Abstract
To evaluate the clinical use and economic aspects of negative pressure wound therapy (NPWT) after dorsal stabilisation of spinal fractures. This study is a prospective randomised evaluation of NPWT in patients with large surgical wounds after surgical stabilisation of spinal fractures by internal fixation. Patients were randomised to either standard wound dressing treatment (group A) or NPWT (group B). The wound area was examined by ultrasound to measure seroma volumes in both groups on the 5th and 10th day after surgery. Furthermore, data on economic aspects such as nursing time for wound care and material used for wound dressing were evaluated. A total of 20 patients (10 in each group) were enrolled. Throughout the whole study, mean seroma volume was significantly higher in group A than that in group B (day 5: 1·9 ml versus 0 ml; P = 0·0007; day 10: 1·6 ml versus 0·5 ml; P <0·024). Furthermore, patients of group A required more wound care time (group A: 31 ± 10 minutes; group B 13·8 ± 6 minutes; P = 0·0005) and more number of compresses (total number; group A 35 ± 15; group B 11 ± 3; P = 0·0376). NPWT reduced the development of postoperative seroma, reduced nursing time and reduced material required for wound care.
Keywords: NPWT, Spinal fracture, Wound complication, Wound healing
Introduction
In recent years, negative pressure wound therapy (NPWT) became a widely used therapy for many different indications in the treatment of wounds 1, 2, 3, 4. Recently, NPWT has also been used in the treatment of closed surgical wounds. The incisional NPWT (iNPWT) has shown beneficial effects when administered after severe trauma 5, 6, 7, 8. The indications and the evidence for the efficacy of iNPWT have increased recently 9, 10, 11. However, only a few prospective randomised studies have been published on those indications mostly dealing with hip arthroplasty 11, 12. The mode of action of iNPWT is still not completely understood. This is thought to be mediated by increased oxygen delivery to the tissue as result of enhanced tissue perfusion and promotion of angiogenesis 5, 9, 13.
The purpose of this study was to evaluate the different aspects of wound healing in spinal fractures treated by internal fixation. We compared a standard wound dressing group with an iNPWT group in the context of postoperative seroma formation in the wound area, the total time of secretion, the total time needed for the wound care (dressing changes) and the material needed for dressing changes.
Materials and methods
A total of 20 patients with spinal fractures were scheduled for internal fixation. They were randomised into two groups. Group A (10 patients) received the standard wound dressing of our department, consisting of a dry wound coverage (compresses attached to the skin).
Group B (10 patients) was treated with iNPWT over the sutured wound area. The surgical intervention was identical in both the groups. An open reduction technique with internal fixation system was performed for all the patients, which was obtained from the same manufacturer (Synthes, West Chester, PA). All patients received two Redon® drains, one on each side of the spinal column subcutaneously. Postoperative physiotherapy and mobilisation were also identical for both the groups.
The iNPWT group (group B) was treated with a PICO™ system (Smith & Nephew plc, London, UK). The PICO™ system was left on the wound for 5 days including the day of surgery. In addition to daily clinical examination, all wounds/seroma were analysed by ultrasonography on the 5th and 10th day after surgery.
Before surgery, plasmatic coagulation was assessed in all patients using the Quick prothrombin time test. Postoperatively, the immediate amount of wound secretion in the Redon drain canisters was quantified. In addition, the length of the incision was measured. The duration of secretion from the wounds was monitored and the total number of dressing changes and the time required to perform the dressing changes were also assessed. The material used for dressing changes was also quantified (compresses and gloves).
Statistical significance was calculated with the Prism v6.0 GraphPad Software, Inc. (La Jolla, CA). For Gaussian distributed data, the Student's t‐test was used. For non‐Gaussian distributed data, the Mann–Whitney test was used.
Informed consent was obtained from each patient. The study was approved by the local ethics committee (Re‐No.139_12 B) and conforms to the principles of the Declaration of Helsinki.
Results
In this study, 10 patients (mean age 57·80 ± 15·24 years) were randomised to group A and 10 patients (mean 52·30 ± 16·32 years) to group B. Both groups displayed normal coagulation times according to the Quick prothrombin time test (group A: 94·20 ± 9·52%; group B: 96·00 ± 7·80%; P = 0·73). There was no significant difference in the postoperative wound size between both groups (group A: 17·25 ± 5·70 cm; group B: 14·60 ± 4·38 cm; P = 0·26). Furthermore, both groups displayed almost equal volumes of wound secretion in the Redon® drain canisters after 2 days (group A: 621·5 ± 286·5 ml; group B: 454·0 ± 229·6 ml; P = 0·16). The seroma volume underneath the surgical wound was significantly lower at day 5 and day 10 in the iNPWT group (day 5: group A: 1·9 ± 2·7 ml versus group B: 0 ± 0 ml (P = 0·0007); day 10: group A: 1·6 ± 2·6 ml versus group B: 0·5 ± 1·0 ml; P = 0·024).
The patients treated with iNPWT required fewer dressing changes: 48 dressing changes in group B patients, equating to 4·8 per patient and 79 dressing changes in group A patients equating to 7·9 per patient (Figure 1). Group B patients also had lesser number of days of wound secretion (Figure 2) and required lesser time for wound care (Figure 3) and lesser material for dressing changes (Figures 4 and 5).
Figure 1.
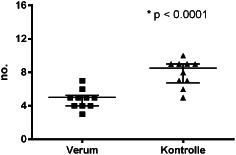
Distribution of number of dressing changes (P < 0·0001).
Figure 2.
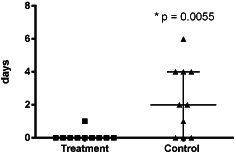
Distribution of number of days of wound secretion.
Figure 3.
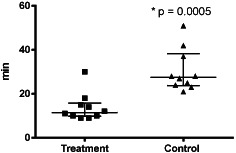
Distribution of wound care time.
Figure 4.
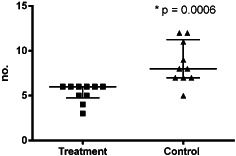
Distribution of number of used gloves for dressing changes.
Figure 5.

Distribution of number of used compresses for dressing changes.
Discussion
Since the development of NPWT, the indications for the use of NPWT have been mainly acute and chronic wounds 1, 2, 3, 14, 15, 16, 17, 18; however, the indications have increased over time 2, 19. NPWT exerts a positive effect on wound healing resulting in a reduction in wound healing complications. Furthermore, NPWT treatment excels because of its ease of application and a low risk of side effects 20, 21. Recent studies using NPWT showed a reduction of seromas in wounds after hip surgery. The beneficial effect of NPWT was found after elective total hip arthoplasty and after arthroplasty of femoral neck fractures 11, 12. A recently published review confirmed the reduction of wound complications in high‐risk wounds 9. In our study, we evaluated for the first time the possible effects of iNPWT in spinal fractures treated with open reduction and internal fixation. Besides the anatomical area of application, it is the first prospective randomised study in orthopaedics using the PICO™ system (Figure 6) (Smith & Nephew plc).
Figure 6.
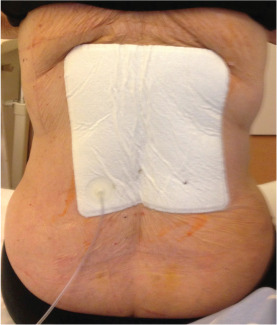
Application of a PICO system to the wound.
This study provides compelling evidence that iNPWT may be useful to treat large surgical incision wounds after surgical treatment of spinal fractures. To the best of our knowledge, significant reduction of wound complications in fractures of the spine treated with open reduction and internal fixation by using iNPWT has not been previously reported. In addition, iNPWT treatment reduced the time and dressing material needed for postoperative wound care.
The present study used ultrasound as a standardised imaging modality to detect seromas in the wound area. The high sensitivity of this imaging modality allowed for the detection of seroma volumes directly underneath the surgical incision. This method was previously described for the evaluation of iNPWT after surgical interventions of the hip and femoral neck fracture 11, 12. In line with these studies, we found a significant reduction of wound draining days in the iNPWT group compared with that in the control group. In addition to reduced wound secretion, Stannard et al. showed that iNPTW treatment reduced haematoma size in patients suffering from high‐energy trauma injuries 5, 22. Given the fact that haemotomas might favour as nutrient‐rich environments for bacterial replication and that persisting wound drainage might facilitate the entrance of bacteria into the wounds, the reduction of wound secretion and the haematoma size might reduce the risk of a wound infection.
Haematomas are thought to serve as rich nutrient sources for infection 23. In our opinion, a prolonged secretion is an important risk factor of early postoperative infections as well. However, to the best of our knowledge, we are not aware of a study that has addressed this issue. Hence, further investigations into these mechanisms are warranted.
Furthermore, it is not fully understood how iNPWT leads to a reduced seroma and haematoma formation in the wounded tissue. Horch et al. suggested that NPWT results in a significantly increased tissue perfusion and oxygenation 13. In addition to altering tissue perfusion, treatment with iNPWT might reduce wound edge tension and thereby promote healing 24.
Our results in this study are consistent with the findings of other studies regarding the reduction of wound healing complications after the treatment with iNPWT.
We and others have demonstrated that wound treatment with iNPWT after elective total hip arthroplasty for the treatment of osteoarthritis of the hip and the treatment with iNPWT after surgical treatment of femoral neck fractures reduced the wound healing complications 11, 12. In addition, patients who suffered from severe soft tissue damage after trauma displayed a faster recovery when the iNPWT device was placed on the wounded tissue at early time points 13, 25. In our study, we attached the iNPWT device immediately after surgical wound closure to the skin over the wound.
In a previous study, we observed that the time dedicated to the wound care and the consumption of wound care material were significantly shorter than those in the control group. In agreement with our previous study 11, we found that iNPWT treatment reduced the time needed for wound care and the consumption of wound care material. The 48 dressing changes in the iNPWT group also included the removal of the redon drains and the removal of the device. To avoid a bias, these dressing changes were also documented; the number would have been even smaller if this study‐related dressing changes were not taken into account.
Limitations of the present study are the relatively small number of enrolled patients and the use of an iNPWT device from a single manufacturer. The difference between the different manufacturers seems to be relatively small and the principles of NPWT seem to be transferable between the different manufacturers. However, this has to be confirmed by comparative studies.
Conclusion
In summary, our results support the use of iNPWT after spinal surgery. Apart from its economic benefits, iNPWT promotes wound healing and might prevent wound infections, which are the dreaded complications of spine surgery.
References
- 1. Kanakaris NK, Thanasas C, Keramaris N, Kontakis G, Granick MS, Giannoudis PV. The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury 2007;38(Suppl 5):S9–18. [DOI] [PubMed] [Google Scholar]
- 2. Krug E, Berg L, Lee C, Hudson D, Birke‐Sorensen H, Depoorter M, Dunn R, Jeffery S, Duteille F, Bruhin A, Caravaggi C, Chariker M, Dowsett C, Ferreira F, Martínez JM, Grudzien G, Ichioka S, Ingemansson R, Malmsjo M, Rome P, Vig S, Runkel N, Martin R, Smith J. Evidence‐based recommendations for the use of Negative Pressure Wound Therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus International Expert Panel on Negative Pressure Wound Therapy [NPWT‐EP]. Injury 2011;42(Suppl 1):S1–12. DOI: 10.1016/S0020-1383(11)00041-6. [DOI] [PubMed] [Google Scholar]
- 3. Lehner B, Fleischmann W, Becker R, Jukema GN. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop 2011;35:1415–20. DOI: 10.1007/s00264-011-1274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stannard JP, Singanamala N, Volgas DA. Fix and flap in the era of vacuum suction devices: what do we know in terms of evidence based medicine? Injury 2010;41:780–6. DOI: 10.1016/j.injury.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 5. Stannard JP, Robinson JT, Anderson ER, McGwin G Jr, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 6. DeCarbo WT, Hyer CF. Negative‐pressure wound therapy applied to high‐risk surgical incisions. J Foot Ankle Surg 2010;49:299–300. DOI: 10.1053/j.jfas.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 7. DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, Ward WG, Teasdall RG. The use of vacuum‐assisted closure therapy for the treatment of lower‐extremity wounds with exposed bone. Plast Reconstr Surg 2001;108:1184–91. [DOI] [PubMed] [Google Scholar]
- 8. Fleischmann W, Russ M, Marquardt C. Closure of defect wounds by combined vacuum sealing with instrumental skin expansion. Unfallchirurg 1996;99:970–4. [DOI] [PubMed] [Google Scholar]
- 9. Karlakki S, Brem M, Giannini S, Khanduja V, Stannard J, Martin R. Negative pressure wound therapy for managementof the surgical incision in orthopaedic surgery: a review of evidence and mechanisms for an emerging indication. Bone Joint Res 2013;2:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Altintas B, Biber R, Brem MH. The accelerating effect of negative pressure wound therapy with Prevena™ on the healing of a closed wound with persistent serous secretion. Int Wound J 2014; doi: 10.1111/iwj.12198 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pauser J, Nordmeyer M, Biber R, Jantsch J, Kopschina C, Bail HJ, Brem MH. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures –reduction of wound complications. Int Wound J 2014; doi: 10.1111/iwj.12344 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P, Pauser J, Gelse K, Brem MH. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 2012;36:719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horch RE, Münchow S, Dragu A. Erste Zwischenergebnisse der Perfusionsbeeinflussung durch Prevena: Gewebsperfusionsmessung. Z Wundheilung 2011;A 16:19–20. [Google Scholar]
- 14. Dedmond BT, Kortesis B, Punger K, Simpson J, Argenta J, Kulp B, Morykwas M, Webb LX. The use of negative‐pressure wound therapy (NPWT) in the temporary treatment of soft‐tissue injuries associated with high‐energy open tibial shaft fractures. J Orthop Trauma 2007;21:11–7. [DOI] [PubMed] [Google Scholar]
- 15. Lehner B, Bernd L. V.A.C.‐instill therapy in periprosthetic infection of hip and knee arthroplasty. Zentralbl Chir 2006;131(Suppl 1):S160–4. [DOI] [PubMed] [Google Scholar]
- 16. Brem MH, Blanke M, Olk A, Schmidt J, Mueller O, Hennig FF, Gusinde J. The vacuum‐assisted closure (V.A.C.) and instillation dressing: limb salvage after 3 degrees open fracture with massive bone and soft tissue defect and superinfection. Unfallchirurg 2008;111:122–5. [DOI] [PubMed] [Google Scholar]
- 17. Loos B, Kopp J, Kneser U, Weyand M, Horch RE. The importance of vacuum therapy in the treatment of sternal osteomyelitis from the plastic surgeons point of view. Zentralbl Chir 2006;131(Suppl 1):S124–8. [DOI] [PubMed] [Google Scholar]
- 18. Stannard JP, Volgas DA, Stewart R, McGwin G Jr, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 2009;23:552–7. [DOI] [PubMed] [Google Scholar]
- 19. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 20. Horch RE, Gerngross H, Lang W, Mauckner P, Nord D, Peter RU, Vogt PM, Wetzel‐Roth W, Willy C. Indications and safety aspects of vacuum‐assisted wound closure. MMW Fortschr Med 2005;147(Suppl 1):1–5. [PubMed] [Google Scholar]
- 21. Willy C, von Thun‐Hohenstein H, von Lubken F, Weymouth M, Kossmann T, Engelhardt M. Experimental principles of the V.A.C.‐therapy – pressure values in superficial soft tissue and the applied foam. Zentralbl Chir 2006;131(Suppl 1):S50–61. [DOI] [PubMed] [Google Scholar]
- 22. Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009;55:58–66. [PubMed] [Google Scholar]
- 23. Verner EF, Musher DM. Spinal epidural abscess. Med Clin North Am 1985;69:375–84. [DOI] [PubMed] [Google Scholar]
- 24. Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 2012;19:67–75. [DOI] [PubMed] [Google Scholar]
- 25. Kaplan M, Daly D, Stemkowski S. Early intervention of negative pressure wound therapy using vacuum‐assisted closure in trauma patients: impact on hospital length of stay and cost. Adv Skin Wound Care 2009;22:128–32. [DOI] [PubMed] [Google Scholar]


