularizes and integrates with human tissue in suboptimal wound conditions. We aimed to evaluate these properties in Strattice™ (Life Cell Inc., Branchburg, NJ) following ventral hernia repair. A retrospective review of patients with high‐risk ventral hernia repair utilising Strattice™ as an onlay after open component separation was conducted. Patients with postoperative wound exploration and exposure of the onlay were included in this review. One patient underwent punch biopsy for histological analysis. Eleven patients with wound complications necessitating postoperative debridement and exposure of Strattice™ onlay were identified. The onlay was partially debrided in two cases, and one case required complete excision. Vascularisation was clinically evident in 10 of 11 cases (91%) as demonstrated by the presence of granulation tissue and/or the ability to support a skin graft. Histological analysis of one onlay 3 months postoperatively showed neovascularisation and collagen remodelling with minimal inflammatory response. Strattice™ demonstrated resistance to rejection, ability to undergo vascularisation and incorporation into host tissues in sub‐optimal wound conditions following ventral hernia repair.
Keywords: Abdominal wall, Components separation, Hernia, Porcine acellular dermal matrix, Strattice
Introduction
Synthetic meshes are not recommended for ventral hernia repair in patients at risk of wound complications because of a susceptibility to bacterial colonisation which may contribute to the development of chronic wounds 1. Materials with efficacy in contaminated environments are valuable alternatives. Biological products represent an increasingly utilised option for this purpose. Acellular dermal matrix (ADM) products possess the ability to support vascular ingrowth and incorporate native tissues 2, 3, 4, 5. This allows the host immune system to access the repair site, which increases resistance to infection by controlling necrotic debris and bacteria that contribute to the development of chronic wounds 1.
Human acellular dermal matrix (HADM) has performed well in abdominal wall reconstruction with subsequent wound complications including postoperative graft exposure 6. Concerns have arisen over the use of HADM because of laxity and the high incidence of bulging in bridging repairs 6. Additionally, the limited supply of human cadaveric tissue and high cost of HADM has fueled the search for xenogeneic substitutes. Porcine ADM (PADM) is an excellent alternative to HADM because of an abundance of source tissue, lower material costs and structural similarities to human collagen.
PADM contains immunogenic disaccharides that can lead to host rejection and/or encapsulation. One method employed to decrease the immunogenicity of PADM is cross‐linking. While cross‐linking improves the immunogenicity of the scaffold by chemically concealing its antigenic epitope, it reduces the matrix metalloprotease‐mediated scaffold degradation and cell infiltration required for matrix remodeling 7. This may lead to suboptimal outcomes 8. Strattice™ (Life Cell Inc, Branchburg, NJ), a non‐cross‐linked PADM, is processed by enzymatic cleavage of the antigenic epitope 7, 8. Strattice™ displayed successful integration with primate tissue in an abdominal wall defect model 8, 9. Recent clinical reports describe successful use of Strattice™ as both an underlay and bridging mesh in abdominal wall reconstruction (AWR). In these studies, Strattice™ did not require an explanation following complications including exposure of the mesh, and all the patients eventually healed 10, 11. Despite showing promise in AWR, the ability of Strattice™ to vascularise and integrate with human tissue in the setting of an open wound has not been previously reported. The following case series aimed to evaluate these properties in high‐risk patients following ventral hernia repair.
Methods
This study design received approval from our institutional review board. Patients included in this case series are a subset from a previously published series of high‐risk patients who underwent ventral hernia repair with open components separation reinforced with Strattice™ onlay between 2008 and 2010. The selected subset experienced wound complications that required exploration resulting in exposure of the PADM onlay. One patient underwent punch biopsy of exposed PADM. Biopsy results were evaluated by a blinded pathologist.
Case 1
A 61‐year‐old, morbidly obese female with a history of recurrent incisional hernias that healed secondarily with resulting hypertrophic scars presented to the plastic surgery service. Preoperative comorbidities included morbid obesity, chronic obstructive pulmonary disease and smoking. On examination, significant loss of abdominal wall domain (>400 cm2) was appreciated, and prior skin graft was found to be directly adherent to bowel (Figure 1).
Figure 1.
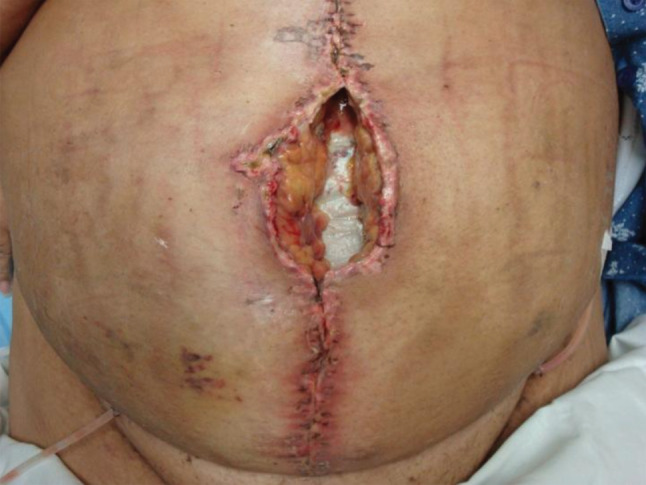
Wound dehiscence with wound cellulitis, underlying fat necrosis and exposed mesh.
The ventral hernia repair (VHR) consisted of skin graft takedown (Figure 2), lysis of adhesions, bilateral components separation with primary closure of muscle (Figure 3), reinforcement with a 20 × 20 cm2 Strattice™ onlay (Figure 4) and an infra‐umbilical panniculectomy via a fleur‐de‐lis incision. The operation was uneventful. Postoperatively, she developed skin and fat necrosis at the inverted T‐junction of her incisions. Operative wound debridement was performed on postoperative day six. The hernia repair was noted to be intact at that time. Negative pressure vacuum‐assisted closure was placed over the wound, and the patient was uneventfully discharged 2 days later.
Figure 2.
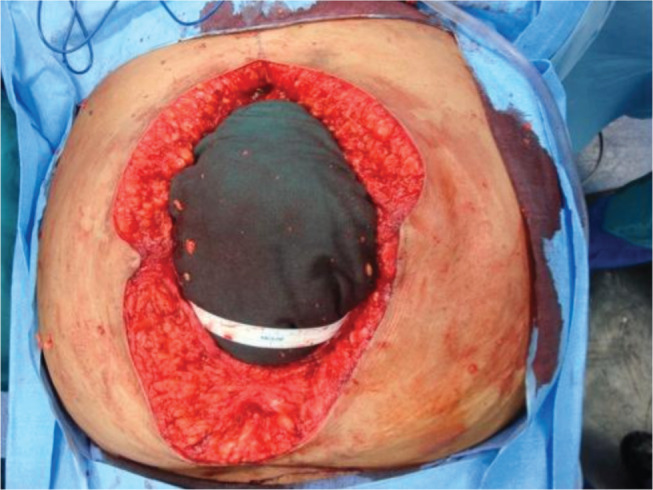
Wound following excision of necrotic tissue and removal of mesh demonstrating healthy tissue. Exposed bowel is directly beneath the blue towel.
Figure 3.
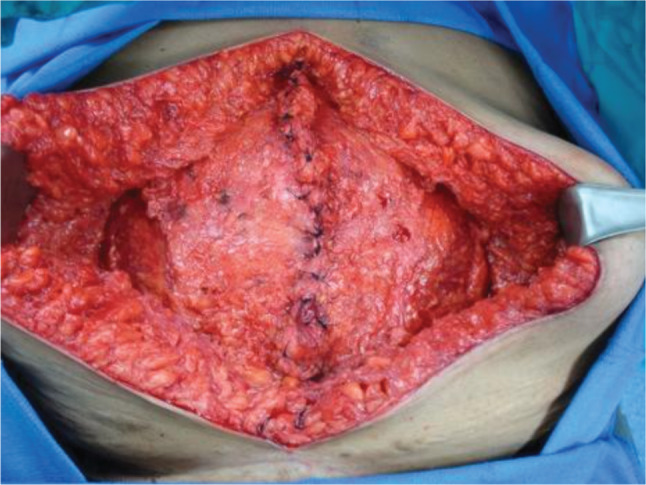
Primary closure of the muscle following lysis of adhesions and bilateral components separation.
Figure 4.
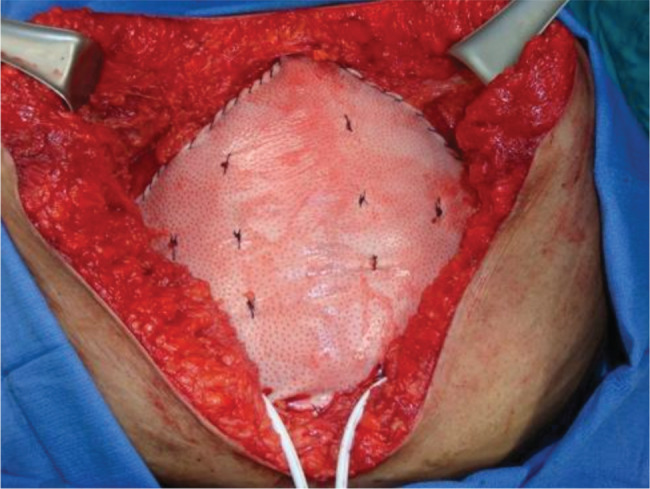
Reinforcement of the muscle with a 20 × 20 cm2 piece of firm non‐cross‐linked PADM mesh onlay and an infra‐umbilical panniculectomy via a fleur‐ de‐lis incision.
The patient followed up in clinic regularly over the next 3 months. During this period, the senior author noted progressive granulation tissue formation overlying the implanted PADM. Upon discontinuation of her negative pressure wound therapy, healthy granulation tissue was noted throughout the base of the wound (Figure 5). Definitive closure was then accomplished with application of a split‐thickness skin graft (Figure 6). A punch biopsy of the PADM onlay was taken at that time. Following blinded pathological review, the specimen was described as ‘fibrofatty tissue with granulation tissue and scar formation’. The PADM has not been explanted.
Figure 5.
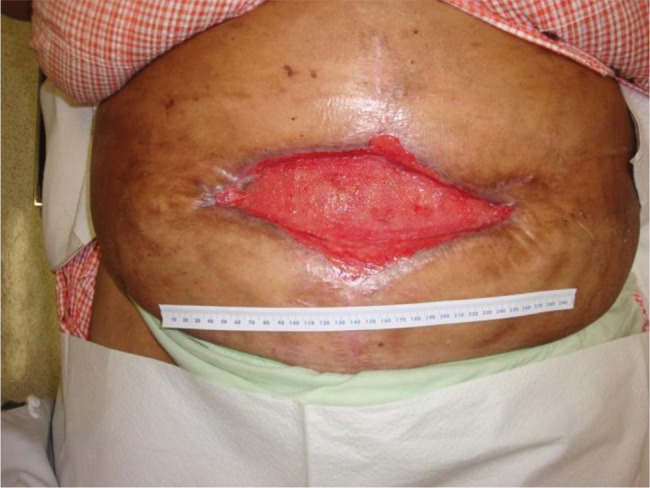
At the discontinuation of negative pressure wound therapy, healthy granulation tissue was noted throughout the base of the wound.
Figure 6.
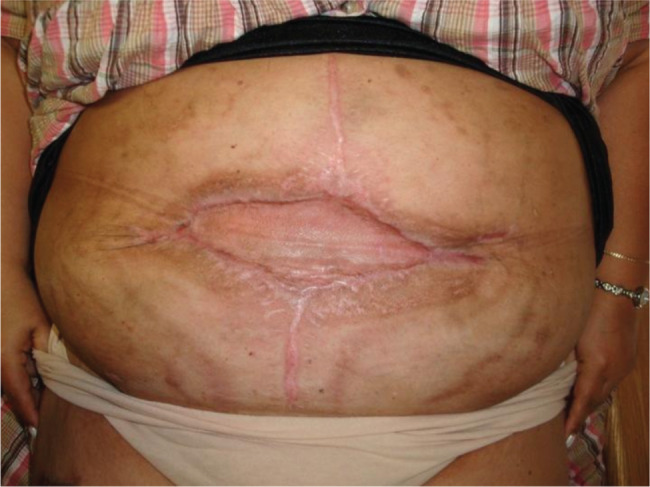
Definitive closure was accomplished by a split‐thickness skin graft.
Results
Wound complications requiring exploration occurred in 11 cases when Strattice™ was used as an onlay for complex hernia repair between 2008 and 2010. Reasons for exploration in these 11 cases included skin and fat necrosis requiring extensive debridement (72·7%), purulent drainage (18·2%) and seroma requiring surgical drainage (9·1%). Patient demographics are listed in Table 1. The average time to wound exploration was 30 ± 23·2 days postoperatively. In all cases, the fascia was found to be intact with no evidence of hernia recurrence. In 8 of the 11 (72·7%) cases, the mesh was observed to be intact with observed granulation tissue. In 2 of the 11 (18·2%) cases, a small portion of PADM was debrided because of partial separation from the fascia. In one case (9·1%), the patient required neoadjuvant radiotherapy and developed persistent drainage from an enterocutaneous fistula. This complication occurred within 1 week postoperatively and led to separation of the onlay from the surrounding tissue with necrosis of overlying granulation tissue. Outcomes following granulation tissue formation included re‐epithelialisation in four patients (36·4%), successful split‐thickness skin grafting in four patients (36·4%), definitive closure with rotational skin flaps in one patient (9·1%) and one patient (9·1%) who expired because of unrelated causes before definitive closure was attempted.
Table 1.
Patient demographics
| Demographic | n (%) |
|---|---|
| Patients | 11 (19) |
| Male | 5 (45·5) |
| Female | 6 (55·4%) |
| Mean age (Years) | 59·8 ± 12·3 |
| Mean BMI (Kg/m2) | 41·2 ± 12·1 |
| Obese | 9 (82) |
| Diabetic | 6 (55) |
| Smoker | 3 (27) |
The biopsy specimen demonstrated decreasing acute inflammation from superficial to deep tissue. Vascularisation was evident throughout the entire depth of the biopsy. There was no evidence of chronic inflammation in the skin graft (Figure 7).
Figure 7.
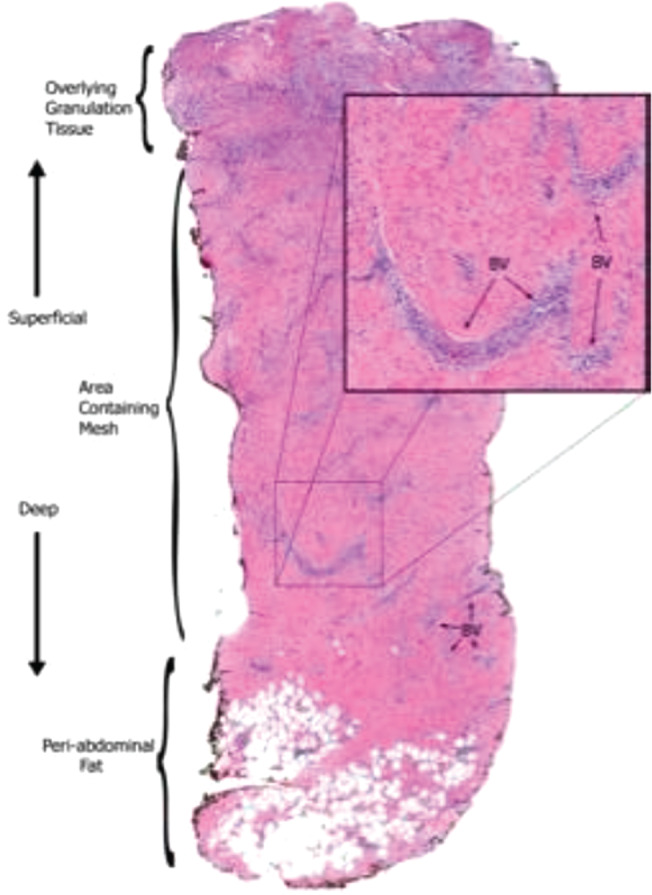
Haematoxylin‐ and eosin‐stained biopsy of Strattice within the wound. Extensive vascular integration is seen throughout the entire thickness of the mesh.
Discussion
Preventing infection of implanted material is crucial in patients at high risk for wound complications. Exposure of synthetic mesh mandates explanation that compromises abdominal wall repair 12. Acellular dermal matrices, which act as a scaffold to support native cellular migration and vascular ingrowth, are an increasingly utilised alternative to synthetic meshes. Primate models studying HADM demonstrate immune cell infiltration at 2 weeks and mature vascular structures at 4 weeks following implantation 13. ADM prevents infection by permitting immune cell access to necrotic debris and bacteria 1. Investigation of xenogeneic alternatives to HADM is important because of the short supply and high cost of human cadaveric tissue as well as uncertainty regarding the long‐term durability of HADM in abdominal wall reconstruction. Porcine tissue, given its abundant availability and its structural similarities to human dermis, may be an ideal alternative.
In this series, Strattice™ contributed to successful single‐stage ventral hernia repair in high‐risk patients. Intact fascia was found in all patients at the time of exploration. We interpret granulation tissue formation with re‐epithelialisation in four cases and granulation tissue formation with successful split‐thickness skin grafting in four cases as clinical evidence of PADM vascularisation. Necrotic granulation tissue seen in one case likely resulted from compromised blood supply following separation of the onlay from surrounding tissue because of persistent drainage from an enterocutaneous fistula that developed following neoadjuvant radiotherapy. Biopsy results in one patient supported our clinical observations, displaying vessel development throughout the tissue.
This study was not designed as a prospective trial, and the interpretation of the results is hindered by a small sample size. Histological analysis only occurred in one patient, preventing identification of variable patterns in immune response, tissue integration and vascularisation. Additionally, no immunological analysis was performed to assess the rejection status of the PADM. Further investigation may better characterise these physiological processes. Prospective studies and long‐term results are needed to further validate PADM use in AWR although we believe it is a durable and safe option.
Conclusion
Non‐cross‐linked PADM (Strattice™) successfully vascularises and integrates into human tissue following ventral hernia repair in high‐risk patients. Evidence for vascularisation and tissue incorporation were seen both clinically and histologically. Non‐cross‐linked PADM (Strattice™) offers durable biological reinforcement in AWR as well as a safety profile that allows use in high‐risk patients where synthetic meshes may not be an option.
Acknowledgements
Jamil A. Matthews is research fellow sponsored by LifeCell Corporation. Devinder P. Singh is a consultant for Acelity Incorporated. Ronald P. Silverman is the Chief Medical Officer for Kinetic Concepts Incorporated. Products used: Strattice®, a LifeCell Corporation product.
References
- 1. Silverman RP. Acellular dermal matrix in abdominal wall reconstruction. Aesthet Surg J 2011;31(7 Suppl):24S–9. [DOI] [PubMed] [Google Scholar]
- 2. Holton LH III, Chung T, Silverman RP, Haerian H, Goldberg NH, Burrows WM, Gobin A, Butler CE. Comparison of acellular dermal matrix and synthetic mesh for lateral chest wall reconstruction in a rabbit model. Plast Reconstr Surg 2007;119:1238–46. [DOI] [PubMed] [Google Scholar]
- 3. Armour AD, Fish JS, Woodhouse KA, Semple JL. A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro. Plast Reconstr Surg 2006;117:845–56. [DOI] [PubMed] [Google Scholar]
- 4. Bottino MC, Jose MV, Thomas V, Dean DR, Janowski GM. Freeze‐dried acellular dermal matrix graft: effects of rehydration on physical, chemical, and mechanical properties. Dent Mater 2009;25:1109–15. [DOI] [PubMed] [Google Scholar]
- 5. Wong AK, Schonmeyr B, Singh P, Carlson DL, Li S, Mehrara BJ. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg 2008;121:1144–52. [DOI] [PubMed] [Google Scholar]
- 6. Broyles JM, Abt NB, Sacks JM, Butler CE. Bioprosthetic tissue matrices in complex abdominal wall reconstruction. Plast Reconstr Surg Glob Open 2013;1:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlson TL, Lee KW, Pierce LM. Effect of cross‐linked and non‐cross‐linked acellular dermal matrices on the expression of mediators involved in wound healing and matrix remodeling. Plast Reconstr Surg 2013;131:697–705. [DOI] [PubMed] [Google Scholar]
- 8. Connor J, McQuillan D, Sandor M, Wan H, Lombardi J, Bachrach N, Harper J, Xu H. Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen Med 2009;4:185–95. [DOI] [PubMed] [Google Scholar]
- 9. Holton LH 3rd, Kim D, Silverman RP, Rodriguez ED, Singh N, Goldberg NH. Human acellular dermal matrix for repair of abdominal wall defects: review of clinical experience and experimental data. J Long Term Eff Med Implants 2005;15:547–58. [DOI] [PubMed] [Google Scholar]
- 10. Patel KM, Nahabedian MY, Albino F, Bhanot P. The use of porcine acellular dermal matrix in a bridge technique for complex abdominal wall reconstruction: an outcome analysis. Am J Surg 2013;205:209–12. [DOI] [PubMed] [Google Scholar]
- 11. Patel KM, Nahabedian MY, Gatti M, Bhanot P. Indications and outcomes following complex abdominal reconstruction with component separation combined with porcine acellular dermal matrix reinforcement. Ann Plast Surg 2012;69:394–8. [DOI] [PubMed] [Google Scholar]
- 12. Newman MI, Samson MC, Berho M. AlloDerm in breast reconstruction: 2 years later. Plast Reconstr Surg 2009;123:205e–6. [DOI] [PubMed] [Google Scholar]
- 13. Xu H, Wan H, Zuo W, Sun W, Owens RT, Harper JR, Ayares DL, McQuillan DJ. A porcine‐derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose‐alpha‐(1,3)‐galactose and retention of matrix structure. Tissue Eng Part A 2009;15:1807–19. [DOI] [PubMed] [Google Scholar]


