ABSTRACT
This evaluation involves an innovative muscle pump‐activating device (geko™) as an adjunctive therapy with best practices for non‐healing venous leg ulcers (VLUs). Stimulating the common peroneal nerve (at the fibular head), the geko™ device creates a response that acts as foot and calf muscle pumps, increasing venous, arterial and microcirculatory flow. The aim was to evaluate and determine if the geko™ is effective in this population and if it should be added to the medical supply formulary. In all, 12 patients with 18 recalcitrant VLUs (defined as less than 30% reduction in wound size in 30 days with best practices) in two community settings in Ontario consented to the evaluation and were treated with the geko™ for up to 20 weeks.
A total of 44% of wounds healed, and 39% decreased in size. One patient non‐adherent with the geko™ and best practices had deterioration in his or her wounds. With the patients as their own control, the mean weekly healing rate with the geko™ was 9·35% (±SD 0·10) compared to 0·06% (±SD 0·10) prior to baseline, which was statistically significant (P < 0·01). Three patients not in optimal therapy increased compression due to decreased pain, further enabling healing. This study was not a randomised investigation, although the patients acted as their own controls. A pragmatic evaluation reflects the reality of the community sector; in spite of best practices or evidence‐based care, therapy is not uniformly applied, with some participants unable to tolerate or indeed comply with optimal compression therapy. Rash occurred under the devices in 7 of 12 (58%) patients. One patient stopped the device due to rash, while another had to take breaks from using the device. Subsequently, the manufacturer (FirstKind Ltd) has developed a new device and protocol specific to the requirements of wound therapy to minimise this response.
This small case series demonstrated the highly significant effectiveness of the geko™ device in these hard‐to‐heal VLUs. Further evaluations to determine dose and patient selection criteria are underway.
Keywords: Blood flow, Geko, Muscle pump activator, Non‐healing venous leg ulcer
Introduction
Chronic venous insufficiency (CVI) is a term used to describe the clinical changes to the skin and subcutaneous tissue that occur because of chronic venous disease 1, caused by venous reflux and post‐thrombotic syndrome. The primary risk factors are advanced age, obesity, previous leg injuries, deep venous thrombosis and phlebitis, legs in the dependent position for long periods of time and female gender 2, 3, 4. Calf muscle pump failure occurs in up to 55% of patients with CVI 5, 6, 7 and is one of the contributing factors, along with skin changes, to the development of venous leg ulcers (VLUs). In Canada, where the prevalence of venous and arterial leg ulcers is reported as 0·7–2·6% of compromised wounds 8, VLUs occur in a population where 30% already have three or more comorbidities, 85% live with leg ulcer pain, 53% have issues with mobility, 24% have problems washing or dressing, 58% had difficulty performing usual activities, and one‐third reported moderate anxiety or depression 9. Obesity, arthritis, neurological disorders or conditions affecting mobility impair the normal mechanics of the legs when walking, further contributing to CVI and the risk of ulceration or non‐healing ulcers 10. VLU complications can include wound infection, cellulitis, osteomyelitis, contact and/or allergic dermatitis, bony ankyloses of the ankle and squamous cell malignancy. Healing can take from 6 months to many years 11, with recurrence rates up to 69% 12, all with incremental increases in cost 13. In the USA, an estimated 600 000 people are affected by VLUs each year at a cost of $1·5–3·5 billion 14, or up to 1% of health care budgets in some industrialised countries 15. Wound care has been estimated to consume 25–65% of the time of community nurses 16, and family physicians see an average of 1·5 patients with a chronic leg ulcer per week 17. Compression therapy is widely recognised as key to the management of VLUs, having been shown to increase healing rates in comparison with no compression 12, 18, 19. Post‐healing, compression reduces the recurrence rates 8. However, despite existing guidance, many patients with a VLU are not able to tolerate compression therapy. Although the overall prevalence is relatively low, the refractory nature of these ulcers increases the risk of morbidity and mortality and has a significant impact on patient's quality of life 20.
It appears expedient that all resources that would potentiate healing should be considered early or at the time of admission in the care of these patients. Phillips et al. 21 recommended that ‘ulcers that are large, of long duration, and slow to heal after 3 to 4 weeks of optimal therapy might benefit from alternative therapeutic measures’.
The geko™ neuromuscular electrostimulation (NMES) muscle pump activator (MPA) device (Figure 1) stimulates the common peroneal nerve in the lower leg, causing isometric muscle activation of the tibialis anterior and peroneus longus, extensor hallucis brevis and the medial gastrocnemius 22 (Figures 2 and 3). The extensor muscles are then activated with an additional stretch of the antagonistic flexor muscles, which pull in a distal direction during dorsiflexion, compressing the flexor muscles by the fascial envelopes 22. The passive motion of the flexor muscle acts as a calf muscle pump, which may enhance venous return by increasing intramuscular pressure, possibly reducing venous stasis and oedema and influencing muscle oxygenation 22. In healthy volunteers, it increases femoral vessel peak velocity (cm/second) to 216%, ejected volume per stimulus (ml) to 113% and volume flow during muscle contraction to 36% 23, 24. In patients with claudication, the geko™ device has been shown to be statistically significant in increasing venous and arterial volume and microcirculatory flow after just 60 minutes of stimulation 25. Venous volume flow increased by 0·034 l/minute (mean) in the active limb compared to 0·002 l/minute (mean) in the passive limb [P < 0·001]; arterial volume flow increased by 0·68 l/minute (mean) compared to the passive limb, 0·004 l/minute (mean) [P < 0·001], and microcirculatory flow increased by 22·25 flux units (mean) [P < 0·001] 25. For patients with CVI, the geko™ device was used for 4–6 hours per day for 6 weeks, compared to baseline, increased the mean femoral vein peak velocity by 60% (P = 0·05), the time averaged peak velocity (TAMV) by 27% (P = 0·07), volume flow by 51% (P = 0·15) and reduced oedema by 16% (P < 0·05) 26, 27, with incremental improvements linked to the length of time (6 weeks) that the device was used.
Figure 1.
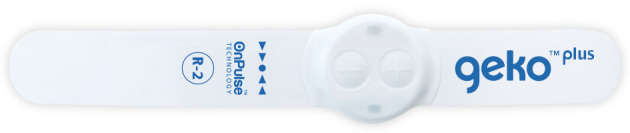
geko™ device.
Figure 2.
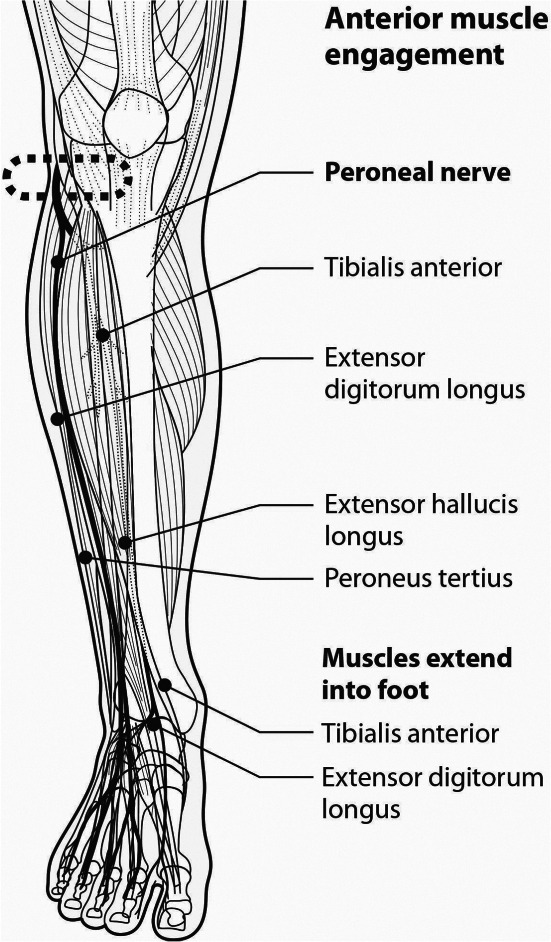
Anterior muscle engagement.
Figure 3.
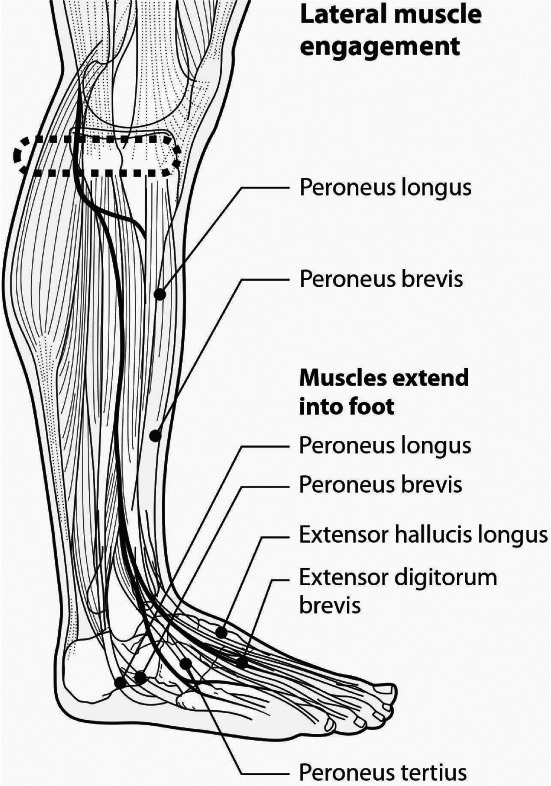
Lateral muscle engagement.
Two Community Care Access Centres (CCACs), providers of community health care in Ontario, Mississauga Halton (MH), Mississauga and South West (SW), London, Canada wished to evaluate the geko™ device as an adjunctive to best practices to determine if these circulatory gains could improve healing rates for patients with recalcitrant VLUs, and if so, which patients might most benefit? Depending on the results, a decision would be made regarding adding the device to the medical supply formulary. This study reports the results in an observational case series approach.
Materials and methods
The R‐2 geko™ devices, manufactured by Firstkind Ltd., High Wycombe, UK, are wearable, self‐contained and adhesive, small (weighs 10 g), disposable, internal battery‐powered, insulated neuromuscular electrostimulation (NMES) muscle pump‐activating (MPA) devices that are applied to the leg at the fibular head. The integral electrodes apply an electrical stimulus of 54 mA at a rate of once per second (1 Hz) to the lateral popliteal or common peroneal nerve. The geko™ device has a range of stimulation levels to balance the maximal effect of stimulation with subject comfort, without affecting normal movement of the limb or mobility of the subject. They were provided at no cost to the patients or CCACs by Perfuse Medtec Inc. London, Canada and Firstkind Ltd.
Participants and procedures
Patients who had chronic VLUs not responding to the current standard of care (i.e. advanced wound dressings, compression therapy if tolerated, pain management and antibiotics as required), whose wounds had less than 30% reduction in surface area following best practice for 30 days and who could or could not tolerate compression therapy were identified.
Ethics review was obtained from the Regional Centre for Excellence in Ethics, Homewood Health Centre, 150 Delhi Street, Guelph, Ontario, N1E 6K9.
Consenting patients were provided with daily pairs of geko™ devices at no cost to themselves or the CCAC. They were to be an adjunct to their standard of care treatment 5 days per week for 6 hours each day, on both legs. On weekends, all patients continued with standard of care but no geko™ therapy. Once the wound closed, the device was to be used 6 hours per day twice weekly for 4 weeks. The therapy would be discontinued for other reasons, such as lack of response, non‐adherence or onset of comorbidity that resulted in hospitalisation. Nurses, the patient and/or family member were educated in the use of the geko™ device. The nursing and CCAC manager participated in bi‐weekly to monthly phone calls to review progress, address issues etc.
Electronic data collection
To expedite data collection for the nurses, a new geko™ data collection document was created by the nursing agency, CarePartners, Kitchener, ON, which was tablet‐fillable and could embed photographs into the document (Figure 4). This allowed the nurses to quickly capture images of the wound, leg and application sites, and the completed wound flow sheets were used to capture wound size, appearance, exudate, local treatment, level of pain etc., part of usual wound assessment and documentation. Oedema, where present, was measured at the mid‐foot, smallest ankle and widest part of the calf using a measuring tape. Reports were due at baseline, every 2 weeks x 4 and then every 4 weeks after that.
Figure 4.
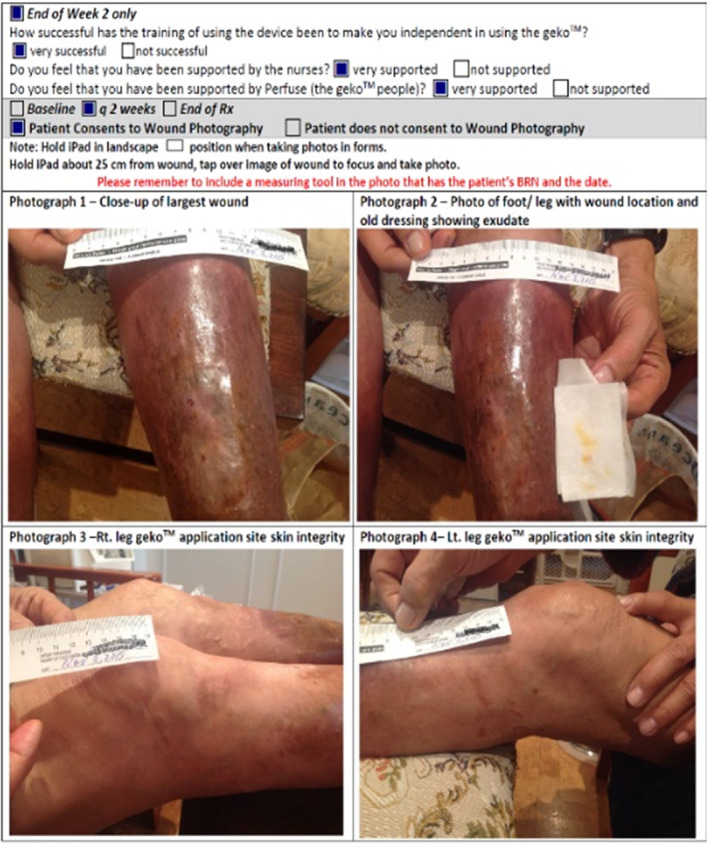
Electronic data collection.
Patient satisfaction and QoL
To understand the patient experience using the geko™ device, a Quality of Life (QoL) screen was used 28. Although not a validated QoL tool, it used a scale of 0–10, with 0 being ‘Delighted’ and 10 being ‘Terrible’ and was recorded at baseline, at 6 weeks and at the end of treatment (Figure 5). It was also important to know whether the patient was satisfied with the training to use the device independently, so a short questionnaire was created asking whether they felt very supported or not supported by (i) the nurses and (ii) the Perfuse consultant (Figure 5).
Figure 5.
Quality of life (QoL) screen.
The anticipated time for the evaluations was 6–9 months, with the opportunity for patients to continue with the geko™ if the wound was responding but not yet healed at the end of the evaluation.
Statistical analysis
The average mean value of the total number of wounds with the number of wounds healed or decreased in surface area was analysed. Box 1 shows the method used to calculate the percentage change in ulcer surface area (SA) over time 29. The weekly ulcer healing rate was determined by dividing these data by the number of weeks between the initial ulcer at time of admission to the point of geko™ implementation (baseline) and from then with the geko™ until complete reepithelialisation (wound closure), patient discharge or at the last point of evaluation. The cumulative proportion of ulcers healed was determined. Ulcers not healed at the final assessment were determined to be improving or deteriorating. A comparison t‐test was used to compare the difference in healing rate per week between the two stages of the study before and after the geko™ was applied.
BOX 1. Percentage change in surface area (cm2) 29 .
*SA = Surface area calculated as longest length × perpendicular widest width.
Results
In all, 12 patients with 18 VLUs, with a combined 33+ year history of living with lower leg ulcers, consented and enrolled between October and December 2015. All had wounds that were considered to be non‐healing prior to using the geko™ device, 9 with VLUs and 3 with veno‐lymphoedema. The range of duration of treatment with regards to community nursing service was 1–73 weeks, with a mean of 24 weeks per patient. Table 1 provides their demographics. The evaluation was considered concluded at 20 weeks for reporting purposes.
Table 1.
Patient demographics
| Demographic | Results |
|---|---|
| Number of patients | 12 |
| Average age | 64 years (range: 41–83 years) |
| Gender | 58% were male and 41% female. |
| ABPI values | Eight ≥0·8 |
| One was >0·7–<0·8 | |
| One was reported as ‘normal’ | |
| One had angioplasty 6 months prior but no ABPI results | |
| One could not have ABPI due to pain. | |
| Comorbidities | Diabetes Type I and II, Hypertension, Chronic Renal Failure, Lung Disease, Cancer, Spinal Cord Injury, Smoker, Lymphoedema, Peripheral Arterial Disease, High BMI, CVI. |
| All had at least 1 comorbidity, and one had more than 10. | |
| Status of leg ulcer | 100% non‐healing |
| Average size of leg ulcer (SA) | 15 cm2 (range: 0·09–85 cm2) |
| Average duration of leg ulcer(s) | 2·6 years (range: 6 weeks to 20 years) |
| Use of compression therapy | 91% were wearing compression of some type/strength |
| Use of advanced wound products | 100% were using advanced wound care products. As this was to be ‘care as per usual’, we did not factor in changes in products (e.g. as exudate decreased, they could use less absorptive dressings, etc.) |
| BMI > 33 kg/m2 | Six had BMI > 33 kg/m2 |
| Mobility less than 200 metres per day | Seven walked less than 200 metres per day Two patients did not have this completed None out of 10 had ulcers should heal in 24 weeks: Nine out of 10 had a combination of indicators that will heal > 24 weeks + may never heal One out of 10 had indicators that may never heal |
| Prognostic indicators of healing: | |
| May have delayed healing (>24 weeks): | |
| >10 cm2 and/or if the wound is older than 12 months 34, 35 | |
| History of venous ligation or vein stripping 34 | |
| History of hip or knee surgery 34 | |
| ABPI < 0·80 34, 36 | |
| >50% covered in fibrin 33, 36 | |
| Obesity (BMI > 33 kg/m2) 37 | |
| Walking < 200 metres/day 37 | |
| History of surgical debridement of ulcer 37 | |
| Depth > 2 cm 37 | |
| Poor compliance with compression systems 35, 38 | |
| May never heal: | |
| Calf–ankle circumference ratio < 1·3 37 | |
| Fixed ankle joint 37 | |
| Decreased ability to flex and dorsiflex foot 37 |
Patient demographics
Prior to adding the geko™ treatment, the average weekly SA reduction was negligible at 0·06% (±SD 0·1). When the geko™ was added, the average weekly healing rate in all patients (adherent and non‐adherent) was a statistically significant 9·35% (±SD 0·1) reduction in SA (P < 0·01) over 20 weeks, with a mean of 11 weeks (Figure 6). Complete time to healing varied, with 22% of patients healed by 11 weeks, and a cumulative 44% healed by 20 weeks (Figure 7). Of the wounds included, 39% (7/18) of wounds had complete closure, 44% (8/18) decreased in size, and 17% (3/18) deteriorated in the 20 weeks of the evaluation (Figure 8).
Figure 6.
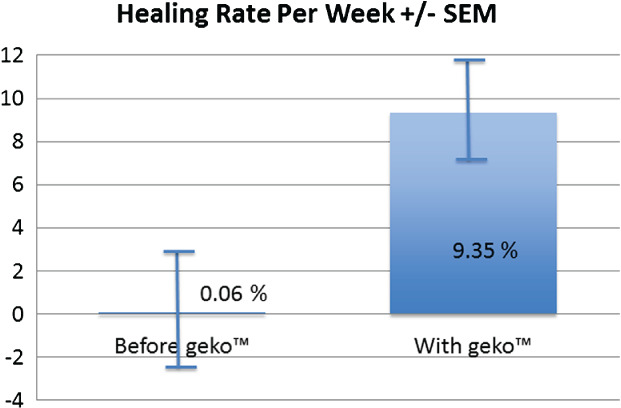
Healing rate per week.
Figure 7.
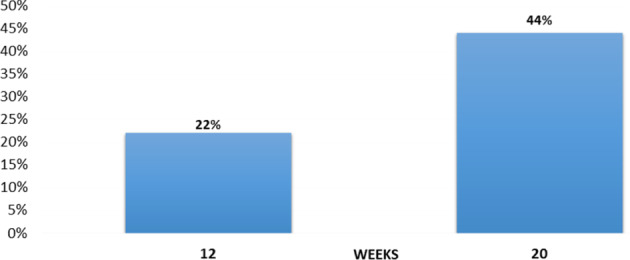
Cumulative proportion healed (all patients).
Figure 8.
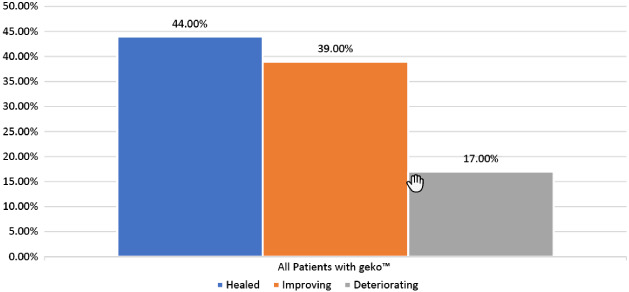
Ulcer healing rates. All patients with geko™ (ulcer healing status through 20 weeks).
Six patients did not stay on the device for the entire evaluation for various reasons, with an average geko™ treatment time of 8 weeks. While none of their eight wounds healed, there was a mean 59% reduction in SA. The reasons for stoppage were various, including discharge to a palliative care facility, self‐discharge from care due to family situation, deceased due to comorbid conditions, family request, increased pain and oedema not thought to be related to geko™ and rash under the devices (one patient for each circumstance). One patient who was deemed non‐adherent to the geko™ and best practices had three wounds that deteriorated. Three patients continued with the device over the 20 weeks: one had healed but continued to develop blisters, one had healed but the wounds re‐opened when compression bandaging applied elsewhere slipped down, and the one who had been non‐adherent had become adherent and wished to continue for a while longer.
Four patients were reported to have pain at baseline, ranging from 3/10 to 10/10, with 10 being the worst pain. Two had reduction, and two had resolution of nociceptive pain. Two patients had documentation or anecdotal reports of pain. In addition, two patients reported a reduction in neuropathic symptoms of tingling, burning, shooting and stabbing pains with the geko™ device. One of these patients needed to stop the geko™ treatment for a period of time due to skin rash and reported that the neuropathic symptoms increased without the geko™ and reduced when he resumed the device. A third patient reported an increase in ‘normal’ sensory function, in that she could feel light touch again.
Oedema changes were inconsistent. Of the 12 patients, 10 had sequential leg measurements recorded; oedema decreased in 7 of 10 but was not uniform for the three sites measured (mid‐foot, smallest ankle and widest calf) and varied by as little as 3% to as much as 26% reduction in size. Oedema measurements increased in the remaining three patients. One of those patients, with chronic bilateral veno‐lymphoedema, reported that his legs were softer and more pliable, with improved ability to dorsiflex both feet and wiggle his toes 2 weeks into the evaluation. It was easier for him to walk, and his legs felt less cumbersome.
From the patient experience perspective, eight patients completed the questionnaire at week 6 and/or at end of treatment. Seven of the eight (88%) were comfortable wearing the device, and one was not. Eight patients completed the questions at week 2 regarding support from nursing and the Perfuse staff; all eight felt very supported by both groups. Patients appeared generally satisfied with the device, with a mean score of 3/10 (0 = ‘delighted’, 10 = ‘terrible’). Rash occurred under the devices in 7 of 12 (58%) patients. One patient stopped the device due to rash, while another had to take breaks from using the device.
Each patient's story was unique. For purposes of brevity, two stories of patients who benefited in a variety of ways from the evaluation are shared here. Patient 1 was a 77‐year‐old male with CVI and diabetes, who had a non‐healing surgical amputation site of one toe on the right foot (Figure 9A), 4·5 months in age, with previous bypass surgery to this leg 7 years prior. Angioplasty was performed 1 month before amputation of the toe. He also had a venous ulcer on the right shin (Figure 9B), which had doubled in size over 3 months. He was in an inelastic Unna's paste boot dressing. His nursing visits went from every 2 days at baseline to every 3 days by week 3. Both wounds closed at 5 weeks (Figure 9C).
Figure 9.
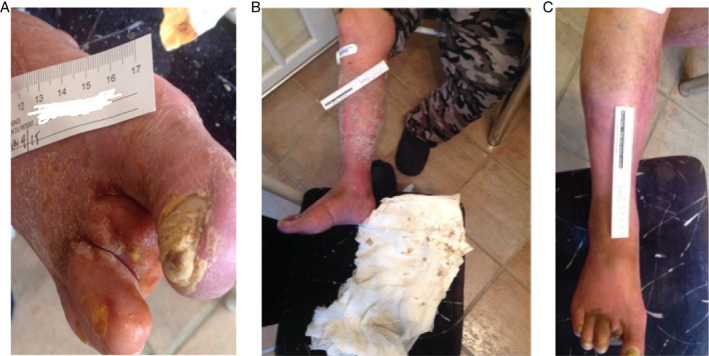
(A) Patient 1 right foot at baseline. (B) Right leg at baseline. (C) Both healed at 5 weeks.
Patient 2 was female, 83‐year‐old, on service for 52 weeks with a 20‐year history of VLU. Her pain was 10/10 during dressing change and 8/10 at other times (decreased with leg elevation), unable to tolerate compression or Ankle Brachial Pressure Index testing (APBI). There were numerous fibrin‐covered ulcers with copious exudate requiring daily dressing changes (Figure 10A) over much of the mid‐anterior and medial right shin, with a total SA of 85 cm2, an increase of 251% since admission. She walked <200 metres/day and had decreased ability to flex and dorsiflex her right foot. Although the nurses reported that patient did not adhere to wearing the geko™ device as per the plan and found the device uncomfortable, more devices were ordered at 7 weeks for another 4 weeks of therapy. At that point, she believed that her wound was healable as it was getting smaller. The exudate decreased; she was moved to dressings every 3rd day; pain had reduced to 3/10; and, of key importance, she was in a 2‐layer‐high‐compression system at 8 weeks. The wounds were clean and granulating from the base (Figure 10B) and on a healing trajectory, compared to approximately 100% fibrin at baseline and non‐healing wounds. The family was seeking an alternate device to promote healing, so the evaluation was stopped, and final measurements were taken at 11 weeks. There was a reduction in the total SA of 85%, or 7·7% decrease in wound size per week – of key importance – she was tolerating a high‐compression system, although she was evidently cutting the bandages on her feet at times due to tightness.
Figure 10.
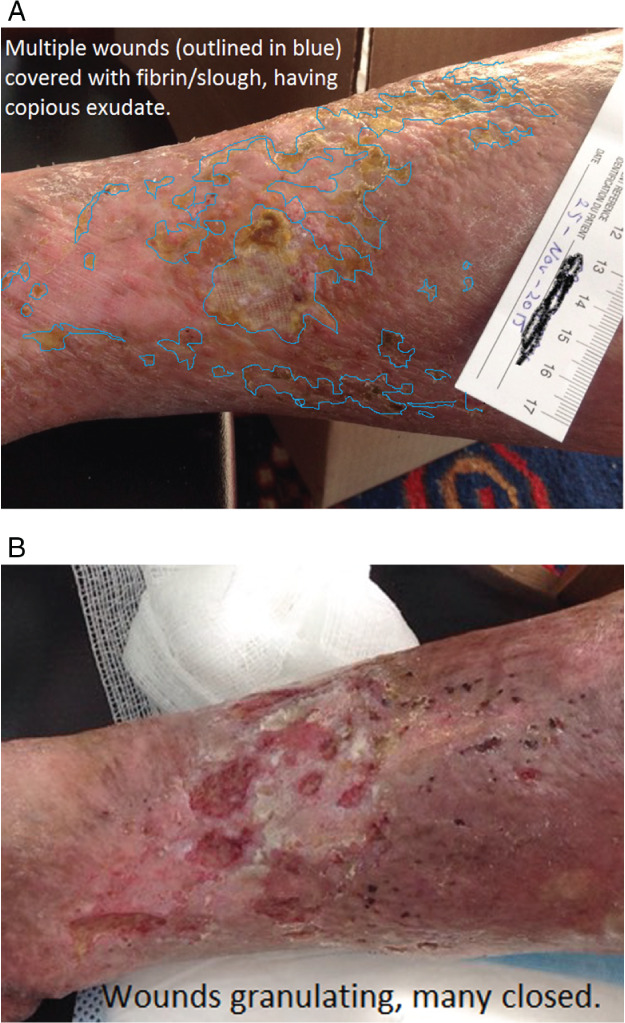
(A) Patient 2 fibrin‐covered wounds at baseline. (B) Wounds at 12 weeks.
Discussion
In this geko™ device evaluation, the focus was primarily on the amount of reduction in SA or wound closure achieved with geko™ and the patient's experience. There was. a significant improvement in the mean weekly healing rate, up to 9·35% ± 0·10 (P < 0·01) in wounds, a very favourable comparison to the baseline healing rate (pre‐geko™) of 0·06% ±0·10 in this previously non‐healing cohort. In new VLUs, an average healing rate of 7.1% per week (28.79% at 4 weeks) is an indicator that the ulcer will close by 24 weeks 30. The geko™ device weekly rate exceeded this with recalcitrant, non‐healing VLUs 30.
Two patients previously deemed non‐adherent to compression therapy could undergo high compression after starting the geko™ device, meaning that they were now adherent to the key best practice intervention.
It was difficult to ascertain why oedema reduction was inconsistent, although it stands to reason that patients with fibrotic chronic veno‐lymphoedema, chronic renal failure or lipodermatosclerosis would be less likely to see changes in volume of the legs. In a patient with bilateral veno‐lymphoedema the change from firm or fibrotic to softer legs and an increased ability to flex and dorsiflex his or her feet suggests that a fibrinolytic benefit was occurring. This was noticed by the patient and nurses within 2 weeks of starting the geko™. Barnes et al. (2016) 31 found a statistically significant LOCAL fibrinolytic effect (P < 0·001) with the geko™ device, which could explain this phenomenon.
Skin inflammation and irritation, including rash, are recognised side effects of electrical stimulation 32, and patients with CVI are at a higher risk of developing reactions to many products 33. This can be further exacerbated by patients scratching or rubbing the skin. When irritation occurred, two alternate fitting locations were used as recommended by the manufacturer: one below the fibular head and one slightly above the knee crease posteriorly. If they required topical care, their nurses or physicians advised or prescribed interventions. The manufacturer of the geko™ device advises caution if the device is to be used for more than 28 consecutive days as may be required for treating the wound care population. Subsequently, the manufacturer (FirstKind Ltd) has developed a new device and protocol specific to the requirements of wound therapy to minimise this response.
The treatment regimen with the geko™ for this evaluation was 6 hours per day, 5 days per week. Two patients had to modify this regimen due to their personal physical inability to manage the device independently. The ‘ideal’ wear time for maximum effect is likely to be subject to many variables.
This evaluation was conducted in part to help determine which patients will benefit from the geko™ therapy and to assess the product's suitability for addition to the medical supply formulary. At the time of writing, both CCACs plan to make the geko(TM) device available for patients with wounds who meet the CCAC criteria. This has been a positive experience for all, as indicated by remarks from each of the CCAC participants, the Nursing Service provider agency and the wound care physician, which can be found in Table 2. It has also enabled MH CCAC to develop a sustainable process for evaluation of new products.
Table 2.
Participants' perspectives on the evaluation
| Mississauga Halton CCAC perspective | South West CCAC perspective |
|---|---|
| MH CCAC is committed to working collaboratively with our partners in the delivery of evidenced‐informed care that supports excellent patient outcomes, enhances the patient experience and balances clinical efficacy with cost efficiency. To evaluate the geko™ device, MH CCAC selected chronic hard‐to‐heal patients with multiple comorbidities. The wound improvements had an overall positive impact on the patients' experience with their wound care. Based on this, MH CCAC is adding the device to their medical supplies and equipment formulary. Furthermore, we believe that its use should not be limited to only those patients with chronic non‐healing wounds, where all other therapies have failed. The implementation of innovative approaches to wound care such as the geko™ device should be considered as an adjunctive therapy. Work is underway to develop a policy and procedure for the use of geko™ to ensure the right patients have access to the device at the appropriate time in their care. Further analysis is also needed to understand whether geko™ has an influence on the frequency of nursing visits. | The South West CCAC appreciated the opportunity to partner in this evaluation of the geko™ device. When enrolled, these chronic hard‐to‐heal patients with venous leg ulcers and multiple comorbidities and the caregivers had low expectations of improvement. However, study participants identified wound improvements as well as an overall positive impact on quality of life. Although SWCCAC has not added the device to their formulary now, they are committed to developing a framework to guide the process of offering and evaluating the effectiveness of alternative and adjunctive wound care therapies. It is important to consider Nursing capacity to develop expertise in this and other alternative and adjunctive wound care therapies. Patient selection criteria need to be clearly established for adjunctive therapy. If the primary wound healing protocol is not being followed, for example off‐loading, compression, nutrition and diabetes management, the adjunctive treatment effectiveness will be suboptimal. Finally, it is necessary to have clear outcome indicators to guide decision making when this device should be discontinued and what next stage protocol is appropriate. |
| An additional benefit of this evaluation is that MH CCAC's medical supply and equipment committee now has a process for evaluation and adoption of new heath care innovations. |
| Nursing service provider perspective | Physician perspective |
|---|---|
| This successful project involved key partners who made a commitment to collaborate in the evaluation, investing time and resources. Factors for success included the number and type of patients and length of time required for the evaluation, co‐ordination and dedication of the Wound Care Specialist and Wound Resource Nurse visits, collaboration with the physician, reporting at set intervals of time, additional education for nurses and patients, keeping patient confidentiality within the parameters of the patient consent, regular teleconferences or face to face meetings as the study progressed, for sharing of information (successes and challenges), performing the evaluation of the patient experience and additional cost to the agency, which reimbursed the nurses to attend meetings, and compensated for longer visits required for the evaluations. The goal was patient centred with the hope that wounds would heal timely resulting in an improved quality of life, and they felt that this was achieved. | Most of the patients evaluated in the South West represent patients with multiple comorbid conditions and complex lower extremity oedema. Most had limited calf muscle pump function and poor walking tolerance. In general, they were intolerant of adequate compression therapy. The device should help to stimulate both venous and lymphatic return through muscle contractions. The trial describes a real‐life evaluation in a home care setting, which presents challenges of consistency of care and patient adherence to care plans. As well, despite the use of a fillable iPad form consistent with forms already in use, with multiple persons entering data there are often missing data points. The only significant adverse effect was rash related to the glue used to adhere the device. This is being addressed by the developer. Given all these challenges the data suggest that it is reasonable to further evaluate this device in a controlled trial. |
Participants' perspectives on the evaluation
Study limitations
This study was not a randomised investigation, although the patients acted as their own controls. There was no pre‐determined treatment duration due to the non‐healing nature of the participants. A pragmatic evaluation reflects the reality of the community sector; in spite of best practices or evidence‐based care, it is not uniformly applied, with some participants unable to tolerate or indeed comply with optimal compression therapy. Timely data collection can be a challenge without an electronic documentation system that tracks metrics or forces responses.
Conclusions
This evaluation provided an opportunity to determine the effectiveness of the geko™ device on a non‐healing VLU cohort. To have achieved an average weekly healing rate of 9·35% (±SD 0·10) with the geko compared to a baseline of 0·06% (±SD 0·10) (P < 0·01) in such recalcitrant patients is exciting and may be the beginning of a paradigm shift in treatment. The ability to add or increase the level of compression in 3 of 12 (25%) of patients is a further positive benefit of geko™ therapy. If the increase in healing and ability to tolerate compression results are reproducible, use of the geko™ device can improve health for many of these patients and may help to reduce health costs. Further evaluation is needed to determine which patients most benefit from the geko™ device as an adjunctive to best practices and when it should be implemented in the treatment timeline.
Acknowledgements
The authors acknowledge Mississauga Halton CCAC; South West CCAC, CarePartners; Mr. Ron Shannon, Health Care Economist and Dr. Duncan Bain who assisted in analysis of the data.
Perfuse Medtec, Inc. London, ON, Canada provided in‐kind support in the way of geko™ devices, software provision to nurses as well as training and support as outlined in this paper.
No direct financial support was received for this study; however, educational support was provided by Perfuse Medtec Inc (London, Canada) and geko™ devices by FirstKind Ltd. (High Wycombe, UK).
For the duration of the evaluations, Connie Harris was the CNS for Wounds at CarePartners but has been a contracted Education and Research Consultant with Perfuse Medtec, Inc. Since 1 April 2016, and with HNHB CCAC since 11 May 2016.
References
- 1. Dissemond J, Körber A, Grabbe S. Differential diagnosis of leg ulcers. J Dtsch Dermatol Ges 2006;4:627–34. [DOI] [PubMed] [Google Scholar]
- 2. Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: a dual case‐control study. J Vasc Surg 1995;22:622–8. [DOI] [PubMed] [Google Scholar]
- 3. Jawien A. The influence of environmental factors in chronic venous insufficiency. Angiology 2003;54:S19–31. [DOI] [PubMed] [Google Scholar]
- 4. Lacroix P, Aboyans V, Preux PM, Houlès MB, Laskar M. Epidemiology of venous insufficiency in an occupational population. Int Angiol 2003;22:172–6. [PubMed] [Google Scholar]
- 5. Araki CT, Back TL, Padberg FT, Thompson PN, Jamil Z, Lee BC, Duran WN, Hobson RW 2nd.. The significance of calf muscle pump function in venous ulceration. J Vasc Surg 1994;20:872–7; discussion 878‐9. [DOI] [PubMed] [Google Scholar]
- 6. Simka M. Calf muscle pump impairment and delayed healing of venous leg ulcers: air plethysmographic findings. J Dermatol 2007;34:537–44. [DOI] [PubMed] [Google Scholar]
- 7. Williams KJ, Ayekoloye O, Moore HM, Davies AH. The calf muscle pump revisited. J Vasc Surg 2014;2:329–34. [DOI] [PubMed] [Google Scholar]
- 8. Canadian Institute for Health (CIHI) Report on compromised wounds 2011‐2012. URL https://secure.cihi.ca/free_products/AiB_Compromised_Wounds_EN.pdf [accessed on 1 September 2016]
- 9. Harrison MB, VanDenKerkhof EG, Hopman WM, Carley ME. Community‐dwelling individuals living with chronic wounds: understanding the complexity to improve nursing care. A descriptive case series evaluation. Clin Nurs Stud 2013;1:43–57. [Google Scholar]
- 10. Back TL, Padberg FT, Araki CT, Thompson PN, Hobson RW. Limited range of motion is a significant factor in venous ulceration. J Vasc Surg 1995;22:519–23. [DOI] [PubMed] [Google Scholar]
- 11. Sauer K, Rothgang H, Glaeske G. BARMER GEK Heil‐ und Hilfsmittel report 2014. URL http://www.zes.uni‐bremen.de/uploads/News/2014/140916_Heil_Hilf_Report_2014.pdf [accessed on 31 March 2017]
- 12. Nelson EA, Bell‐Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2014;9:CD002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tennvall RJ, Hjelmgren J, Öien R. The cost of treating hard‐to‐heal venous leg ulcers: results from a Swedish survey. World Wide Wounds 2006: 1–7. URL http://www.worldwidewounds.com/2006/november/Tennvall/Cost‐of‐treating‐hard‐to‐heal‐venous‐leg‐ulcers.html [accessed on 13 November 2012]. [Google Scholar]
- 14. Hankin CS, Knispel J, Lopes M, Bronstone A, Maus E. Clinical and cost efficacy of advanced wound care matrices for venous ulcers. J Manag Care Pharm 2012;18:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Donnell TF Jr, Balk EM. The need for an Intersociety Consensus Guideline for venous ulcer. J Vasc Surg 2011;54(6 Suppl):83S–90. [DOI] [PubMed] [Google Scholar]
- 16. Probst S, Seppänen S, Gerber V, Hopkins A, Rimdeika R, Gethin G. EWMA Document: Home CareWound Care. J Wound Care 2014;23(5 Suppl):S1–44. [DOI] [PubMed] [Google Scholar]
- 17. Hampton S, Lindsay E. Empowering patients to take control of leg ulcer treatment through individualised management. J Wound Care 2005;14:238–40. [DOI] [PubMed] [Google Scholar]
- 18. Harding, K. , Comerota AJ., Partsch, H. 2008. Chronic venous insufficiency and venous ulceration‐ aetiology and treatment. Compression in venous leg ulcers: a consensus document WUWHS. URL http://www.wuwhs.org/datas/2_1/9/Compression_VLU_English_WEB.pdf [accessed on 1 May 2011]
- 19. Registered Nurses Association of Ontario . Assessment and management of venous leg ulcers supplement. Toronto, Canada: Registered Nurses Association of Ontario. 2007. URL http://rnao.ca/sites/rnao‐ca/files/storage/related/2469_RNAO_Venous_Leg_Ulcer_Supplement.pdf [accessed on 6 January 2017].
- 20. Vishwanath V. Quality of life: venous leg ulcers. Indian Dermatol Online J 2014;5:397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V. Prognostic indicators in venous ulcers. J Am Acad Dermatol 2000;43:627–30. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Q, Styf J, Ekström L, Holm AK. Effects of electrical nerve stimulation on force generation, oxygenation and blood volume in muscles of the immobilized human leg. Scand J Clin Lab Invest 2014;74:369–77. [DOI] [PubMed] [Google Scholar]
- 23. Griffin M, Bond D, Nicolaides A. Measurement of blood flow in the deep veins of the lower limb using the geko™ neuromuscular electrostimulation device. Poster. URL http://www.gekodevices.com/media/126684/13339_geko_poster_a0.pdf [accessed on 31 March 2017]
- 24. Griffin M, Bond D, Nicolaides A. Measurement of blood flow in the deep veins of the lower limb using the geko™ neuromuscular electro‐stimulation device. Int Angiol 2016;35:406–10. [PubMed] [Google Scholar]
- 25. Barnes R, Shahin Y, Tucker A, Chetter I. Haemodynamic augmentation in patients with peripheral arterial disease with eh geko™ transcutaneous neuromuscular electrical stimulation device. ASIT/ASGBI Short Paper Prize Section. Abstracts. Int J Surg 2015;18:239. [Google Scholar]
- 26. Williams KJ, Babber A., Ravikumar R, Ellis M, Davies AH. Pilot trial of neuromuscular stimulation in the management of chronic venous disease. 2 Posters from VEINS Conference, UK. 2014.
- 27. Williams KJ, Davies AH. Pilot trial of neuromuscular stimulation in the management of chronic venous disease. British Journal of Surgery 2015;102:20. [Google Scholar]
- 28. SouthWest Regional Wound Care Framework . URL www.southwesthealthline.ca/healthlibrary_docs/b.1.2.screenandbradenscale.pdf [accessed on 15 September 2015]
- 29. Sussman C. Wound measurements and prediction of healing. In: Sussman C, Bates‐Jensen BM, editors. Wound Care: A Collaborative Practice Manual. Philadelphia: Lippincott Williams & Wilkins, 2007. pp. 134. [Google Scholar]
- 30. Kantor J, Margolis DJ. A multicenter study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 31. Barnes R, Madden LA, Chetter IC. Fibrinolytic effects of peroneal nerve stimulation in patients with lower limb vascular disease. Blood Coagul Fibrinolysis 2016;27:275–80. [DOI] [PubMed] [Google Scholar]
- 32. Orsted HL, O'Sullivan‐Drombolis D, Haley J, LeBlanc K, Parsons L. The effects of low frequency nerve stimulation to support the healing of venous leg ulcers. Can Assoc Wound Care 2016:1–16. [Google Scholar]
- 33. Rietschel RL, Fowler JF. Fischer's Contact Dermatitis, 5th edn. Vol. 39. Philidelphia: Lippincott Williams & Wilkins, 2001. [Google Scholar]
- 34. Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999;135:920–6. [DOI] [PubMed] [Google Scholar]
- 35. Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages? Am J Med 2000;109:15–9. [DOI] [PubMed] [Google Scholar]
- 36. Skene AI, Smith JM, Dore CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992;305:1119–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Milic DJ, Zivic SS, Bogdanovic DC, Karanovik ND, Golubovic ZV. Risk factors related to the failure of venous leg ulcers to heal with compression treatment. J Vasc Surg 2009;49:1242–7. [DOI] [PubMed] [Google Scholar]
- 38. Scotton MF, Abbade LBF, Miot MA. Factors that influence healing of chronic venous leg ulcers: a retrospective cohort. An Bras Dermatol 2014;89:414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


