Abstract
Squamous cell carcinoma (SCC) arising from chronic hidradenitis suppurativa (HS) is rare; however, the morbidity associated with this presentation is high and management has not been standardised or optimised. We present a case of HS of the perineum and buttocks complicated by SCC, requiring multiple extensive surgical resections. Adjuvant radiotherapy was withheld initially because of concern for poor healing of the surgical wound but was eventually initiated after a second recurrence was identified. The patient ultimately expired 4 years after the initial diagnosis of SCC. We also review 80 cases of SCC complicating HS found in the English literature. Case reports and mechanistic studies suggest the possibility that human papilloma virus and smoking may be risk factors associated with SCC in HS. Despite the majority of SCC cases being well‐differentiated tumours in HS, the highly aggressive nature of SCC in HS and its high likelihood for rapid progression, recurrence, metastasis and high mortality suggests the need to advocate for aggressive treatment. We recommend an aggressive approach to management at the time of SCC diagnosis in HS, which includes appropriate imaging to establish the extent of the tumour, large and deep surgical excision, sentinel lymph node evaluation, consultation with radiation oncology for potential adjuvant radiation therapy and close surveillance.
Keywords: Acne inversa, Hidradenitis suppurativa, Skin cancer, Squamous cell carcinoma
Introduction
Hidradenitis suppurativa (HS) is a chronic, recurrent inflammatory disorder of the hair follicles in anatomic areas rich with apocrine glands. Patients develop long‐standing painful subcutaneous nodules, sinus tracts and scarring, mainly in the axillae, groin and buttocks 1. As with other chronic inflammatory disorders, squamous cell carcinoma (SCC) may arise in the setting of chronic inflamed lesions of HS. Although infrequently reported, there is an estimated prevalence of SCC among HS of up to 4·6% 2.
Case report
The patient was a 69‐year‐old African‐American gentleman presenting with continued drainage and tenderness from buttock lesions. He had a 40‐year history of smoking cigars and HS since age 16, requiring multiple surgeries on his buttocks/perineum and resection of invasive well‐differentiated (WD) SCC from a chronic left buttock lesion 2 years prior.
A physical exam showed a large, erythematous, fungating plaque on his left upper buttock, with surrounding induration, a background of extensive scarring, sinus tract formation and purulent, sanguineous drainage (Figure 1A,B). Deep biopsy demonstrated invasive SCC. Plastic surgery performed a wide resection, showing an infiltrating, moderately differentiated SCC (Figure 2A). The SCC measured 18·5 cm in greatest dimension, was present within 1 mm of the deep margin and arose in a background of acute and chronic inflammation, abscess formation and fistula tracts (Figure 2B). The SCC was negative for p16 by immunohistochemistry and negative for high‐risk human papilloma virus (HPV) by in situ hybridisation. An enlarged right inguinal lymph node on imaging was negative on biopsy.
Figure 1.
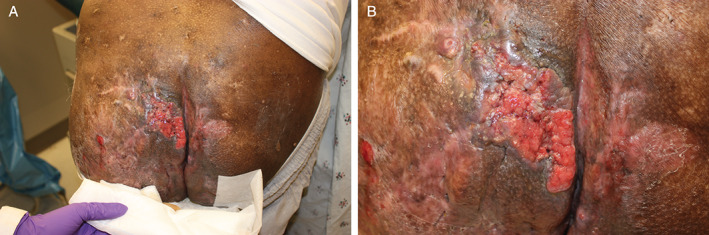
(A,B) Large, erythematous, fungating plaque on the left upper buttock.
Figure 2.
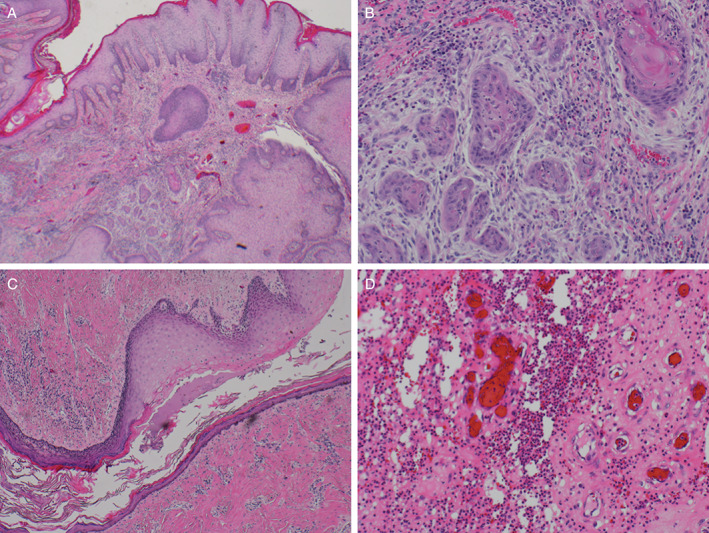
(A, B)The tumour displayed infiltrating nests of moderately differentiated squamous cells with keratinisation and desmoplastic stroma, consistent with infiltrating moderately differentiated squamous cell carcinoma. (C, D) The patient's gluteal tissue also displayed regions of fistula tract formation (left, H&E ×2) and abscess formation (right, H&E ×10), with abundant neutrophils and granulation tissue, consistent with a long‐standing history of hidrandenitis suppurativa.
The surgery was complicated by infection from faecal contamination, requiring additional debridement and a diverting colostomy to prevent further wound infection, and 40% of the split‐thickness graft performed two months later did not take. Adjuvant radiotherapy was postponed for fear of impairing wound healing at the extensive resection site. Eight months after the resection, SCC recurred at the previous resection site. Subsequent surgery showed invasive WD SCC with positive lateral and deep margins. Radiotherapy was again deferred because of concern for impairing wound healing.
Five months later, the patient was admitted after a fall and was found to have severe anaemia. Additional studies and surgical wound exploration showed SCC with highly vascularised progression, resulting in bleeding and requiring emergency radiotherapy. A more defined radiotherapy regimen was subsequently initiated but later discontinued after the patient decompensated with evidence of sepsis. The patient was discharged to hospice and ultimately expired 4 years after initial SCC diagnosis.
Discussion
There are 80 cases of SCC arising from HS reported in the English literature to date, with 76 cases summarised in case reports, series, reviews 2, 3, 4 and three additional case reports 5, 6, 7 in addition to the case presented in this manuscript; however, characteristics were not available for all cases. The average age of the reported cases was 52·4 years, with an average of 25·5 years of HS duration prior to SCC diagnosis (Table 1). Cases reporting SCC location (N = 80) were mainly perianal/perineal (41·3%) and gluteal (53·8%).
Table 1.
Characteristics associated with squamous cell carcinoma (SCC) complicating hidradenitis suppurativa (HS)
| Characteristic | N | Value |
|---|---|---|
| Average age, years (range) | 73 | 52·4 (27·0–78·0) |
| Gender | 74 | 86·5% male |
| Tobacco use status | 15 | 80·0% reported use |
| Duration of HS prior to SCC, years (range) | 69 | 25·5 (3·0–51·0) |
| Location of SCC* | 80 | |
| Perianal/perineal | 33 | 41·3% |
| Buttock/gluteal | 43 | 53·8% |
| Thigh | 5 | 6·3% |
| Groin/inguinal | 3 | 3·8% |
| Male external genitalia | 3 | 3·8% |
| Female external genitalia | 4 | 5·0% |
| Sacrum | 3 | 3·8% |
| Scapula | 1 | 1·3% |
| Histological differentiation | 58 | |
| Verrucous carcinoma | 9 | 15·5% |
| Well‐differentiated | 31 | 53·4% |
| Moderately differentiated | 12 | 20·7% |
| Poorly differentiated | 6 | 10·3% |
| Alpha‐HPV strain | 11 | 72·7% positive |
| Beta‐HPV strain | 8 | 87·5% positive |
| Evaluation for metastasis | 58 | 55·1% confirmed metastasis |
| Vital status | 71 | 53·5% confirmed dead |
HPV, human papilloma virus.
Percentages do not add up to 100% as SCC may involve more than one location.
While cases reporting detailed histology (N = 58) were most often WD (53·4%; Table 1), SCC in HS can be aggressive, with local invasion and increased risk of metastasis and mortality 2. Constantinou et al. reviewed 43 patients with HS complicated by SCC and demonstrated 48% local or distant recurrence, even among patients who had ‘curative’ surgery with negative margins, with mortality approaching 50% 1. Lavogiez et al. found that nearly half of 38 patients with SCC in HS had lymph node metastases on follow‐up, versus 5–10% in SCC of any aetiology, and that 57% of 52 patients with follow‐up expired within 2 years, with only 19% alive and free of recurrence after 1 year 2.
Delay of SCC diagnosis in HS patients is common as differentiating the disease process of HS from malignant transformation is difficult. A painful malignant ulcer could be mistaken for an inflammatory HS lesion 8, and lymphedematous changes resulting from chronic inflammation in HS can manifest focally as verrucous papules and nodules, which are difficult to distinguish clinically from SCC 6. Malignant transformation may also extend along the subcutaneous sinus tracts in HS, showing only atypical pseudoepitheliomatous hyperplasia with superficial biopsies 9. Therefore, a low threshold should exist to biopsy suspicious lesions in HS and repeat deep biopsies in any atypical non‐healing lesions 2.
While HS is more common in women than men, there is male predominance in SCC transformation 2. An explanation may be that SCC in HS mostly occurs in the perineal/perianal/gluteal areas, with higher prevalence of HS in these areas among men and the potential role of bacteria/viruses as a regional cofactor in combination with chronic inflammation in the development of SCC 2. The presence of certain HPV types is a known risk factor for the development of anogenital SCC 10, and a similar association has been hypothesised for the cutaneous SCCs associated with HS. Lavogiez et al. found high‐risk HPV‐16 and various subgroups of beta‐HPV in seven of eight cases of SCC in HS 2. Interestingly, one study found that DNA from the ‘low‐risk’ beta‐HPV species 2 subtypes (but not overall HPV DNA) was more likely to be present in SCC tumoural skin compared with normal skin 10. This finding suggests that the beta‐HPV subtype may be involved in the development of cutaneous SCC, which may warrant testing in future studies. Furthermore, important consideration should be given to the increasing use of biological immunosuppressants in HS and the association between chronic immunosuppression and SCC as beta‐HPV is activated under conditions of immune suppression 11.
Increasing evidence points to impaired Notch signalling as the unifying pathogenic mechanism explaining HS12. Impaired notch signalling is thought to compromise maintenance of the hair follicle and skin appendages, resulting in keratin‐enriched epidermal cysts that may rupture and trigger chronic inflammation. This inflammation, along with toll‐like receptor‐mediated innate immunity secondary to impaired Notch signalling, may promote and further perpetuate Th17‐mediated autoinflammatory reactions. Additionally, Notch has been shown to act as an epidermal tumour suppressor in SCC 13. While HS is associated with smoking, SCC in HS may be particularly associated with heavy smoking, as seen in our case and others 2. Smoking has been reported to downregulate Notch signalling in airway epithelial cells 14. Thus, the effects of smoking may augment pre‐existing impairment of Notch signalling in patients with HS 12, which may increase susceptibility to SCC in these individuals.
Once a diagnosis of SCC in HS has been made, an aggressive approach to management is critical in preventing significant morbidity and mortality. Thus, large and deep surgical excision is recommended, with a minimum margin of 2 cm when possible 2. Magnetic resonance imaging or positron emission tomography imaging may be helpful in establishing the true extent of the disease before surgery and for surveillance after surgery 15. Considering the high prevalence of lymph node metastasis, we also recommend sentinel lymph node evaluation, with surgical resection of the SCC to help diagnose subclinical lymph node metastasis 2. Additionally, consultation with radiation oncologists for adjuvant radiotherapy may be particularly important in this subset of patients and thus should not be postponed.
Conclusions
Case reports and mechanistic studies suggest the possibility that HPV and smoking may be risk factors associated with SCC in HS. Despite the majority of SCC cases being WD tumours in HS, the highly aggressive nature of SCC in HS and its high likelihood for rapid progression, recurrence, metastasis and high mortality suggests the need to advocate for aggressive treatment. We recommend an aggressive approach to management at the time of SCC diagnosis in HS, which includes appropriate imaging to establish the extent of the tumour, large and deep surgical excision, sentinel lymph node evaluation, consultation with radiation oncology for potential adjuvant radiation therapy and close surveillance.
References
- 1. Constantinou C, Widom K, Desantis J, Obmann M. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg 2008;74:1177–81. [PubMed] [Google Scholar]
- 2. Lavogiez C, Delaporte E, Darras‐Vercambre S, Martin De Lassalle E, Castillo C, Mirabel X, Laurent F, Patenotre P, Gheit T, Talmant JC, Beylot‐Barry M, Martinot V, Piette F, Aubin F, Mortier L. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology 2010;220:147–53. [DOI] [PubMed] [Google Scholar]
- 3. Perez‐Diaz D, Calvo‐Serrano M, Martinez‐Hijosa E, Fuenmayor‐Valera L, Munoz‐Jimenez F, Turegano‐Fuentes F, Del Valle E. Squamous cell carcinoma complicating perianal hidradenitis suppurativa. Int J Colorectal Dis 1995;10:225–8. [DOI] [PubMed] [Google Scholar]
- 4. Pena ZG, Sivamani RK, Konia TH, Eisen DB. Squamous cell carcinoma in the setting of chronic hidradenitis suppurativa: report of a patient and update of the literature. Dermatol Online J 2015;21:1–8. [PubMed] [Google Scholar]
- 5. Chang JB, Kung TA, Cederna PS. Acute Marjolin's ulcers: a nebulous diagnosis. Ann Plast Surg 2014;72:515–20. [DOI] [PubMed] [Google Scholar]
- 6. Chu EY, Kovarik CL, Lee RA. Lymphedematous verrucous changes simulating squamous cell carcinoma in long‐standing hidradenitis suppurativa. Int J Dermatol 2013;52:808–12. [DOI] [PubMed] [Google Scholar]
- 7. Verdelli A, Antiga E, Bonciani D, Bonciolini V, Volpi W, Caproni M. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol 2015;55:e52–3. [DOI] [PubMed] [Google Scholar]
- 8. Pagliarello C, Paradisi A. The perils of a defective medical communication: fatal neglected squamous cell carcinoma arising in perineal hidradenitis suppurativa. Case Rep Dermatol 2011;3:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Humphrey LJ, Playforth H, Leavell UW Jr. Squamous cell carcinoma arising in hidradenitis suppurativum. Arch Dermatol 1969;100:59–62. [PubMed] [Google Scholar]
- 10. Asgari MM, Kiviat NB, Critchlow CW, Stern JE, Argenyi ZB, Raugi GJ, Berg D, Odland PB, Hawes SE, de Villiers EM. Detection of human papillomavirus DNA in cutaneous squamous cell carcinoma among immunocompetent individuals. J Invest Dermatol 2008;128:1409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Villiers EM, Fauquet C, Broker TR, Bernard HU, Hausen HZ. Classification of papillomaviruses. Virology 2004;324:17–27. [DOI] [PubMed] [Google Scholar]
- 12. Melnik BC, Plewig G. Impaired Notch signalling: the unifying mechanism explaining the pathogenesis of hidradenitis suppurativa (acne inversa). Br J Dermatol 2013;168:876–8. [DOI] [PubMed] [Google Scholar]
- 13. Panelos J, Massi D. Emerging role of Notch signaling in epidermal differentiation and skin cancer. Cancer Biol Ther 2009;8:1986–93. [DOI] [PubMed] [Google Scholar]
- 14. Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O'Connor TP, Crystal RG. Down‐regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;179:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wortsman X, Jemec GB. Real‐time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg 2007;33:1340–2. [DOI] [PubMed] [Google Scholar]


