Abstract
Chitin and chitosan are biopolymers with excellent bioactive properties, such as biodegradability, non‐toxicity, biocompatibility, haemostatic activity and antimicrobial activity. A wide variety of biomedical applications for chitin and chitin derivatives have been reported, including wound‐healing applications. They are reported to promote rapid dermal regeneration and accelerate wound healing. A number of dressing materials based on chitin and chitosan have been developed for the treatment of wounds. Chitin and chitosan with beneficial intrinsic properties and high potential for wound healing are attractive biopolymers for wound management. This review presents an overview of properties, biomedical applications and the role of these biopolymers in wound care.
Keywords: Biopolymer, Chitin, Chitosan, Healing, Wound dressing
Introduction
Chitin is the second most ubiquitous natural polysaccharide after cellulose on earth. It is a high molecular weight linear homopolymer of β‐(1, 4) linked N‐acetylglucosamine (N‐acetyl‐2‐amino‐2‐deoxy‐D‐glucopyranose) units. Chitosan, the principal derivative of chitin, is a copolymer of glucosamine and N‐acetylglucosamine units linked by 1–4 glucosidic bonds. Chitin is the major constituent of the shells of crustaceans, the exoskeletons of insects and the cell walls of fungi where it provides strength and stability. On the other hand, chitosan only occurs naturally in some fungi, Mucoraceae 1. Chitin was first discovered in the cell walls of mushrooms by Professor Henri Braconnot in 1811. Henri Braconnot's name for chitin was fungine. In 1823, Odier found the same material in insects and plants and named it chitin 2. Professor C. Rouget discovered chitosan in 1859, and over the next century, much fundamental research took place on these compounds. An intense interest in new applications grew in the 1930s and early 1940s. However, commercial development was hampered due to the lack of adequate manufacturing facilities and competition from synthetic polymers. Revived interest in the 1970s encouraged scientists worldwide to explore the more distinct properties of chitin and its derivatives. Since then, numerous research studies have been undertaken to understand the potential of these natural polymers.
The role of chitin and chitosan as biomaterials is indisputable, as evidenced by the scientific literature and patents published over the last 40 years. Chitin is one of the most abundant renewable biopolymers on earth that can be obtained at a low cost from marine sources. Chitosan is obtained by N‐deacetylation of chitin to a varying extent that is characterised by the degree of deacetylation. As functional materials, chitin and chitosan offer a unique set of characteristics and have been extensively exploited in biomaterials research. Chitin and chitosan are currently receiving a great deal of interest with regards to medical and pharmaceutical applications, owing to their distinct chemical and biological properties 3, 4. Chitin is generally an insoluble material but can be deacetylated to form the soluble polymer chitosan. Chitosan preparations of various molecular weights, degrees of deacetylation and with further molecular derivatization patterns have attracted much attention because of their potentially beneficial biological properties 5, 6. Chitin and chitosan are biopolymers endowed with an excellent biocompatibility, which strongly recommends their use as an innovative biomatrix intended for clinical applications, such as drug‐delivery devices, scaffolds for tissue engineering and bioactive dressings. Other interesting characteristics include a non‐toxic, non‐immunogenic nature with excellent biodegradability, antimicrobial activity and accelerated wound‐healing properties 7, 8. Chitin and chitosan are attracting increasingly more attention recently as their inherent biological and physicochemical characteristics are being better understood. Chitin and chitosan with beneficial intrinsic properties and high potential for wound healing are attractive biopolymers for wound management.
Chitin and chitosan polymers
Chemical structure
Chitin (C8H13O5N)n is a long‐chain polymer of N‐acetylglucosamine, a derivative of glucose. Figure 1 shows the structure of the chitin molecule, showing two of the N‐acetylglucosamine units that repeat to form long chains in β‐1,4 linkage. Chitosan is a high‐molecular copolymer with acetylglucosamine and glucosamine radicals in its chains.
Figure 1.
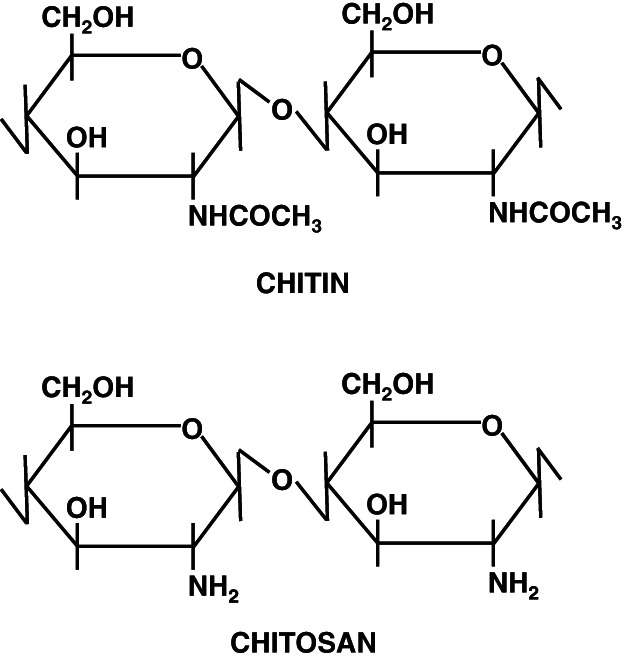
Structure of chitin and chitosan.
Chitin structure is similar to the structure of cellulose, except that acetylamino groups have replaced the hydroxyl groups in position 2. It contains acetamide groups (−NHCOCH3) at the C‐2 positions. Approximately 16% of naturally occurring chitin units are deacetylated. Chitosan is a linear polymer of a 1–4 linked 2‐amino‐2‐deoxy‐D‐glucopyranose and is derived by N‐deacetylation to a varying extent that is, consequently, a copolymer on N‐acetylglucosamine and glucosamine. Deacetylation releases amine groups (NH) and gives the chitosan a cationic characteristic. The various sources of chitin differ in their structure and chitin content. There are three polymorphic forms of chitin, α, β and γ. The most abundant and easily accessible form is α‐chitin. The chitin molecules align in an anti‐parallel fashion in α‐chitin that allows maximum intermolecular hydrogen bonding 9. The chitin molecules are, however, parallel in β‐chitin, which is responsible for the weaker intermolecular forces 10. The structure of α‐ and β‐forms differ only in that the piles of chains are arranged alternately anti‐parallel in α‐ chitin, whereas they are all parallel in β‐chitin 11. The γ‐chitin form has characteristics of both α‐ and β‐forms, where two chains run in one direction and another chain in the opposite direction. γ‐chitin is considered only a variant of the α‐family because it has the same properties as the α‐chitin 12. α‐Chitin is the most abundant and also the most stable thermodynamically 13, 14, and the β‐ and γ‐chitin forms can be irreversibly converted into the α‐form 15. Some characteristics of β‐chitin, such as high affinity for solvents and high reactivity, are attributable to its loose crystalline structure. Chitosan is also crystalline, but the structure is different from that of either α‐ or β‐chitin, as evidenced by the X‐ray diffraction patterns. The crystal structures have been refined for the anhydrous and hydrated forms 16, 17.
Sources and processing
Chitin is one of the most abundant nitrogenous polysaccharides found in nature. It is a tough, semi‐transparent, horny substance found in crustaceans, such as crabs, lobsters and shrimp. It is also found in clams, oysters, krill and squid. It is the principal component of the exoskeletons of arthropods and the cell walls of certain fungi. The shells of marine crustaceans, such as crabs and shrimps, available as waste from the seafood processing industry are used for the commercial production of chitin 18, 19. The shells contain 15–40% chitin, with proteins (20–40%) and calcium carbonate (20–50%) as two other major components. Other possible sources of chitin production include krill, clams, oysters, insects and fungi 20. The nitrogen content in chitin varies from 5% to 8% depending on the extent of deacetylation, whereas the nitrogen in chitosan is mostly in the form of primary aliphatic amino groups. Processing of chitin is primarily from two sources: crab or shrimp shells and fungal mycelia. In the first case, chitin production is associated with food industries such as shrimp canning. In the second case, the production of chitosan–glucan complexes is associated with fermentation processes. The processing of crustacean shells mainly involves the removal of proteins and the dissolution of calcium carbonate that is present in crab shells in high concentrations. The resulting chitin is deacetylated in 40% sodium hydroxide at 120°C for 1–3 hours. This treatment produces 70% deacetylated chitosan, and complete deacetylation can be obtained by repeating the step. Significant efforts have also been devoted for commercialising chitosan extracted from fungal and insect sources to completely replace crustacean‐derived chitosan 21. The processing of the fungal wastes from Aspergillus niger, Mucor rouxii and Streptomyces consists of an alkali treatment that yields chitosan–glucan complexes. The alkali removes the protein and deacetylates chitin simultaneously. Depending on the alkali concentration, some alkali soluble glucans are also removed 22.
Chemical properties
Most of the naturally occurring polysaccharides like cellulose, dextran, pectin, alginic acid, agar, agarose and carrageenan are neutral or acidic in nature. Chitin and chitosan are highly basic polysaccharides. Their unique properties include polyoxysalt formation, ability to form films, chelate metal ions and optical structural characteristics 23. Like cellulose, chitin naturally functions as a structural polysaccharide but differs from cellulose in the properties. Chitosan is the universally accepted non‐toxic N‐deacetylated product of chitin. A sharp nomenclature border has not been defined between chitin and chitosan based on the degree of N‐deacetylation 24, 25. Chitin is highly hydrophobic and is insoluble in water and common organic solvents. Its highly crystalline structure accounts for its poor solubility. The major solvent that dissolves chitin is lithium chloride‐tertiary amides solvent system 26. The most frequent solvents used to make a 5–7% (w/v) lithium chloride solution are N,N‐Dimethylacetamide, N,N‐Dimethylpropionamide, N‐Methyl‐2‐pyrrolidinone and 1,3‐Dimethyl‐2‐imidazolidinone. It is also soluble in hexafluoroisopropanol, hexafluoroacetone 27 and chloroalcohols in conjunction with aqueous solutions of mineral acids 23. It is also possible to dissolve chitin in a narrow range of carboxylic and sulfonic acids. Unlike α‐chitin, β‐chitin is soluble in formic acid and swells in water considerably. Chitosan, the deacetylated product of chitin, is insoluble in organic solvents and water. It is soluble in aqueous acids owing to the presence of free amino groups. Some organic acids, such as formic, acetic, lactic, pyruvic and oxalic acids, are frequently used for dissolution. Mineral acids such as hydrochloric and nitric acids also form chitosan solutions, but phosphoric and sulphuric acids are not suitable.
Biological properties
Chitin and chitosan exhibit a set of unique biological properties. The major attractions are biocompatibility and acceptable biodegradation products by virtue of biopolymer origin 28, 29. Enzymatic cleavage by lysozyme results in the formation of N‐acetylglucosamine and glucosamine. Chitin and chitosan possess excellent antimicrobial properties 30, 31. Chitin products are non‐toxic and non‐allergic 32. Chitin and its deacetylated form, chitosan, also exhibit a haemostatic effect 33, 34, 35. It has been shown that poly‐N‐acetyl glucosamine produces rapid haemostasis by stimulating erythrocyte aggregation. For chitin, the haemostatic effect is believed to be due to vasoconstriction and the mobilisation of erythrocytes, clotting factors and platelets to the site of the injury 36. Chitosan is a polycation and has agglutinating and binding abilities. Chitin and chitosan also promote wound healing 37, 38, 39 and can be fabricated as gels, films, fibres, beads, support matrices and in blends as well. A number of studies have demonstrated that chitin and its derivatives have antibacterial and painkilling properties, induce faster wound healing and stimulate the reconstruction of connective tissue 40, 41, 42, 43.
Biomedical applications of chitin and chitosan
Chitin is a unique biopolymer for versatile applications. Many reviews and articles have been published covering the applications of chitin and its derivatives in the biomedical field 44, 45, 46, 47, 48, 49, 50, 51, 52. Films, beads, intragastric floating tablets, microspheres and nanoparticles of chitin and its derivatives have been formulated for use in pharmaceutical field 53, 54, 55, 56, 57. The combination of chitin's remarkable properties make it extremely versatile. A wide variety of biomedical applications for chitin and chitin derivatives have been reported, including tissue engineering, orthopaedic and periodontal applications, drug‐delivery systems and wound‐healing applications.
Tissue engineering applications
Biodegradable polymeric scaffolds are widely used as a temporary extracellular matrix in tissue engineering. Chitin and chitosan are promising candidate as supporting material for tissue engineering applications owing to their porous structure, gel‐forming properties, ease of chemical modification and high affinity to in vivo macromolecules 58, 59, 60. There are a number of reports on the use of chitin and chitosan as scaffold material in tissue engineering. Chow et al. 61 made a series of porous chitin matrixes by producing chitin gels from chitin solutions followed by lyophilisation to give porous chitin matrixes. Mouse and human fibroblast cell cultures exposed to these chitin matrixes were found to be growing and proliferating, indicating the feasibility of using these porous chitin matrixes for cell transplantation applications to regenerate tissues. Chitosan can be easily processed into porous scaffolds, films and beads for tissue engineering 62. Kast et al. 63 showed that chitosan–thioglycolic acid (Chitosan–TGA) conjugate is a promising candidate as scaffolding material in tissue engineering. Synthesis of porous chitosan scaffolds for tissue engineering have also been reported by Madihally and Matthew 64. Three‐dimensional hydroxyapatite/chitosan gelatin network scaffolds have also been fabricated 65. The scaffold was demonstrated to be suitable for bone tissue engineering applications using osteoblast cells. Macroporous chitosan calcium phosphate composite scaffolds have been synthesised and characterised for tissue engineering by Zhang and Zhang 66. They showed that the role of chitosan was to provide a scaffold form; on the other hand, calcium phosphates bioactivity presumably encourages osteoblast attachment and strengthens the scaffold. The composite scaffold was found to be stronger, bioactive and biodegradable. Wang et al. 67 have developed a novel thermally induced phase separation method to prepare polyglycolic acid PGA‐chitosan hybrid matrices using solvents of low toxicity. The success of seeding cells in this matrix demonstrated the potential of the matrix as new biomaterial for tissue engineering. Chung et al. 68 prepared chitosan‐based scaffolds by combining with alginate. Chitosan was modified with lactobionoic acid to produce galactosylated chitosan, which was added to cross‐linked alginate gel. Hepatocytes attachment to the alginate‐galactosylated chitosan scaffolds was demonstrated. Feng et al. 69 have also fabricated galactosylated chitosan nanofibrous scaffold by electrospinning using formic acid solvent for tissue engineering applications. Injectable chitosan‐based hydrogels have been found promising for cartilage tissue engineering 70, 71. Duarte et al. 72 have reported the preparation of chitosan scaffolds loaded with dexamethasone for the preparation of a drug‐delivery system for bone tissue engineering purposes. In summary, studies reporting the application of chitin and chitosan alone or in combination with other polymers have demonstrated the significant promise of these biopolymers to tissue engineering.
Orthopaedic and periodontal applications
Chitin's role in the exoskeleton of crustaceans is analogous to that of collagen in bone, being the fibrous reinforcement for the support of inorganic calcium mineral. Therefore, chitin possesses the inherent physical and biological characteristics that may render it useful as a component in hard tissue replacement material. A number of studies have shown the strong possibilities of using chitin in orthopaedics as an agent that helps bones to heal. Composites based on polymer matrix–calcium‐based compounds systems have potential use as hard tissue substitute materials. The advantage of such composites is the enhancement of the osteogenic potential accorded by the calcium compounds and the binder characteristic of the polymer matrix in inhibiting migration of the calcium compounds. Hydroxyapatite or other calcium‐containing materials incorporated into chitin or chitosan have been investigated for orthopaedic or bone substitution and periodontal applications 73, 74, 75. The combination of polymer with hydroxyapatite has been shown in vivo to maximise the osteo‐conductive behaviour of hydroxyapatite, allowing bony ingrowths into the implant to occur as the matrix is progressively resorbed. Wan et al. 76 combined chitin with hydroxyapatite to produce a hydroxyapatite–chitin composite material by dispersing hydroxyapatite in chitin to give an intimately blended material. Plastic properties of the polymer favourable for bone substitute applications were retained. Wan et al. 77 investigated the induction of in situ calcification by chitin. It was theorised that if chitin was chemically modified with anionic carboxymethyl groups, a chitin matrix with surface acidic carboxymethyl residues that would become calcium ions attractor sites could be prepared. Porous chitin matrixes have also been prepared 78 by freeze‐drying chitin gels followed by chemically modifying their surfaces with carboxymethyl and phosphoryl (P‐chitin) ionic groups. P‐chitosan, in combination with monocalcium phosphate and calcium oxide, was found suitable as a phosphate cement to fill bone cavities 79. Histological studies in a rabbit model suggested biocompatibility, bio‐absorbability and osteoinductivity of P‐chitosan–calcium phosphate cement 80. Several studies have focused on the use of chitosan as a component in calcium‐based cements in the development of bone substitutes. Yokoyama et al. 81 used chitosan as a component of the liquid phase, which includes citric acid and glucose in combination with α‐tricalcium phosphate and tetra‐calcium phosphate to produce an easily mouldable cement. Sunny et al. 82 have reported the preparation of hydroxyapatite–chitosan microspheres as potential bone and periodontal filling materials. A chitosan sponge has also been reported to release a growth factor suitable for periodontal bone regeneration 83. Chitosan, both as a carrier in gel form and as an active agent, has been suggested for the treatment of chronic periodontitis 84. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects demonstrated that chitosan gel alone or its combination with demineralised bone matrix/collagenous membrane is promising for periodontal regeneration 85. Chen et al. 86 studied the effects of chitosan‐coated pressed calcium sulphate pellets combined with recombinant human bone morphogenetic protein‐2 in a rabbit femoral condyle‐contained bone defect model. The results indicated that chitosan‐coated pressed calcium sulphate pellets exhibited relatively slower resorption, which closely coincides with the growth rate of new bone and enhanced osteogenesis.
Drug‐delivery applications
Chitin and chitosan have also received increasing attention for a wide range of drug‐delivery applications 87, 88, 89, 90. They have been used as a carrier for various active agents, including drugs and biologics. Chitin and chitosan incorporated as a component of interpenetrating networks (IPN) has been one of the approaches. Lee et al. 91 reported the synthesis of poly(ethylene glycol) macromer and β‐chitosan that was subsequently cross‐linked with a UV‐active agent to give semi‐IPN hydrogels. Drug‐containing poly(ethylene glycol)/chitosan microspheres with glutaraldehyde as the cross‐linking agent have also been reported 92. A novel inorganic–organic pH‐sensitive membrane based on an IPN utilising inorganic silicate and organic chitosan has been proposed for drug delivery 93. Light‐initiated polymerisation of acrylic acid in the presence of chitosan was used to derive a mucoadhesive membrane suitable for transmucosal drug‐delivery applications 94. Chitosan–xanthan microspheres that have potential as drug‐delivery systems in simulated gastric and intestinal fluids has been investigated 95, 96. There has also been continued interest in N‐succinyl‐chitosan as a possible drug carrier 97. The in vivo biodisposition was studied using mice to assess the bioaccumulation at the tumour site as well as elimination of the chitosan derivative. The loading of a β‐ emitter onto a chitosan hydrogel that was, in turn, coated onto a PET balloon surface has been described 98. Chitosan gels have also been investigated and are shown to be a potentially good delivery system to the buccal mucosa for protein drugs 99. Chenite et al. 100 have developed novel injectable solutions comprising a combination of chitosan with glycerol‐phosphate useful for drug delivery. Zhao et al. 101 have reported chitosan–gelatin drug‐containing films incorporating herbal extract for delivery in the abdominal cavity. The system has potential for drug delivery to treat anastomosis and muscle/ tissue healing by inserting the membrane at the abdominal cavity by surgical procedure. Chitosan microspheres and nanospheres have also been formulated for drug delivery 102, 103. The use of chitosan to produce designed nanocarriers and to enable microencapsulation techniques is under increasing investigation for the delivery of drugs, biologics and vaccines 89. Spray drying as a method of preparing chitosan microcores containing vitamin D2 has also been reported 104. The preparation of chitosan‐based nanospheres has also been performed by utilising the in situ polymerisation of acrylic acid in the presence of chitosan 105. An implant controlled‐release system for protein drug delivery based on a polyion complex device composed of chitosan and sodium hyaluronate has been reported by Surini et al. 106. Bernardo et al. 107 reported a bupivacaine‐loaded chitosan system as a drug release device with an analgesic action. Chitosan hydrogel has also been proposed as a promising carrier for drugs such as fibroblast growth factor‐2 and paclitaxel to control vascularisation 108. It has also been investigated as a suitable polymer coating for oral delivery of proteins/peptide drugs 109, 110, 111, 112 as well as immobilisation/delivery of living cells 113, 114. The use of polymeric nanoparticles composed of natural polymers is also an interesting approach in drug delivery, mainly because of the advantages offered by their small dimensions. Chitosan–carrageenan nanoparticles have shown promising properties that allow them to be used as carriers of therapeutic macromolecules, with potential application in drug delivery 115. Hari and Kumpati 90 have reported chitosan‐reduced gold nanospheres as biocompatible, non‐toxic nanoconjugates for targeted drug delivery.
Other biomedical applications
Fibres made of chitin and chitosan are useful as absorbable sutures. These chitin sutures resist attack in bile, urine and pancreatic juice, which other absorbable sutures find difficult. Other medical uses reported for chitin and chitosan are antibacterial sponges and hospital dressings, dental plaque inhibition, artificial blood vessels, inhibition of tumour cells and reduction of blood cholesterol level 116. Deacetylated chitin, or chitosan, has been shown to aggressively bind to a variety of mammalian and microbial cells. This property of chitosan may lead to a variety of biomedical applications. These possible applications will use chitosan as a haemostatic, bacteriostatic and spermicidal agent. Chitosan has also been used as a substitute for the synthetic polymers in opthalmological applications. Chitosan possess the ideal characteristics, such as optical clarity, mechanical stability, sufficient optical correction, gas permeability, wettability and immunological compatibility, required for an ideal contact lens. Antimicrobial and wound‐healing properties of chitosan, along with excellent film‐forming capability, make chitosan suitable for the development of ocular bandage lens 117. Chitosan can also be used in dental specialities due to its antibacterial and regenerative‐inducing properties as well as high biocompetency 118. The cationic nature of chitosan allows it to complex with DNA molecules, making it an ideal candidate for gene delivery strategies. The ability to manipulate and reconstitute tissue structure and function using this material has tremendous clinical implications and is likely to play a key role in cell and gene therapies 119. Chitosan oligosaccharides are also a promising candidate for anti‐tumour functional food or pharmaceutical adjuvant in oncotherapy, especially for patients after surgical resection 120.
Chitin and chitosan have many distinctive bioactive properties and provide a diverse spectrum of uses in the biomedical arena. The wound‐healing property of chitin and its derivatives is of immense biomedical and therapeutic significance. Properties and application of chitin and chitosan for wound healing are discussed below.
Wound‐healing properties of chitin and chitosan
Chitin and chitosan have an accelerating effect on the wound‐healing process. A number of studies have demonstrated that chitin and chitosan accelerated wound healing in clinical and veterinary cases, and remedies using chitin and chitosan for treatment of wounds are being marketed. Chitin and chitosan have been used as filaments, powders, granules, sponges or as a composite with cotton or polyester. Non‐woven chitin fabrics and chitin threads are used as artificial skins and sutures, with the advantages of wound healing, biocompatibility and biodegradability. The main biochemical activities of chitin‐ and chitosan‐based materials in wound healing are polymorphonuclear cell activation, fibroblast activation, cytokine production, giant cell migration and stimulation of type IV collagen synthesis 121. Chitin and chitosan evidently promote wound healing and enable high‐quality cosmetic restoration 122. The increasing interest in chitin and its deacetylated forms is due to the biological activity resulting from its susceptibility to degradation under the influence of enzymes present in body fluids, such as lysozyme and N‐acetylglucosoaminidase. The degradation products, called chito‐oligomers, are able to stimulate macrophages and positively influence collagen deposition, thus accelerating the wound‐healing process 123. Chitin's monomeric unit, N‐acetylglucosamine, occurs in hyaluronic acid, an extracellular molecule that is important in wound repair. Therefore, chitin possesses the characteristics favourable for promoting rapid dermal regeneration and accelerated wound healing. Chitin and chitosan will gradually depolymerise to release N‐acetyl‐β‐D‐glucosamine, which initiates fibroblast proliferation, helps in ordered collagen deposition and stimulates an increased level of natural hyaluronic acid synthesis at the wound site. It helps in faster wound healing and scar prevention. Furthermore, it also possesses other biological activities and affects macrophage function that helps in faster wound healing 124. It also has an aptitude to stimulate cell proliferation and histoarchitectural tissue organisation 125. A number of studies have confirmed that chitin promotes the ordered healing of tissues, activates macrophages and works as a bacteriostatic, anti‐stereoporotic and immunoadjuvant agent 53, 126. Chitin and chitosan have a beneficial influence on the various phases of wound healing, such as fibroplasia, collagen synthesis and contraction, resulting in faster healing. Enhanced healing of wounds by chitosan film has been attributed to its stimulating activity and/or its capacity to stimulate fibroblast proliferation, resulting in the progression of wound healing 127. It was reported that chitosan stimulated the migration of polymorphonuclear as well as mononuclear cells and accelerated reepithelialisation and normal skin regeneration 128. Ishihara et al. 129 have demonstrated that the chitosan hydrogel in the presence of foetal bovine serum has a chemo‐attractive ability and stimulates the migration and proliferation of dermal fibroblast cells. It is possible that the positively charged chitosan molecules adsorb some substances involved in cell proliferation and migration, such as growth factors and cytokines, from blood plasma or exudates in the wound and that the adsorbed substances stimulate cell proliferation and migration. Fibroblast growth factor‐2 or heparin‐binding epidermal growth factor containing chitosan hydrogel also showed significant inductions of fibroblast cell migration and proliferation. In fact, chitosan molecules have been reported to induce the migration of fibroblasts and inflammatory cells, which are activated to produce multiple cytokines and accelerate wound healing 128, 130, 131, 132. Furthermore, it has been observed that chitosan in wound healing in animal studies stimulated not only the migration and proliferation of fibroblasts 43, 133, 134 but also the production of collagen 134. It has also been reported that fibroblasts stimulated by chitosan molecules secrete interleukin‐8, IL‐8 133. IL‐8 is known to be angiogenic and chemo‐attractive to both endothelial and epidermal cells 135. Fibroblasts adhering to a chitosan hydrogel may secrete IL‐8 and other cytokines, which in turn could induce angiogenesis, fibrosis and epithelialisation.
Chitin and chitosan wound dressings
Chitin wound dressings
Chitin has been shown to be useful as a wound dressing material. Extensive research being conducted is aimed at the wider use of chitin in wound dressings. Treatment with chitin demonstrated a substantial decrease in treatment time, with minimum scar formation in various animals 38. Cho et al. 136 reported water‐soluble chitin, prepared by controlling the degree of acetylation and molecular weight of chitin through alkaline and ultrasonic treatment, as a wound‐healing accelerator. Chitin beads with fluid‐absorbing properties useful for the absorption of wound exudates have potential as a component of wound dressings. Chitin beads were prepared using a non‐solvent coagulant, ethanol, which was subsequently activated to introduce a carboxymethylated surface layer to the chitin beads 40. Hirano et al. 137 reported the preparation of novel chitin–acid glycosaminoglycan fibres that released a portion of the glycosaminoglycans in animal body fluids for use as novel biocompatible dressing materials in the veterinary and clinical fields. Chitin fibres containing 5–33% glycosaminoglycans was prepared using sodium N‐acetylchitosan salt (alkaline chitin) mixed with sodium hyaluronate, sodium heparin, sodium chondroitin 4‐sulfate, sodium chondroitin 6‐sulfate or sodium dermatan sulphate. Dung et al. 138 prepared chitin membranes, named Vinachitin, by decrystallising ricefield crab shells. A clinical study of 300 patients demonstrated wound‐healing properties of chitin for deep burns, orthopaedic, trauma and ulcer conditions. Kifune et al. 139 patented a wound dressing composed of a non‐woven fabric of chitin fibres. The dressing, which was compatible with the living body and had a good adherence property to the surface of wounds, was found suitable for the protection of skin defect wounds. Chitin artificial skin, Beschitin W, has been proven to be beneficial in clinical practice 140. Ohshima et al. 141 evaluated chitin non‐woven fabric for dressing of donor sites, skin graft areas, raw areas under pedicle flaps, skin abrasions, burns and skin ulcers in 91 patients. Chitin dressing showed excellent results with regards to satisfactory pain relief, adherence to the wound and drying without dissolution or other adverse effects. Pielka et al. 142 have demonstrated textile dressings containing dibutyrylchitin or regenerated chitin as valuable dressing materials that accelerate wound healing. Dibutyrylchitin or regenerated chitin caused no cytotoxic effects or primary irritation and had a positive influence on the wound‐healing process. Jang et al. 143 have studied the effect of electrospun non‐woven mats of dibutryl chitin/poly(lactic acid) blends on wound healing in hairless mice. The results demonstrated that dibutryl chitin efficiently accelerated the proliferation of keratinocytes. Acrylic‐grafted chitin with hydrogel characteristic has also been prepared for wound dressing application 144. The water sorption ability and the cytocompatibility suggested chitin poly (acrylic acid) film as a potential wound dressing. Singh et al. 145 developed and characterised chitin membranes for wound dressing application. The antimicrobial efficacy, fluid‐handling properties and dermal irritancy of the chitin membranes were investigated. The chitin dressing provided an effective barrier to microbial penetration and exerted a broad bacteriostatic action against Gram‐positive and Gram‐negative organisms. The results suggested chitin membranes as a promising wound dressing material with optimal performance characteristics. Singh and Singh 146 have also reported papain‐incorporated chitin dressings sterilised by gamma radiation. Papain as a proteolytic enzyme was immobilised using biopolymer chitin for wound debridement application. The effects of a chitin nanofibril‐based gel on the rate and quality of wound healing was investigated in a clinical model 121. Chitin nanofibril‐based gel promoted rapid and physiological healing of different types of wounds and prevented hypertrophic scarring and keloid scarring.
Chitosan wound dressings
Chitosan, the partially deacetylated form of chitin, has been widely studied as a wound dressing material. Healing at split‐skin graft donor sites using chitosan and a conventional dressing was compared. An observation was made that chitosan facilitated rapid wound reepithelialisation and the regeneration of nerves within a vascular dermis. Early returns to normal skin colour at chitosan‐treated areas were demonstrated 147. Mizuno et al. 148 have demonstrated that chitosan facilitates wound repair and that the incorporation of a basic fibroblast growth factor accelerated the rate of healing. Ishihara 42 reported the preparation of a photocrosslinkable chitosan hydrogel through short ultraviolet light irradiation for application to various kinds of wounds. Application of the photocrosslinkable chitosan hydrogel on full‐thickness skin incisions made on the backs of mice significantly induced wound contraction and accelerated wound closure and healing compared with the untreated controls 129, 149, 150. Howling et al. 151 evaluated the effect of chitin and chitosan on fibroblast and keratinocyte proliferation. Chitosan with relatively high degrees of deacetylation strongly stimulated fibroblast proliferation, while samples with lower levels of deacetylation showed less activity. Similarly, Chatelet et al. 152 showed that chitosan films were cytocompatible with keratinocytes and fibroblasts and that lowering the degree of acetylation led to improvements in keratinocyte proliferation. Alemdaroglu et al. 153 investigated burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Better and faster epithelialisation was observed in the epidermal growth factor containing chitosan gel formulation group as compared to silver sulfadiazine or untreated control groups. A modified chitosan membrane, with antibacterial potential over chitosan, was prepared by chemical modification to graft extra amine groups to native chitosan 154. FT‐IR analysis and solubility test confirmed the occurrence of amination process. Using glycerol as a plasticiser improved the surface roughness, water uptake, water vapour permeability and the mechanical properties of aminated chitosan membranes for use as wound dressing. Ong et al. 155 have developed a chitosan‐based wound dressing with potent haemostatic and antimicrobial properties. A composite film consisting of chitosan and chitin nanofibres with improved tensile strength and elasticity has been reported for wound‐healing applications 156. The tensile strength of the chitosan films was increased up to a significant level by incorporating chitin nanofibres without appreciable change in water vapour permeability. Khan and Peh 157 investigated the wound‐healing efficacy of two chitosan films in comparison with a commercial preparation, Omiderm® (Omikron Scientific Ltd., Rehovot, Israel), using punch biopsy wounds in rats. The two chitosan films and Omiderm® films were comparable in terms of transparency, flexibility, adherence property, ease of film removal from wounds without damaging underlying tissues and fluid accumulation. The chitosan films were able to promote wound healing with minimal scar formation. Qin 158 studied chitosan wound dressings with different degrees of acetylation for the absorption capacity and antimicrobial properties, as well as the dry and wet strength. Partially acetylated chitosan wound dressing had a much higher absorption capacity than the original untreated chitosan samples, while there was a reduction in the wet strength and antimicrobial property for the partially acetylated chitosan non‐woven dressing. Biagini et al. 159 developed a chitosan derivative, N‐carboxybutyl chitosan, used in dressing for treating plastic surgery donor sites. The solution of N‐carboxybutyl chitosan was dialysed and freeze‐dried to produce a soft and flexible pad, which was sterilised and applied to the wound. They reported that N‐carboxybutyl chitosan leads to the formation of regularly organised cutaneous tissue and reduces anomalous healing of donor sites in patients undergoing plastic surgery. Compared to control donor sites, better histoarchitectural order, better vascularisation and the absence of inflammatory cells were observed at the dermal level. Chen et al. 160 studied the effect of carboxymethyl chitosan on proliferation and collagen secretion of normal and keloid skin fibroblasts. Carboxymethyl chitosan promoted the proliferation of the normal skin fibroblast significantly but inhibited the proliferation of keloid fibroblast. Ribeiro et al. 161 evaluated the applicability of chitosan hydrogel for the treatment of dermal burns through the induction of full‐thickness transcutaneous dermal wounds in Wistar rats. Macroscopic and histological analysis demonstrated the role of chitosan hydrogel in the re‐establishment of skin architecture.
Chitin/chitosan – polymer composite wound dressings
Chitosan has been surface‐reacted with polyanion aqueous solutions to give polyelectrolyte complexes. Yan et al. 162 prepared chitosan–alginate polyelectrolyte complex (PEC) films and evaluated them as potential wound dressing materials. The PEC films exhibited good in vitro biocompatibility with mouse and human fibroblasts. These water‐insoluble but biodegradable chitosan–alginate polyelectrolyte membranes displayed greater stability to pH changes and were more effective as controlled‐release membranes than either the chitosan or alginate separately 163. The chitosan, in combination with alginate as PEC films, were found to promote accelerated healing of incisional wounds in a rat model compared with conventional gauze dressing. The closure rate and appearance of PEC membrane‐treated wounds were comparable with Opsite1‐treated wounds 164. Mature epidermal architecture with a keratinised surface of normal thickness and a subsided inflammation in the dermis was observed. Chitosan–alginate PECs, prepared in situ in beads and microspheres, cast as films showed good wound dressing capability 163. The potential application of chitosan–cellulose blends as wound dressing is reported 165, 166, 167. As an effective wound dressing agent with antibacterial properties, chitosan–cellulose blend membranes have been prepared. They may protect wounds from excessive dehydration and infection 168. Dressing sponges based on chitosan and chitosan–alginate fibrids with physical (absorption ability) and biological features (cytotoxic and haemostatic properties) suitable for the healing of wounds at different phases have been prepared 169. De Castro et al. 170 have studied the efficacy of modified chitosan sponge dressing in a lethal arterial injury model in swine. Hydrophobically modified chitosan sponge was found to be superior to unmodified chitosan sponges or standard gauze for controlling bleeding. Sparkes and Murray 171 developed a surgical dressing comprising a blend of chitosan and gelatin in a weight ratio of about 3:1–1:3. A claim was made that in contrast to the conventional biological dressings, this experimental dressing displayed excellent adhesion to subcutaneous fat. The dressing was particularly useful for the protection of wounds during the healing process. A series of toxicological evaluations, antibacterial properties and animal wound model studies have testified the safe reliability, antibacterial property and wound‐healing ability of the chitosan–gelatin sponge wound dressing for clinical application 172. Keratin–chitosan composite films, as well as chitosan films, were found to support fibroblast attachment and proliferation, suggesting a role in wound healing 173.
Poly (vinyl alcohol) sponges containing chitooligosaccharide have been found to be very effective as a wound‐healing accelerator in the early stage of wound healing 174. The wound in rats, as investigated by macroscopic examination and measurement of wound area, was almost reepithelialised and granulation tissues in the wound were considerably replaced by fibrosis at 8 days after initial wounding. Semi‐interpenetrating polymer networks composed of chitosan and poloxamer have also been prepared for wound dressing application 175. The wound‐healing efficacy of chitosan/poloxamer semi‐interpenetrating polymer networks as a wound dressing was demonstrated on experimental full‐thickness wounds in a mouse model. Chen et al. 176 have fabricated composite nanofibrous membranes of type I collagen, chitosan and polyethylene oxide by elecrtospinning, which could be further cross‐linked by glutaraldehyde vapour. The membranes showed no cytotoxicity towards growth of 3T3 fibroblasts and had good in vitro biocompatibility. Animal studies demonstrated a better wound‐healing rate with an electrospun matrix compared to gauze and commercial collagen sponge wound dressing. Xu et al. 177 have demonstrated a chitosan–hyaluronic acid hybrid film as an inexpensive wound dressing with good properties for practical applications. Chitosan‐containing polyurethane and N‐isopropylacrylamide thermosensitive membrane has also been evaluated as a wound dressing. The membranes exhibited very low cytotoxicity, could support cell adhesion and growth and had antibacterial ability 178. Kang et al. 179 evaluated the effect of chitosan‐coated poly (vinyl alcohol) nanofibres on open wound healing in mice. The histological examination and mechanical stability revealed the chitosan‐coated poly (vinyl alcohol) nanofibrous matrix to be more effective as a wound‐healing accelerator in the early stages of wound healing than the heat‐treated poly (vinyl alcohol) nanofibrous matrix. Chitosan/polyamine biopolymers have also been used to treat cotton dressings for antimicrobial properties 180. Chitosan and polyvinyl amine had a synergistic bacteriostatic effect on the treated cotton wound dressing. Abdel‐Mohsen et al. 181 fabricated non‐woven microfibre mats as wound dressing using chitin/chitosan–glucan complex isolated from Schizophyllum commune. The biological activity of the non‐woven fabric was tested against different types of bacteria, exhibiting excellent antibacterial activity. Chitin/chitosan–glucan wound dressing also exhibited excellent surgical wound‐healing ability when tested using rat models. Chitosan–gelatin sponge was prepared using a chitosan/ascorbic acid solution blend containing gelatin, followed by cross‐linking with tannin acid and freeze‐drying 182. Chitosan–gelatin sponge loaded with platelet‐rich plasma resulted in accelerated healing of skin wounds. Chitin–chitosan membrane has been designed for clinical duroplasty and exhibited the ability to support cell growth and inhibit microbial growth 183.
Antimicrobial wound dressings
Chitin‐ and chitosan‐based wound dressings incorporating antimicrobial agents have also been developed. β‐chitin was combined with polyethylene glycol to form a partial gel 184. Silver sulfadiazine was then added to the partial gel, with subsequent precipitation in a non‐solvent, producing the combination gel that was finally freeze‐dried to give the dressing. Animal studies carried out to evaluate the wound‐healing effect of β‐chitin‐based semi‐interpenetrating polymer networks confirmed the proliferation of fibroblasts in the wound bed and a distinct reduction in infectious cells. Sponge‐like asymmetric chitosan membrane fabricated by the immersion‐precipitation phase‐inversion method was evaluated as wound covering 185. The membrane showed controlled evaporative loss and excellent oxygen permeability and promoted fluid drainage ability. Histological examination revealed that the epithelialisation rate was increased, and the deposition of collagen in the dermis was well organised by covering the wound with this asymmetric chitosan membrane. The bi‐layer chitosan wound dressing was found suitable for use as a topical delivery method of silver sulfadiazine for the control of wound infections 103. Silver sulfadiazine dissolved from bi‐layer chitosan dressings to release silver and sulfadiazine. The release of sulfadiazine from the bi‐layer chitosan dressing displayed a burst release on the first day and then tapered off to a much slower release. However, the release of silver from the bi‐layer chitosan dressing displayed a slow release profile with a sustained increase of silver concentration. In vivo antibacterial tests confirmed that this wound dressing is effective for long‐term inhibition of the growth of Pseudomonas aeruginosa and Staphylococcus aureus at an infected wound site. Singh and Singh 186 have developed chitin membranes containing silver nanoparticles for wound dressing application. Silver nanoparticles were synthesised by gamma irradiation in the presence of sodium alginate as a stabiliser. The chitin membranes with nanosilver showed promising antimicrobial activity against common wound pathogens. The effect of gamma radiation on the structural and functional characteristics of the chitin–nanosilver membranes was evaluated 187. The antimicrobial efficacy and impermeability of the chitin–nanosilver membranes was not affected by gamma irradiation. Vimala et al. 188 have reported fabrication of porous chitosan films impregnated with silver nanoparticles for wound dressing and antibacterial application. A three‐step process consisting of silver ion–poly (ethylene glycol) matrix preparation, addition of chitosan matrix and removal of poly (ethylene glycol) from the film matrix was used. Madhumathi et al. 189 have also reported the development of chitin/nanosilver composite scaffolds. The antibacterial, blood clotting and cytotoxicity studies suggested that chitin/nanosilver composite scaffolds could be used for wound healing applications. Wound dressing composed of nanosilver and chitosan was evaluated for treatment of deep partial‐thickness wounds, with silver sulfadiazine and chitosan film dressings as controls 190. Compared with the controls, the silver nanocrystalline chitosan dressing significantly increased the rate of wound healing and was associated with silver levels in blood and tissues lower than levels associated with the silver sulfadiazine dressing. The study demonstrated the wide application of silver nanocrystalline chitosan wound dressing in clinical settings. In vitro cytotoxicity of chitosan–silver composite for potential wound management applications has been reported to be within the acceptable range for human use 191.
Loke et al. 192 have developed a non‐adherent wound dressing with sustained antimicrobial capability for treating mustard burn‐induced septic wound injuries. The dressing consists of a carboxymethyl chitin hydrogel and a chitosan acetate foam incorporated with the antimicrobial agent. The bi‐layered dressing was fabricated by combining two separate films, a carboxymethylated chitin hydrogel, which provided the exudate‐absorbing component, pressed together with a chlorhexidine‐loaded chitosan film. Chlorhexidine‐containing chitosan‐based wound dressing displayed antibacterial efficacy towards the primary wound bed bacteria, Pseudomonas aeruginosa and Staphylococcus aureus. When placed on open wounds, this dressing serves the dual function of controlling fluid and heat loss from the wound and acting as prophylaxis against wound sepsis. Because the antimicrobial effects are derived directly from the dressing, frequent changes of the dressing material to re‐apply antimicrobial creams or ointments is minimised, and wound healing is expedited. Muzzarelli et al. 193 prepared formulations including chitin nanofibrils, chitosan glycolate, and chlorhexidine as spray, gel and gauze. The gauze included non‐woven dibutyryl chitin as a biocompatible support and was used to treat 75 patients for a variety of traumatic wounds, with good results in all cases. The spray could be used as a first‐aid tool on bleeding abrasions, and the gel enhanced physiological repair and was recommended for areas within thin epidermal layers. Chitosan wound dressing loaded with minocycline has also been suggested as a useful formulation for the treatment of severe burn wounds 194.
Dressings based on fungal chitin
Fungal mycelia as the source of chitin and their application as a skin substitute for wound healing have also been investigated 195. A weavable skin substitute (Sacchachitin) from the residual fruiting body of Ganoderma tsugaue was developed by first processing the mycelia to remove protein and pigments followed by the isolation of fibres in the 10–50 µm diameter range and final consolidation into a freeze‐dried membrane under aseptic conditions. Su et al. 196 observed that the sacchachitin membrane significantly enhanced the proliferation and migration of fibroblast cells. The in vitro cell culture using rat fibroblast and in vitro immunogenicity evaluations indicated that sacchachitin was a safe biomaterial for use as a wound dressing for skin healing 197. Its promoting action for wound healing might be due to its chemotactic effect for inflammatory cells, which in turn may facilitate subsequent angiogenesis, granulation tissue formation and faster new tissue formation. A research group at the British Textile Technology Group (BTTG) have also investigated the potential application of fungal filaments in wound management 198, 199.
Commercial dressings
Few commercial dressings based on chitin and chitosan are available in the market 169, 200, 201. Chitin dressings in sponge form (Chitopack S®), non‐woven dressing made of chitin‐modified PET (Chitopack P®) and cotton–chitosan non‐woven dressing (Chitopack C®) are commercial wound remedies offered by Eisai Co. in Tokyo, Japan. The Japanese concern Unitika Co. sells a non‐woven dressing made of chitosan fibres 202. Beschitin ®, Unitika (Osaka, Japan), which is a non‐woven fabric manufactured from chitin filaments, is also commercially available in Japan. The dressing Beschitin is recommended for the successful and fast healing of burns, skin abrasions, postoperative wounds, bed sores, ulcers and several other injuries. The American firm 3 M offers a chitosan preparation in gel Tegasorb® (3M Health Care, St. Paul, MN) or hydrocolloid form Tegaderm®, (3M Health Care, St. Paul, Minnesota, USA), designed for the healing of wide internal wounds 202. In 2002, a chitosan dressing HemCon Bandage™ for battlefield use was devised by Tricol Biomedical Inc. (Portland, OR) in a joint effort with the U.S. Army. The HemCon Bandage is a pliable, sterile, chitosan dressing capable of stopping severe external haemorrhage following trauma and penetrating injury. The bandage was the result of a 3‐year collaborative effort to address the leading cause of battlefield mortality bleeding. The HemCon Bandage controls bleeding by becoming extremely sticky when in contact with blood, and this adhesive‐like action seals the wound. The HemCon Bandage is even effective on high‐ pressure, high‐flow arterial bleeds. The HemCon Bandage has been demonstrated to act as an effective barrier between an open wound and dangerous bacteria. It gained importance following the Iraqi war and terrorist threat. After testing at the US Army Institute of Surgical Research, the Brooke Army Medical Center and the Oregon Medical Laser Center, it was included in US Army equipment. HemCon was used by US forces in Iraq and Afghanistan, and an assessment of 64 cases of military use indicates that it has been successful in 97% of instances 203. A study of HemCon application by civilian EMS found that it controlled bleeding in 27 of 34 (79%) cases 204. Another advantage of chitosan is that its polycationic nature at acidic pH (like in HemCon bandages, which include acetic acid) allows it to disrupt the membranes of Gram‐negative bacteria, giving it natural antimicrobial properties 205, 206. Small pieces of HemCon bandage applied to mouse wounds contaminated with bacteria were highly microbicidal and prevented bacterial access to the bloodstream and subsequent death from sepsis 207, 208. One of the most prominent chitin dressings is the Rapid Deployment Hemostat (RDH) Bandage made by Marine Polymer Technologies, USA in collaboration with the office of Naval Research for the treatment of bleeding because of extremity trauma 208. It is intended as both a battlefield and civilian severe trauma wound dressing. RDH applied to splenic lacerations in swine led to coagulation in 23 seconds, significantly faster than the 173 seconds with fibrin glue 210. Also available is a chitosan‐modified cellulose non‐woven dressing under the trade name Syvek Patch (Marine Polymer Technologies, Danvers, MA) 211. Clo‐Sur PAD (Medtronic‐Scion, Miami, FL), Chito‐Seal (Abbott, Lake Forest, IL), the M‐Patch and Trauma DEX (Medafor, Minneapolis, MN) are the other haemostatic dressings containing chitin and chitosan as bioactive agents.
Conclusion
Chitin and chitosan possess inherent biological characteristics that render them useful as a wound‐healing material. A number of studies have shown strong possibilities of using chitin in wound management. Processing chitin and its derivatives into films and fibres is a promising direction in the practical application of these polymers. Chitin and its derivatives are potential polymers to make dressings for burns, surface wounds and skin graft donor sites to accelerate healing. Accordingly, chitin and its derivatives have received increasing attention for a wide range of wound healing applications. Interest in this abundant biopolymer has increased enormously in recent years. The research activity for chitin is now surprisingly high worldwide in both academia and industry, as evidenced by the rapid increase in the numbers of relevant research papers and patents. Consequently, basic and applied research has made great progress, and it is evident that chitin and chitosan exhibit an unlimited application potential for use in wound care. Studies reported on the application of chitin and chitosan demonstrate significant promise of these biopolymers for wound management.
Acknowledgements
The authors are grateful to Director, Defence Laboratory, Jodhpur for the encouragement and support. The authors declare that there is no conflict of interest.
References
- 1. Roberts G. Chitin Chemistry. London: Macmillan, 1998. [Google Scholar]
- 2. Muzzarelli RAA, Muzzarelli C. Chitin and chitosan hydrogels. In: Phillips GO, Williams PA, editors. Handbook of Hydrocolloids. Cambridge: Woodhead Publishing Ltd, 2009:849–88. [Google Scholar]
- 3. Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm 1998;24:979–93. [DOI] [PubMed] [Google Scholar]
- 4. Paul W, Sharma CP. Chitosan, a drug carrier for the 21st century: a review. STP Pharmaceut Sci 2000;10:5–22. [Google Scholar]
- 5. Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev 2004;104:6017–84. [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz E. Chitosan: a versatile biomaterial. Adv Exp Med Biol 2004;553:59–68. [DOI] [PubMed] [Google Scholar]
- 7. Peter M. Applications and environmental aspects of chitin and chitosan. J Macromol Sci Pure Appl Chem 1995;A32:629–40. [Google Scholar]
- 8. Tharanathan RN, Kittur FS. Chitin‐the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr 2003;43:61–87. [DOI] [PubMed] [Google Scholar]
- 9. Blackwell J, Gardner KH, Kolpak FJ, Minke R, Classey WB. Refinement of cellulose and chitin structures. ACS Symp Ser 1980;141:315–34. [Google Scholar]
- 10. Gardner KH, Blackwell J. Refinement of the structure of β‐chitin. Biopolymers 1975;14:1581–95. [DOI] [PubMed] [Google Scholar]
- 11. Sugiyama J, Boisset C, Hashimoto M, Watanabe T. Molecular directionality of beta‐chitin biosynthesis. J Mol Biol 1999;286:247–55. [DOI] [PubMed] [Google Scholar]
- 12. Atkins E. Conformation in polysaccharides and complex carbohydrates. J Biosci 1985;8:375–87. [Google Scholar]
- 13. Jang MK, Kong BG, Jeong YI, Lee CH, Nah JW. Physicochemical characterization of alpha‐chitin, beta‐chitin, and gamma‐chitin separated from natural resources. J Polym Sci A Polym Chem 2004;42:3423–32. [Google Scholar]
- 14. Kameda T, Miyazawa M, Ono H, Yoshida M. Hydrogen bonding structure and stability of α‐chitin studied by C‐13 solid‐state NMR. Macromol Biosci 2005;5:103–6. [DOI] [PubMed] [Google Scholar]
- 15. Rudall KM, Kenchington W. The chitin system. Biol Rev Camb Philos Soc 1973;48:597–633. [Google Scholar]
- 16. Yui T, Imada K, Okuyama K, Obata Y, Suzuki K, Ogawa K. Molecular and crystal structure of the anhydrous form of chitosan. Macromolecules 1994;27:7601–5. [Google Scholar]
- 17. Okuyama K, Noguchi K, Miyazawa T, Yui T, Ogawa K. Molecular and crystal structure of hydrated chitosan. Macromolecules 1997;30:5849–55. [Google Scholar]
- 18. Al Sagheer FA, Al‐Sughayer MA, Muslim S, Elsabee MZ. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr Polym 2009;77:410–9. [Google Scholar]
- 19. Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 2015;13:1133–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurita K. Chitin and chitosan: functional biopolymers from marine crustaceans. Marine Biotechnol 2006;8:203–26. [DOI] [PubMed] [Google Scholar]
- 21. Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl Biochem Biotechnol 2017;181:1314–37. [DOI] [PubMed] [Google Scholar]
- 22. Muzzarelli RAA, Tanfani F, Scarpini G. Chelating film forming and coagulating ability of the chitosan‐glucan complex from Aspergillus niger industrial wastes. Biotechnol Bioeng 1980;22:885–96. [DOI] [PubMed] [Google Scholar]
- 23. Austin PR, Brine CJ, Castle JE, Zikakis JP. Chitin: new facets of research. Science 1981;212:749–53. [DOI] [PubMed] [Google Scholar]
- 24. Muzzarelli RAA. Chitosan. In: Muzzarelli RAA, editor. Natural chelating polymers. Oxford: Pergamon Press, 1973:144–76. [Google Scholar]
- 25. Zikakis JP. Chitin, Chitosan and Related Enzymes. Orlando: Academic Press, 1984. [Google Scholar]
- 26. Rutherford FA, Austin PR. Marine chitin properties and solvents. In: Muzzarelli RAA, Pariser ER, editors. Proceedings of the First International Conference on Chitin/Chitosan. Cambridge: MIT‐Sea Grant Program, 1978:182–92. [Google Scholar]
- 27. Kurita K. Controlled functionalization of the polysaccharide chitin. Progr Polym Sci 2001;26:1921–71. [Google Scholar]
- 28. Muzzarelli RAA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci 1997;53:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev 2010;62:3–11. [DOI] [PubMed] [Google Scholar]
- 30. No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 2002;74:65–72. [DOI] [PubMed] [Google Scholar]
- 31. Lim SH, Hudson SM. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J Macromol Sci Pol Rev 2003;43:223–69. [Google Scholar]
- 32. Bhaskara Rao S, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res 1997;34:21–8. [DOI] [PubMed] [Google Scholar]
- 33. Abhay SP. Hemostatic wound dressing. Patent 5836970, USA, 1998.
- 34. King K, Neuffer MC, McDivitt J, Rose D, Cloonan CC, Vayer JS. Hemostatic dressings for the first responder: a review. Mil Med 2004;169:716–20. [DOI] [PubMed] [Google Scholar]
- 35. Whang HS, Kirsch W, Zhu YH, Yang CZ, Hudson SM. Hemostatic agents derived from chitin and chitosan. Polym Rev 2005;45:309–23. [Google Scholar]
- 36. Kulling D, Vournakis JN, Woo S, Demcheva MV, Tagge DU, Rios G, Finkielsztein S, Hawes RH. Endoscopic injection of bleeding esophageal varices with a poly‐N‐acetyl glucosamine gel formulation in the canine portal hypertension model. Gastrointest Endosc 1999;49:764–71. [DOI] [PubMed] [Google Scholar]
- 37. Biagini G, Muzzarelli RAA, Giardino R, Castaldini C. Biological materials for wound healing. In: Brine CJ, Sandford PA, Zikakis JP, editors. Advances in Chitin and Chitosan. London: Elsevier Applied Science, 1992:16–24. [Google Scholar]
- 38. Muzzarelli RA, Mattioli‐Belmonte M, Pugnaloni A, Biagini G. Biochemistry, histology and clinical uses of chitins and chitosans in wound healing. EXS 1999;87:251–64. [DOI] [PubMed] [Google Scholar]
- 39. Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev 2001;52:105–15. [DOI] [PubMed] [Google Scholar]
- 40. Yusof NL, Lim LY, Khor E. Preparation and characterization of chitin beads as a wound dressing precursor. J Biomed Mater Res 2001;54:59–68. [DOI] [PubMed] [Google Scholar]
- 41. Zhishen I, Dongfen S, Weiliang X. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res 2001;333:1–6. [DOI] [PubMed] [Google Scholar]
- 42. Ishihara M. Photocrosslinkable chitosan hydrogels as a wound dressing and a biological adhesive. Trends Glycosci Glycotechnol 2002;14:331–41. [Google Scholar]
- 43. Okamoto Y, Kawakami K, Miyatake K, Morimoto M, Shigemasa I, Minami S. Analgesic effects of chitin and chitosan. Carbohydr Polym 2002;49:249–52. [Google Scholar]
- 44. Pariser ER, Lombadi DP. Chitin Source Book: a Guide to Research Literature. New York: Wiley, 1980. [Google Scholar]
- 45. Singh DK, Ray AR. Biomedical applications of chitin, chitosan, and their derivatives. JMS‐Rev Macromol Chem Phys 2000;C40:69–83. [Google Scholar]
- 46. Khor E. Chitin: a biomaterial in waiting. Curr Opin Solid State Mater Sci 2002;6:313–7. [Google Scholar]
- 47. Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials 2003;24:2339–49. [DOI] [PubMed] [Google Scholar]
- 48. Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm 2004;57:19–34. [DOI] [PubMed] [Google Scholar]
- 49. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res 2006;133:185–92. [DOI] [PubMed] [Google Scholar]
- 50. Harish Prashanth KV, Tharanathann RN. Chitin/ chitosan: modifications and their unlimited application potential ‐ an overview. Trends Food Sci Technol 2007;8:117–31. [Google Scholar]
- 51. Cardenas G, Anaya P, von Plessing C, Rojas C, Sepulveda J. Chitosan composite films. Biomedical applications. J Mater Sci Mater Med 2008;19:2397–405. [DOI] [PubMed] [Google Scholar]
- 52. Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Therapeut 2017;170:80–97. [DOI] [PubMed] [Google Scholar]
- 53. Tomihata K, Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997;18:567–75. [DOI] [PubMed] [Google Scholar]
- 54. Mao HQ, Roy K, Troung‐Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan‐DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release 2001;70:399–421. [DOI] [PubMed] [Google Scholar]
- 55. Ma ZS, Yeoh HH, Lim LY. Formulation pH modulates the interaction of insulin with chitosan nanoparticles. J Pharm Sci 2002;91:1396–404. [DOI] [PubMed] [Google Scholar]
- 56. Kim TH, Ihm JE, Choi YJ, Nah JW, Cho CS. Efficient gene delivery by urocanic acid‐modified chitosan. J Control Release 2003;93:389–402. [DOI] [PubMed] [Google Scholar]
- 57. Yoen JS, Jang JS, Cho YW. Biodistribution and anti‐tumor efficacy of doxorubicin loaded glycol‐chitosan nanoaggregates by EPR effect. J Control Release 2003;91:135–45. [DOI] [PubMed] [Google Scholar]
- 58. Malafaya PB, Silva GA, Reis RL. Natural‐origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev 2007;59:207–33. [DOI] [PubMed] [Google Scholar]
- 59. Laurencin CT, Jiang T, Kumbar SG, Nair LS. Biologically active chitosan systems for tissue engineering and regenerative medicine. Curr Top Med Chem 2008;8:354–64. [DOI] [PubMed] [Google Scholar]
- 60. Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 2008;26:1–21. [DOI] [PubMed] [Google Scholar]
- 61. Chow KS, Khor E, Wan ACA. Porous chitin matrices for tissue engineering: Fabrication and in‐vitro cytotoxic assessment. J Polym Res 2001;8:27–35. [Google Scholar]
- 62. Jarry C, Chaput C, Chenite A, Renaud MA, Buschman M, Leroux JC. Effects of steam sterilization on thermogelling chitosan‐based gels. J Biomed Mater Res 2001;58:127–35. [DOI] [PubMed] [Google Scholar]
- 63. Kast CE, Frick W, Losert U, Schnurch AB. Chitosan‐thioglycolic acid conjugate: A new scaffold material for tissue engineering. Int J Pharm 2003;256:183–9. [DOI] [PubMed] [Google Scholar]
- 64. Madihally SV, Matthew HWT. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999;20:1133–42. [DOI] [PubMed] [Google Scholar]
- 65. Zhao F, Yin Y, Lu WW, Leong JC, Zhang W, Zhang J, Zhang M, Yao K. Preparation and histological evaluation of biomimetic three–dimensional hydroxyapatite/ chitosan gelatin network composite scaffolds. Biomaterials 2002;23:3227–34. [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Zhang M. Synthesis and characterization of macroporous chitin calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res 2001;55:304–12. [DOI] [PubMed] [Google Scholar]
- 67. Wang YC, Lin MC, Wang DM, Hsieh HJ. Fabrication of a novel porous PGA‐ chitosan hybrid matrix for tissue engineering. Biomaterials 2003;24:1047–57. [DOI] [PubMed] [Google Scholar]
- 68. Chung TW, Yang J, Akaike T, Cho KY, Nah JW, Kim SI, Cho CS. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002;23:2827–34. [DOI] [PubMed] [Google Scholar]
- 69. Feng ZQ, Chu X, Huang NP, Wang T, Wang Y, Shi X, Ding Y, Gu ZZ. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009;30:2753–63. [DOI] [PubMed] [Google Scholar]
- 70. Jin R, Teixeira LSM, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, Feijen J. Injectable chitosan‐based hydrogels for cartilage tissue engineering. Biomaterials 2009;30:2544–51. [DOI] [PubMed] [Google Scholar]
- 71. Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009;30:2499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duarte ARC, Mano JF, Reis RL. Preparation of chitosan scaffolds loaded with dexamethasone for tissue engineering applications using supercritical fluid technology. Eur Polymer J 2009;45:141–8. [Google Scholar]
- 73. Higashi S, Yamamuro T, Nakamura T, Ikada Y, Hyon SH, Jamshidi K. Polymer‐hydroxyapatite composites for biodegradable bone fillers. Biomaterials 1986;7:183–7. [DOI] [PubMed] [Google Scholar]
- 74. Ito M. In vitro properties of a chitosan‐bonded hydroxyapatite bone‐filling paste. Biomaterials 1991;12:41–5. [DOI] [PubMed] [Google Scholar]
- 75. Yamaguchi I, Tokuchi K, Fukuzaki H, Koyama Y, Takakuda K, Monma H, Tanaka J. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J Biomed Mater Res 2001;55:20–7. [DOI] [PubMed] [Google Scholar]
- 76. Wan ACA, Khor E, Hastings GW. Hydroxyapatite modified chitin as potential hard tissue substitute material. J Biomed Mater Res 1997;38:235–41. [DOI] [PubMed] [Google Scholar]
- 77. Wan ACA, Khor E, Wong JM, Hastings GW. Promotion of calcification on carboxymethylchitin disc. Biomaterials 1996;17:1529–34. [DOI] [PubMed] [Google Scholar]
- 78. Wan ACA, Khor E, Hastings GW. Preparation of a chitin‐apatite composite by in situ precipitation onto porous chitin scaffolds. J Biomed Mater Res 1998;41:541–8. [DOI] [PubMed] [Google Scholar]
- 79. Wang X, Ma J, Wang Y, He B. Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials 2001;22:2247–55. [DOI] [PubMed] [Google Scholar]
- 80. Wang X, Ma J, Wang Y, He B. Bone repair in radii and tibias of rabbits with phosphorylated chitosan reinforced calcium phosphate cements. Biomaterials 2002;23:4167–76. [DOI] [PubMed] [Google Scholar]
- 81. Yokoyama A, Yamamoto S, Kawasaki T, Kohgo T, Nakasu M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials 2002;23:1091–101. [DOI] [PubMed] [Google Scholar]
- 82. Sunny MC, Ramesh P, Varma HK. Microstructured microspheres of hydroxyapatite ceramic. J Mater Sci Mater Med 2002;13:623–32. [DOI] [PubMed] [Google Scholar]
- 83. Park YJ, Lee YM, Park SN, Sheen SY, Chung CP, Lee SJ. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials 2000;21:153–9. [DOI] [PubMed] [Google Scholar]
- 84. Akincibay H, Senel S, Ay ZY. Application of chitosan gel in the treatment of chronic periodontitis. J Biomed Mater Res 2007;80B:290–6. [DOI] [PubMed] [Google Scholar]
- 85. Boynuegri D, Ozcan G, Şenel S, Uç D, Uraz A, Oguş E, Cakilci B, Karaduman B. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: A pilot study. J Biomed Mater Res 2009;90B:461–6. [DOI] [PubMed] [Google Scholar]
- 86. Chen H, Cui X, Yu X, Tian X, Zhang B, Tang P, Wang Y. Effects of chitosan‐coated pressed calcium sulfate pellets combined with recombinant human bone morphogenetic protein 2 on bone formation in femoral condyle‐contained bone defects. J Craniofac Surg 2010;21:188–97. [DOI] [PubMed] [Google Scholar]
- 87. Prabaharan M, Rodriguez‐Perez MA, de Saja JA, Mano JF. Preparation and characterization of poly(L‐lactic acid)‐chitosan hybrid scaffolds with drug release capability. J Biomed Mater Res 2007;81B:427–34. [DOI] [PubMed] [Google Scholar]
- 88. Varshosaz J. The promise of chitosan microspheres in drug delivery systems. Expert Opin Drug Deliv 2007;4:263–73. [DOI] [PubMed] [Google Scholar]
- 89. Elieh‐Ali‐Komi D, Hamblin MR. Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res 2016;4:411–27. [PMC free article] [PubMed] [Google Scholar]
- 90. Hari K, Kumpati P. Chitosan tethered colloidal gold nanospheres for drug delivery applications. J Nanosci Nanotechnol 2016;16:229–41. [DOI] [PubMed] [Google Scholar]
- 91. Lee YM, Kim SS, Kim SH. Synthesis and properties of poly(ethylene glycol) macromer/ β‐chitosan hydrogels. J Mater Sci Mater Med 1997;8:537–41. [DOI] [PubMed] [Google Scholar]
- 92. Gupta KC, Ravi Kumar MNV. pH dependent hydrolysis and drug release behavior of chitosan/ poly(ethylene glycol) polymer network microspheres. J Mater Sci Mater Med 2001;12:753–9. [DOI] [PubMed] [Google Scholar]
- 93. Park SB, You JO, Park HY, Haam SJ, Kim WS. A novel pH‐sensitive membrane from chitosan‐TEOS IPN: preparation and its drug permeation characteristics. Biomaterials 2001;22:323–30. [DOI] [PubMed] [Google Scholar]
- 94. Ahn JS, Choi HK, Cho CS. A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan. Biomaterials 2001;22:923–8. [DOI] [PubMed] [Google Scholar]
- 95. Chellat F, Tabrizian M, Dumitriu S, Chornet E, Magny P, Rivard CH, Yahia L. In vitro and in vivo biocompatibility of chitosan‐xanthan polyionic complex. J Biomed Mater Res 2000;51:107–16. [DOI] [PubMed] [Google Scholar]
- 96. Chellat F, Tabrizian M, Dumitriu S, Chornet E, Rivard CH, Yahia L. Study of biodegradability behavior of chitosan‐xanthan microspheres in simulated physiological media. J Biomed Mater Res 2000;53:592–9. [DOI] [PubMed] [Google Scholar]
- 97. Kato Y, Onishi H, Machida Y. Evaluation of N‐succinyl‐chitosan as a systemic long‐circulating polymer. Biomaterials 2000;21:1579–85. [DOI] [PubMed] [Google Scholar]
- 98. Qu X, Weinberger J. Novel β‐emmiting poly (ethylene terephthalate) surface modification. J Biomed Mater Res 2000;52:492–7. [DOI] [PubMed] [Google Scholar]
- 99. Senel S, Kremer MJ, Kas S, Wertz PW, Hincal AA, Squier CA. Enhancing effect of chitosan on peptide drug delivery across buccal mucosa. Biomaterials 2000;21:2067–71. [DOI] [PubMed] [Google Scholar]
- 100. Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoeman CD, Leroux JC, Atkinson BL, Binette F, Selmani A. Novel injectable neutral solutions of chitosan from biodegradable gels in situ. Biomaterials 2000;21:2155–61. [DOI] [PubMed] [Google Scholar]
- 101. Zhao HR, Wang K, Zhao Y, Pan LQ. Novel sustained‐release implant of herb extract using chitosan. Biomaterials 2002;23:4459–62. [DOI] [PubMed] [Google Scholar]
- 102. Mi FL, Shyu SS, Chen CT, Schoung JY. Porous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: preparation of antigen adsorbed microsphere and in vitro release. Biomaterials 1999;20:1603–12. [DOI] [PubMed] [Google Scholar]
- 103. Mi FL, Tan YC, Liang HF, Sung HW. In vivo biocompatibility and degradability of a novel injectable chitosan‐ based implant. Biomaterials 2002;23:181–91. [DOI] [PubMed] [Google Scholar]
- 104. Shi XY, Tan TW. Preparation of chitosan/ ethylcellulose complex microcapsule and its application in controlled release of Vitamin D2 . Biomaterials 2002;23:4469–73. [DOI] [PubMed] [Google Scholar]
- 105. Hu Y, Jiang X, Ding Y, Ge H, Yuan Y, Yang C. Synthesis and characterization of chitosan‐poly(acrylic acid) nanoparticles. Biomaterials 2002;23:3193–201. [DOI] [PubMed] [Google Scholar]
- 106. Surini S, Akiyama H, Morishita M, Nagai T, Takayama K. Release phenomena of insulin from an implantable device composed of a polyion complex of chitosan and sodium hyaluronate. J Control Release 2003;90:291–301. [DOI] [PubMed] [Google Scholar]
- 107. Bernardo MV, Blanco MD, Sastre RL, Teijon C, Teijon JM. Sustained release of bupivacaine from devices based on chitosan. Il Farmaco 2003;58:1187–91. [DOI] [PubMed] [Google Scholar]
- 108. Ishihara M, Obara K, Nakamura S. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J Artif Organs 2006;9:8–16. [DOI] [PubMed] [Google Scholar]
- 109. McKnight CA, Ku A, Goosen MFA, Sun D, Penny C. Synthesis of chitosan‐alginate microcapsule membrane. J Bioactive Compat Polym 1988;3:335–54. [Google Scholar]
- 110. Polk A, Amsden B, De Yao K, Peng T, Goosen MFA. Controlled release of albumin from chitosan‐alginate microcapsules. J Pharm Sci 1994;83:178–85. [DOI] [PubMed] [Google Scholar]
- 111. Arhewoh IM, Okhamafe AO. An overview of site‐specific delivery of orally administered proteins/ peptides and modelling considerations. J Med Biomed Res 2004;3:7–20. [Google Scholar]
- 112. Arhewoh IM, Ahonkhai EI, Okhamafe AO. Optimising oral systems for the delivery of therapeutic proteins and peptides. Afr J Biotech 2005;4:1591–7. [Google Scholar]
- 113. Takechi H, Yamamoto H, Niwa T, Hinos T, Kawashima Y. Enteral absorption of insulin in rats from mucoadhesive chitosan–coated liposome. Pharm Res 1996;13:896–901. [DOI] [PubMed] [Google Scholar]
- 114. Okhamafe AO, Goosen MFA. Modulation of membrane permeability. In: Mattheus G, editor. Cell Encapsulation Technology and Therapeutics. Boston: Birkhäuser, 1999:53–62. [Google Scholar]
- 115. Grenha A, Gomes ME, Rodrigues M, Santo VE, Mano JF, Neves NM, Reis RL. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J Biomed Mater Res 2010;92A:1265–72. [DOI] [PubMed] [Google Scholar]
- 116. Dunn QL, Sugita ET, Grandmaison EW, Goosen MFA. Applications and properties of chitosan. J Bioactive Compat Polym 1992;7:371–97. [Google Scholar]
- 117. Jiang H, Su W, Caracci S, Bunning TG, Copper T, Adams W. Optical wave guiding and morphology of chitosan thin films. J Appl Polym Sci 1996;61:1163–71. [Google Scholar]
- 118. Wieckiewicz M, Boening KW, Grychowska N, Paradowska‐Stolarz A. Clinical application of chitosan in dental specialities. Mini Rev Med Chem 2017;17:401–9. [DOI] [PubMed] [Google Scholar]
- 119. Di MA, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue‐engineering. Biomaterials 2005;26:5983–90. [DOI] [PubMed] [Google Scholar]
- 120. Zou P, Yang X, Zhang Y, Du P, Yuan S, Yang D, Wang J. Antitumor effects of orally and intraperitoneally administered chitosan oligosaccharides (COSs) on S180‐bearing/residual mouse. J Food Sci 2016;81:H3035–42. [DOI] [PubMed] [Google Scholar]
- 121. Mezzana P. Clinical efficacy of a new chitin nanofibrils‐based gel in wound healing. Acta Chir Plast 2008;50:81–4. [PubMed] [Google Scholar]
- 122. Degim Z, Celebi N, Sayan H, Babul A, Erdogan D, Take G. An investigation on skin wound healing in mice with a taurine‐chitosan gel formulation. Amino Acids 2002;22:187–98. [DOI] [PubMed] [Google Scholar]
- 123. Muzzarelli RAA. Biochemical significance of exogenous chitins and chitosans in animals and patients. Carbohydr Polym 1993;20:7–16. [Google Scholar]
- 124. Balassa LL, Prudden JF. Applications of chitin and chitosan in wound healing acceleration. In: Zikakis JP, editor. Chitin, Chitosan and Related Enzymes. San Diego: Academic Press, 1984:296–305. [Google Scholar]
- 125. Muzzarelli RAA. Amphoteric derivatives of chitosan and their biological significance. In: Skjak‐Braek G, Anthonsen T, Sanford P, editors. Chitin and Chitosan. London: Elsevier Applied Science, 1989:87–99. [Google Scholar]
- 126. Lloyd LL, Kennedy JF, Methacanon P, Paterson M, Knill CJ. Carbohydrate polymers as wound management aids. Carbohydr Polym 1998;37:315–22. [Google Scholar]
- 127. Diegelmann RF, Dunn JD, Lindblad WJ, Cohen IK. Analysis of the effects of chitosan on inflammation, angiogenesis, fibroplasia, and collagen deposition in polyvinyl alcohol sponge implants in rat wounds. Wound Repair Regen 1996;4:48–52. [DOI] [PubMed] [Google Scholar]
- 128. Usami Y, Okamoto Y, Minami S, Matsuhashi A, Kumazawa NH, Tanioka S, Shigemasa Y. Chitin and chitosan induce migration of bovine polymorphonuclear cells. J Vet Med Sci 1994;56:761–2. [DOI] [PubMed] [Google Scholar]
- 129. Ishihara M, Ono K, Sato M, Nakanishi K, Saito Y, Yura H, Matsui T, Hattori H, Kikuchi M, Kurita A. Acceleration of wound contraction and healing with photocrosslinkable chitosan hydrogel. Wound Repair Regen 2001;9:513–21. [DOI] [PubMed] [Google Scholar]
- 130. Nishimura K, Ishihara C, Ukei S, Tokura S, Azuma I. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine 1986;4:151–6. [DOI] [PubMed] [Google Scholar]
- 131. Peluso G, Petillo O, Ranieri M, Santin M, Ambrosic L, Calabro D, Avallone B, Balsamo G. Chitosan‐mediated stimulation of macrophage function. Biomaterials 1994;15:1215–20. [DOI] [PubMed] [Google Scholar]
- 132. Usami Y, Okamoto Y, Minami S, Matsuhashi A, Kumazawa NH, Tanioka S, Shigemasa Y. Migration of canine neutrophils to chitin and chitosan. J Vet Med Sci 1994;56:1215–6. [DOI] [PubMed] [Google Scholar]
- 133. Mori T, Okumura M, Matsuura M, Ueno K, Tokura S, Okamoto Y, Minami S, Fujinaga T. Effect of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials 1997;18:947–51. [DOI] [PubMed] [Google Scholar]
- 134. Ueno H, Yamada H, Tanaka I, Kaba N, Matsuura M, Okumura M, Kadosawa T, Fujinaga T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999;20:1407–14. [DOI] [PubMed] [Google Scholar]
- 135. Koch AE, Polverini PJ, Kunkel SL, Harlow LA, Dipietro LA, Elner VM, Elner SG, Strieter RM. Interleukin‐8 as a macrophage‐derived mediator of angiogenesis. Science 1992;258:1798–801. [DOI] [PubMed] [Google Scholar]
- 136. Cho YW, Cho YN, Chung SH, Yoo G, Ko SW. Water‐soluble chitin as a wound healing accelerator. Biomaterials 1999;20:2139–45. [DOI] [PubMed] [Google Scholar]
- 137. Hirano S, Zhang M, Nakagawa M. Release of glycosaminoglycans in physiological saline and water by wet‐spun chitin‐acid glycosaminoglycan fibers. J Biomed Mater Res 2001;56:556–61. [DOI] [PubMed] [Google Scholar]
- 138. Dung PL, Dong NT, Mai PT, Binh T, Diem CV. Vinachitin, an artificial skin for wound healing. In: Uragami T, Kurita K, Fukamizo T, editors. Chitin and Chitosan in Life Science. Tokyo: Kodansha Scientific Ltd, 2001:27–230. [Google Scholar]
- 139. Kifune K, Yamaguchi Y and Tanae H. Wound dressing. Patent 4651725, USA, 1987.
- 140. Kifune K. Clinical application of chitin artificial skin (Beschitin W). In: Brine CJ, Sanford PA, Zikakis JP, editors. Advances in Chitin and Chitosan. London: Elsevier Applied Science, 1992:9–15. [Google Scholar]
- 141. Ohshima Y, Nishino K, Yonekura Y, Kishimoto S, Wakabayashi S. Clinical application of chitin non‐woven fabric as wound dressing. Eur J Plast Surg 1987;10:66–9. [Google Scholar]
- 142. Pielka S, Paluch D, Staniszewska‐Kus J, Zywicka B, Solski L, Szosland L, Czarny A, Zaczynska E. Wound Healing acceleration by a textile dressing containing dibutyrylchitin and chitin. Fibres Text East Europe 2003;11:79–84. [Google Scholar]
- 143. Jang SI, Moke JY, Jeon IH, Park KH, Nguyen TTT, Park JS, Hwang HM, Song MS, Lee D, Chai KY. Effect of electrospun non‐woven mats of dibutryl chitin/poly (lactic acid) blends on wound healing in hairless mice. Molecules 2012;17:2992–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tanodekaew S, Prasitsilp M, Swasdison S, Thavornyutikarn B, Pothsree T, Pateepasen R. Preparation of acrylic grafted chitin for wound dressing application. Biomaterials 2004;25:1453–60. [DOI] [PubMed] [Google Scholar]
- 145. Singh R, Chacharkar MP, Mathur AK. Chitin membrane for wound dressing application – preparation, characterisation and toxicological evaluation. Int Wound J 2008;5:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Singh D, Singh R. Papain incorporated wound debridement sterilized by gamma radiation. Radiat Phy Chem 2012;81:1781–5. [Google Scholar]
- 147. Minami S, Okamoto Y, Hamada K, Fukumoto Y, Shigemasa Y. Veterinary practice with chitin and chitosan. In: Jolles P, Muzzarelli RAAA, editors. Chitin and Chitinases. Vol. 87. Basel: Birkhauser Verlag, 1999:265–77. [DOI] [PubMed] [Google Scholar]
- 148. Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, Sakurai T, Nimura Y. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res 2003;64:177–81. [DOI] [PubMed] [Google Scholar]
- 149. Stone CA, Wright H, Clarke T, Powell R, Devaraj VS. Healing at skin graft donor sites dressed with chitosan. Br J Plast Surg 2000;53:601–6. [DOI] [PubMed] [Google Scholar]
- 150. Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yura H, Matsui T, Hattori H, Uenoyama M, Kurita A. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002;23:833–40. [DOI] [PubMed] [Google Scholar]
- 151. Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001;22:2959–66. [DOI] [PubMed] [Google Scholar]
- 152. Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001;22:261–8. [DOI] [PubMed] [Google Scholar]
- 153. Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 2006;32:319–27. [DOI] [PubMed] [Google Scholar]
- 154. Eldin MSM, Soliman EA, Hashem AI, Tamer TM. Chitosan modified membranes for wound dressing applications: preparation, characterization and bio‐evaluation. Trends Biomater Artif Organs 2008;22:154–64. [Google Scholar]
- 155. Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan‐based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008;29:4323–32. [DOI] [PubMed] [Google Scholar]
- 156. Shelma R, Paul W, Sharma CP. Chitin nanofibre reinforced thin chitosan films for wound healing application. Trends Biomater Artif Organs 2008;22:107–11. [Google Scholar]
- 157. Khan TA, Peh KK. A preliminary investigation of chitosan film as dressing for punch biopsy wounds rats. J Pharm Pharmaceut Sci 2003;6:20–6. [PubMed] [Google Scholar]
- 158. Qin Y. The preparation and characterization of chitosan wound dressings with different degrees of acetylation. J Appl Polym Sci 2008;107:993–9. [Google Scholar]
- 159. Biagini G, Bertani A, Muzzarelli R, Damadei A, DiBenedetto G, Belligolli A, Riccotti G, Zucchini C, Rizzoli C. Wound management with N‐carboxybutyl chitosan. Biomaterials 1991;12:281–6. [DOI] [PubMed] [Google Scholar]
- 160. Chen XG, Wang Z, Liu WS, Park HJ. The effect of carboxymethyl‐chitosan on proliferation and collagen secretion of normal and keloid skin fibroblasts. Biomaterials 2002;23:4609–14. [DOI] [PubMed] [Google Scholar]
- 161. Ribeiro MP, Espiga A, Silva D, Baptista P, Henriques J, Ferreira C, Silva JC, Borges JP, Pires E, Chaves P, Correia IJ. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen 2009;17:817–24. [DOI] [PubMed] [Google Scholar]
- 162. Yan XL, Khor E, Lim LY. Chitosan‐alginate films prepared with chitosans of different molecular weights. J Biomed Mater Res 2001;58:358–65. [DOI] [PubMed] [Google Scholar]
- 163. Yan XL, Khor E, Lim LY. PEC films prepared from chitosan‐alginate coacervates. Chem Pharm Bull 2000;48:941–6. [DOI] [PubMed] [Google Scholar]
- 164. Wang LS, Khor E, Wee A, Lim LY. Chitosan ‐ alginate PEC membrane as a wound dressing: assessment of incisional wound dressing. J Biomed Mater Res 2002;63:610–8. [DOI] [PubMed] [Google Scholar]
- 165. Tran CD, Duri S, Harkins AL. Recyclable synthesis, characterization and antimicrobial activity of chitosan‐based polysaccharide composite materials. J Biomed Mater Res A 2013;101A:2248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Harkins AL, Duri S, Kloth LC, Tran CD. Chitosan‐cellulose composite for wound dressing material. Part 2. Antimicrobial activity, blood absorption ability, and biocompatibility. J Biomed Mater Res B 2014;102:1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Abdul Khalil HPS, Saurabh CK, Adnan AS, Nurul Fazita MR, Syakir MI, Davoudpour Y, Rafatullah M, Abdullah CK, Haafiz MK, Dungani R. A review on chitosan‐cellulose blends and nanocellulose reinforced chitosan biocomposites: properties and their applications. Carbohydr Polym 2016;150:216–26. [DOI] [PubMed] [Google Scholar]
- 168. Wu YB, Yu SH, Mi CW, Shyu SS, Pong CK, Chao AC. Preparation and characterization of mechanical and antibacterial properties of chitosan/cellulose blends. Carbohydr Polym 2004;57:435–40. [Google Scholar]
- 169. Kucharska M, Niekraszewicz A, Wisniewska‐Wrona M, Brzoza‐Malczewska K. Dressing sponges made of chitosan and chitosan‐alginate fibrids. Fibres Text East Europe 2008;16:109–13. [Google Scholar]
- 170. De Castro GP, Dowling MB, Kilbourne M, Keledjian K, Driscoll IR, Raghavan SR, Hess JR, Scalea TM, Bochicchio GV. Determination of efficacy of novel modified chitosan sponge dressing in a lethal arterial injury model in swine. J Trauma Acute Care Surg 2012;72:899–907. [DOI] [PubMed] [Google Scholar]
- 171. Sparkes BG, Murray DG. Chitosan based wound dressing materials. Patent 4572906, USA, 1986.
- 172. Deng CM, He LZ, Zhao M, Yang D, Liu Y. Biological properties of the chitosan‐gelatin sponge wound dressing. Carbohydr Polym 2007;69:583–9. [Google Scholar]
- 173. Tanabe T, Okitsu N, Tachibana A, Yamauchi K. Preparation and characterization of keratin–chitosan composite film. Biomaterials 2002;23:817–25. [DOI] [PubMed] [Google Scholar]
- 174. You Y, Park WH, Ko BM, Min BM. Effects of PVA sponge containing chitooligosaccharide in the early stage of wound healing. J Mater Sci Mater Med 2004;15:297–301. [DOI] [PubMed] [Google Scholar]
- 175. Kim IY, Yoo MK, Seo JH, Park SS, Na HS, Lee HC, Kim SK, Cho CS. Evaluation of semi‐interpenetrating polymer networks composed of chitosan and poloxamer for wound dressing application. Int J Pharm 2007;341:35–43. [DOI] [PubMed] [Google Scholar]
- 176. Chen JP, Chang GY, Chen JK. Electrospun collagen/chitosan nanofibrous membranes as wound dressing. Colloid Surf Physicochem Eng Aspect 2008;313–4:183–8. [Google Scholar]
- 177. Xu H, Ma L, Shi H, Gao C, Han C. Chitosan–hyaluronic acid hybrid film as a novel wound dressing: in vitro and in vivo studies. Polym Adv Technol 2007;18:869–75. [Google Scholar]
- 178. Yang JM, Yang SJ, Lin HT, Wu TH, Chen HJ. Chitosan containing PU/Poly (NIPAAm) thermosensitive membrane for wound dressing. Mat Sci Eng 2008;28:150–6. [Google Scholar]
- 179. Kang YO, Yoon IS, Lee SY, Kim DD, Lee SJ, Park WH, Hudson SM. Chitosan‐coated poly(vinyl alcohol) nanofibers for wound dressings. J Biomed Mater Res 2010;92:568–76. [DOI] [PubMed] [Google Scholar]
- 180. Fouda MMG, Wittke R, Knittel D, Schollmeyer E. Use of chitosan/polyamine biopolymers based cotton as a model system to prepare antimicrobial wound dressing. Int J Diabet Mellitus 2009;1:61–4. [Google Scholar]
- 181. Abdel‐Mohsen AM, Jancar J, Massoud D, Fohlerova Z, Elhadidy H, Spotz Z, Hebeish A. Novel chitin/chitosan‐glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. Int J Pharm 2016;510:86–99. [DOI] [PubMed] [Google Scholar]
- 182. Lu B, Wang T, Li Z, Dai F, Lv L, Tang F, Yu K, Liu J, Lan G. Healing of skin wounds with a chitosan‐gelatin sponge loaded with tannins and platelet‐rich plasma. Int J Biol Macromol 2016;82:884–91. [DOI] [PubMed] [Google Scholar]
- 183. Pogorielov M, Kravtsova A, Reilly GC, Deineka V, Tetteh G, Kalinkevich O, Pogorielova O, Moskalenko R, Tkach G. Experimental evaluation of new chitin‐chitosan graft for duraplasty. J Mater Sci Mater Med 2017;28:34. DOI: 10.1007/s10856-017-5845-3(online). [DOI] [PubMed] [Google Scholar]
- 184. Lee YM, Kim SS, Park MH, Song KW, Sungm YK, Kang IK. β‐Chitin‐based wound dressing containing silver sulfurdiazine. J Mater Sci Mater Med 2000;11:817–23. [DOI] [PubMed] [Google Scholar]
- 185. Mi FL, Shyu SS, Wu YB, Lee ST, Shyong JY, Ro RNH. Fabrication and characterization of a sponge‐like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001;22:165–73. [DOI] [PubMed] [Google Scholar]
- 186. Singh R, Singh D. Chitin membranes containing silver nanoparticles for wound dressing application. Int Wound J 2014;11:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Singh R, Singh D, Singh A. Effect of gamma radiation on chitin‐nanosilver membranes. Macromol Symp 2015;357:116–23. [Google Scholar]
- 188. Vimala K, Mohan YM, Sivudu KS, Varaprasad K, Ravindra S, Reddy NN, Padma Y, Sreedhar B, Mohana RK. Fabrication of porous chitosan films impregnated with silver nanoparticles: a facile approach for superior antibacterial application. Colloids Surf B Biointerfaces 2010;76:248–58. [DOI] [PubMed] [Google Scholar]
- 189. Madhumathi K, Kumar PTS, Abhilash S, Sreeja V, Tamura H, Manzoor K, Nair SV, Jayakumar R. Development of novel chitin/ nanosilver composite scaffolds for wound dressing applications. J Mater Sci Mater Med 2010;21:807–13. [DOI] [PubMed] [Google Scholar]
- 190. Lu S, Gao W, Gu HY. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns 2008;34:623–8. [DOI] [PubMed] [Google Scholar]
- 191. Sabudin S, Derman MA, Zainol I, Noorsal K. In vitro cytotoxicity and cell seeding studies of a chitosan‐silver composite for potential wound management applications. J Eng Sci 2012;8:29–37. [Google Scholar]
- 192. Loke WK, Lau SK, Yong LL, Khor E, Sum CK. Wound dressing with sustained anti‐microbial capability. J Biomed Mater Res 2000;53:8–17. [DOI] [PubMed] [Google Scholar]
- 193. Muzzarelli RAA, Morganti P, Morganti G, Palombo P, Palombo M, Biagini G, Belmonte MM, Giantomassi F, Orlandi F, Muzzarelli C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr Polym 2007;70:274–84. [Google Scholar]
- 194. Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm 2007;330:138–45. [DOI] [PubMed] [Google Scholar]
- 195. Su CH, Sun CS, Juan SW, Hu CH, Ke WT, Sheu MT. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997;18:1169–74. [DOI] [PubMed] [Google Scholar]
- 196. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials 1999;20:61–8. [DOI] [PubMed] [Google Scholar]
- 197. Hung WS, Fang CL, Su CH, Lai WFT, Chang YC, Tsai YH. Cytotoxicity and immunogenicity of sacchachitin and its mechanism of action on skin wound healing. J Biomed Mater Res 2001;56:93–100. [DOI] [PubMed] [Google Scholar]
- 198. Sagar B, Hamlyn P, Wales D. Wound dressing. Patent 4960413, USA, 1990.
- 199. Hamlyn PF. Fabricating fungi. In: Glasman I, LennoxKerr P, editors. Textile Technology International. London: Sterling Publications Ltd, 1991:254–7. [Google Scholar]
- 200. Niekraszewicz A. Chitosan medical dressings. Fibres Text East Europe 2005;13:16–8. [Google Scholar]
- 201. Kardas I, Struszczyk MH, Kucharska M, van den Broek LAM, van Dam JEG, Ciechańska D. Chitin and chitosan as functional biopolymers for industrial applications. In: Navard P, editor. The European Polysaccharide Network of Excellence. Vienna: Springer, 2013:329–73. [Google Scholar]
- 202. Muzzarelli RAA. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohdr Polym 2009;76:167–82. [Google Scholar]
- 203. Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan‐based hemostatic dressing: experience in current combat operations. J Trauma 2006;60:655–8. [DOI] [PubMed] [Google Scholar]
- 204. Brown MA, Daya MR, Worley JA. Experience with chitosan dressings in a civilian EMS system. J Emerg Med 2009;37:1–7. [DOI] [PubMed] [Google Scholar]
- 205. Tsai GJ, Su WH. Antibacterial activity of shrimp chitosan against Escherichia coli. J Food Prot 1999;62:239–43. [DOI] [PubMed] [Google Scholar]
- 206. Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003;4:1457–65. [DOI] [PubMed] [Google Scholar]
- 207. Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano AP, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006;27:4157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Burkatovskaya M, Castano AP, Rice TND, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen 2008;16:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Vournakis JN, Demcheva M, Whitson AB, Finkielsztein S, Connolly RJ. The RDH bandage: hemostasis and survival in a lethal aortotomy hemorrhage model. J Surg Res 2003;113:1–5. [DOI] [PubMed] [Google Scholar]
- 210. Chan MW, Schwaitzberg SD, Demcheva M, Vournakis SJ, Finkielsztein S, Connolly R. Comparison of poly‐N‐acetylglucosamine (P‐GlcNAc) with absorbable collagen (Actifoam), and fibrin sealant (Bolheal) for achieving hemostasis in a swine model of splenic hemorrhage. J Trauma 2000;48:454–7. [DOI] [PubMed] [Google Scholar]
- 211. Fischer TH, Connolly R, Thatte HS, Schwaitzberg SS. Comparison of structural and hemostatic properties of the poly N‐acetylglucosamine Syvek patch with products containing chitosan. Microsc Res Tech 2004;63:168–74. [DOI] [PubMed] [Google Scholar]


