Abstract
Purpose:
To present a perspective on the use of electrotherapeutics in the history of ophthalmology along with the development of novel contemporary ophthalmic instrumentation.
Design:
Perspective study
Methods:
We reviewed historical journals, articles, and books discussing the use of electricity and electrotherapeutics in ophthalmology.
Results:
Electrotherapeutic applications have been researched and employed to treat ocular diseases as far back as the 18th century. By the 20th century, research in electrotherapeutics in ophthalmology had caught the eye of Edward Jackson, first president of the American Academy of Ophthalmology (AAO) and first editor of the American Journal of Ophthalmology (AJO). Edward Jackson published an extensive review on this topic and reported an attempt to use electricity in treating cataract.
Conclusions:
While many early therapeutic uses of electricity did not produce effective and replicable results, studies on electrical stimulation of the eye provided the foundation for the development of clinically significant vision enhancing and restoring instrumentation.
Keywords: Electricity, Electrotherapy, Electrotherapeutics, Electroceuticals, Ophthalmology
This perspective discusses the uses of electrotherapeutics in the history of ophthalmology with reviews by Edward Jackson and its use in contemporary practices.
Introduction
There has been a fascination with the use of electricity in medical practices for centuries. From the use of electric fishes for treating headaches and gout to the employment of artificial electrical devices for the treatment of muscle spasms and tremors,1–5 electrotherapeutics has continued to play a role in modern medicine. The earliest uses of electricity in ophthalmology date to the 18th century with a proliferation of electrical applications in the late 19th and early 20th centuries.6 Edward Jackson chronicled the use of galvanic and faradic currents commonly used to treat ocular diseases in the early 20th century. He argued the efficacy of using electrotherapeutics to treat ophthalmic diseases including, among others, the use of electrolysis and the electromagnet, both of which are still employed in contemporary ophthalmology.7 Nonetheless, while electrotherapy was at the forefront of medicine in the early 20th century, its use fell out of practice with the development of alternative techniques.
In the last 50 years, there has been a resurgence in the popularity of electrotherapeutic applications, many of which were foreseen by ophthalmologists cited in Jackson’s paper in the early 20th century. Electric stimulation of the cornea, lens, choroid, and retina have proved efficacious for the development of devices such as iontophoresis, transcorneal electrical stimulation (TES), the Argus II™ subretinal implant, and the Orion™ cortical implant.8–11 This review looks at the electrical devices used in ophthalmology historically as well as those being employed or developed in contemporary practice.
Electrotherapy in Medicine
Uses of the Electric Fish
One of the earliest accounts of bioelectricity was shown on the wall of an Egyptian tomb of architect, Ti, dating back to 2500 BC, illustrating a man experiencing a painful shock when he hooked an electrical catfish (Malopterurus electricus) while fishing on the Nile river.4 Ancient Greek philosophers and physicians studied the properties of the electric ray (or torpedo fish) extensively, and Aristotle (384 – 322BC) described the torpedo fish stating that, “it necrotizes the creatures that it wants to catch, overpowering them by shock that is resident in its body and feeds upon them.”4 The Greek philosopher, Theophrastus (371 – 287 BC) also discovered that the electricity from the torpedo can be conducted through the metal trident used to spear it.4
Scribonius Largus (1st century AD), a Roman physician to emperor Claudius, is often credited as the first to use electrotherapeutics in medicine, when he described the use of a torpedo fish to treat headache and gout.1 He lists this remedy in a report that Anteros, a freedman of Tiberius, had been successfully treated for this disease and writes, “For any type of gout, a live black torpedo should, when the pain begins, be placed under the feet. The patient must stand on a moist shore washed by the sea, and he should stay like this until this whole foot and leg up to the knee is numb. This takes away present pain and prevents pain from coming on if it has not already arisen.”1 Thirty years after Largus, the famed Greek pharmacologist and physician Dioscorides (40 – 90 AD) described the use of the torpedo fish to treat a prolapsed anus - a practice that continued for centuries up to the end of the 17th century.1 The use of electrical fishes was also found in the East where Ibn-Sidah, a Muslim physician in the 11th century, believed that live electric catfish can be used to treat patients suffering from epilepsy.1 Then, in the mid-16th century, the invention of the Leyden jar sparked similarities between the shock it delivered and the electrical discharge from the electric fish.
In 1774, Alexander Garden, a Scottish physician, botanist, and zoologist, observed and conducted electrical experiments on “eels” in North America. His observations and preservation methods helped advise John Hunter of the Royal Society of London to provide remarkably detailed descriptions of the electric organs of the eels. John Hunter’s descriptions helped provide evidence for proving fish electricity and ultimately the revolutionary notion that human nerves and muscles function through electrical stimulation and signaling.2 The works of Luigi Galvani and Giovanni Aldini on animal and human corpses proved the theory that there are electrical forces found in living organisms – first termed as “animal electricity.”12
Development of Electrotherapy
By the 18th century, there were three main forms of electricity being employed in medicine, Franklinism, Galvanism, and Faradization. Franklinism, or static electricity, is the use of friction to create an electrical charge, and this charge can be stored into a capacitor such as the Leyden jar. A Leyden jar stores a high-voltage electric charge between electrical conductors on the inside and outside of a glass jar, and its use in medicine was popularized due to its mobility. It was used to treat various diseases such as sciatic nerve pain, heart flutters, tremors of the limb, and aphonia.5,12
In 1791, “Galvanism” was discovered and introduced by Luigi Galvani, an Italian physician and physicist, who worked on electrical stimuli in animal models (Figure 1). Galvanism refers to the use of chemical decomposition to create an electrical current to stimulate muscle contractions. Galvanism is characterized by low intensity, but high quantity electrical currents that produce both chemical and thermic sensations, and its uses included the treatment of muscle spasms and coagulation. However, Galvani’s theory of animal electricity was met with skepticism and criticism and was discredited by Alexandro Volta who had reservations about the idea that there was an inherent electricity found in animals. Animal electricity would not be widely accepted until Galvani’s contemporaries including Giovanni Aldini, Benjamin Franklin, and Emil du Bois-Reymond proved his hypothesis true.12 In 1843, Emil du Bois – Reymond pricked his finger and used a galvanometer to measure the skin currents and potentials at the wound site, thus establishing that there were biological electrical forces in living tissue (Figure 2).13,14
Figure 1.
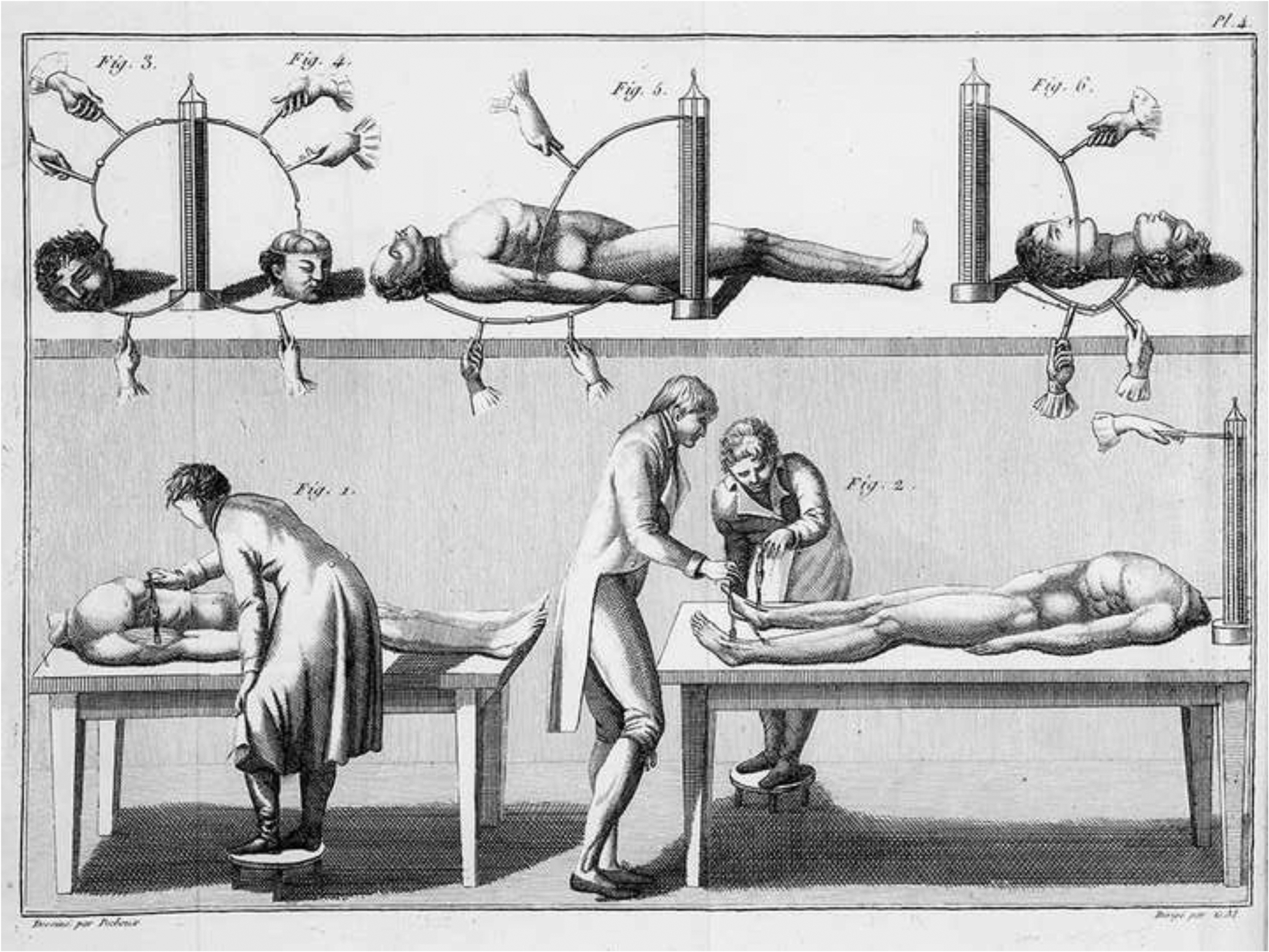
A sketch displaying the use of galvanism to induce muscle contractions.
Figure 2.
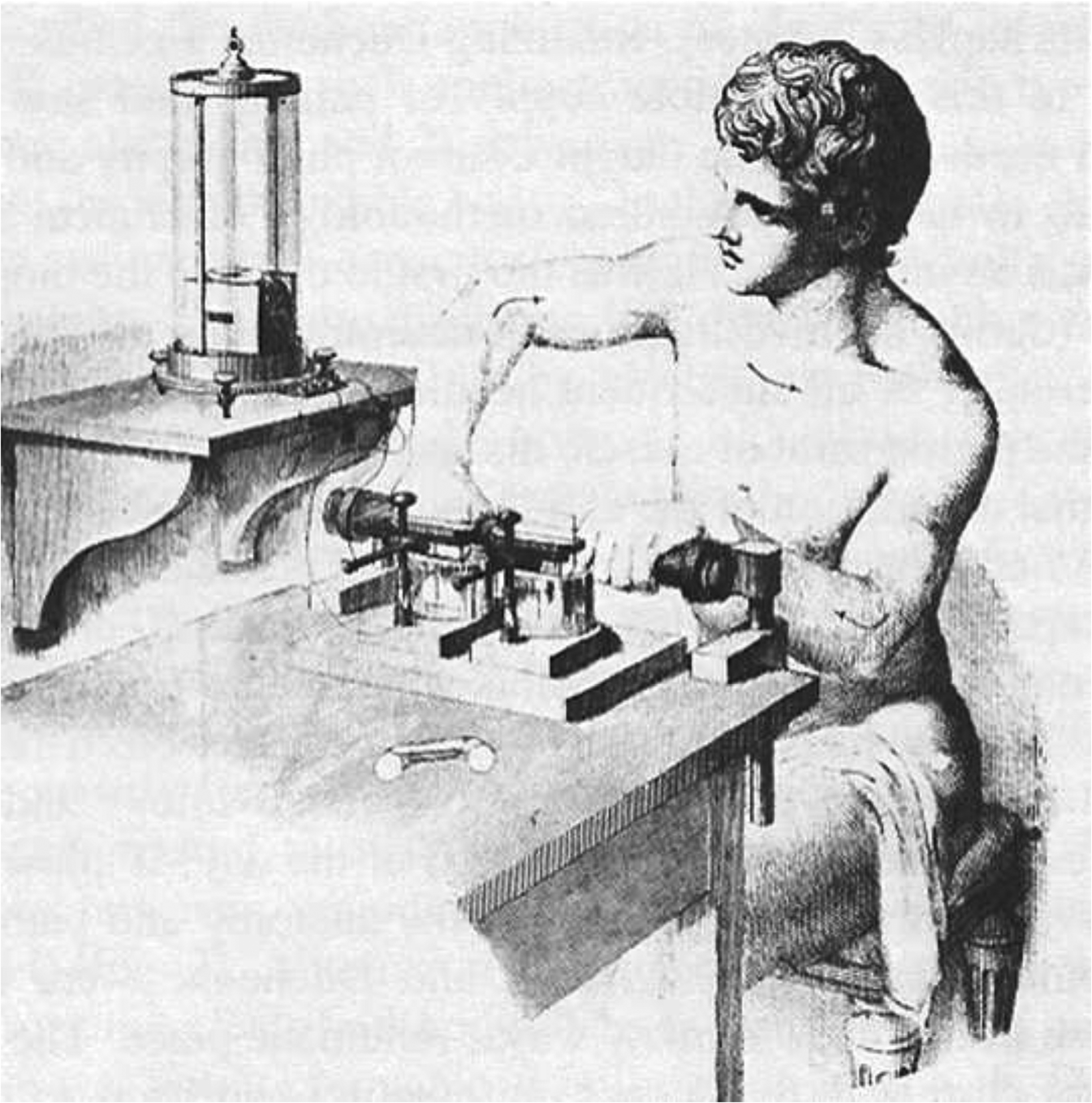
A drawing of Emil Du-Bois Reymond using a galvanometer to measure skin currents and potentials from a wound. The galvanometer used to detect the signal rests on a separate table by his right arm, the one he appears to be tensing. (Courtesy of Dr. Gabriel Finkelstein, University of Colorado, Denver)
In 1831, Michael Faraday, a pioneering British scientist, discovered “faradization”, which is a high-intensity induced alternating current that is localized to stimulate muscle and nerve contractions. The induced current is made momentarily by the making or breaking of a galvanic current or a battery and therefore produces no chemical or thermic sensations as opposed to galvanism. Faradization was used in medicine to treat patients with nerve sensory deficits and various nerve palsies.12
Early Uses of Electricity in Ophthalmology
Cataracts
The application of electricity in ophthalmology paralleled the emergence of electrotherapy in general medicine. In the early 18th century, Benjamin Franklin reported that patients who were struck by lightning developed rapidly progressing cataracts, which ultimately resulted in blindness.6 Despite this report, electric and magnetic therapy was practiced in London for the treatment of patients with cataracts in 1779.6 In 1887, Elliot Colburn, professor of ophthalmology and otology in the Chicago Policlinic, wrote a paper on the use of galvanic currents to treat patients with age – related cortical cataracts. He suggested that patients without choroidal and retinal comorbidities and without systemic complications such as diabetes, nephropathy and cirrhosis should expect to have visual improvement after receiving galvanic treatment. Colburn described placing the negative electrode over the eye and the positive electrode at the nape of the neck for no more than five to ten minutes. He reports using the treatment once daily and then gradually decreasing to once or twice a week to render good results.15 An article co-written by Edward Jackson in 1894 discussed the treatment of cataracts using electricity. The article suggests that while there is no evidence to show that electrical current affect the growth of lenticular opacities, the use of “iodids of soda and potash, sedatives, e.g. bromid of potash, and tonics;… diminish the congestion of the choroid coat…[and] relieve the associated asthenopia and permit the patient reasonable use of his eyes.”16
Edward Jackson
By the 20th century, electrotherapy had become so prominent in medicine that it piqued the interest of Edward Jackson (Figure 3). He wrote an paper on the uses of electricity in diseases of the eye in which he discussed various ocular diseases such as optic atrophy, retinal detachment, trachoma, and trichiasis among many others that were amenable to treatment with electrotherapy.7 Jackson acknowledged the benefits of electrotherapy in treating certain diseases of the eye, despite having some reservations on many other applications. In his paper, he states, “From reading the many articles devoted to this subject…the value of electricity in disease of the eye is clearly and definitely established for a few of its applications; is a possibility worthy of further investigation in a few more; and, with regard to other uses, of which much as been written, is a myth, supported only the hopes or desires of the physician or the patient.”7 Jackson mentions a variety of electrotherapeutics being used to treat ocular diseases:
Figure 3.

Edward Jackson (A) and his article on “Electricity in the Diseases of the Eye” (B)
Galvanization–
Mild galvanic currents were being used to treat patients with retinitis pigmentosa, inflammation, corneal opacities, and asthenopia. H. Derby and Myles Standish reported four cases of retinitis pigmentosa having central and peripheral visual improvement following treatment with mild galvanic currents lasting for five minutes every two to eight days for several months. Jackson furthermore mentions using galvanic currents to reduce of the inflammation of “chronic thickenings of the lids” and the removal of exudates from the clear media. Improvement in corneal opacities following interstitial keratitis and extensive corneal ulceration were also noted, and Beard and Rockwell recommended using mild labile faradization and stable galvanization to treat asthenopia. For the treatment of optic atrophy for which electricity has been most widely tried, Jackson remarked that the general verdict of ophthalmologists is that, “Electricity has failed to vindicate its pretensions to any real value, although, by its capacity for exciting phosphenes, it fosters of the hopes of a credulous incurable.” The idea of activating phosphenes has been used in contemporary ophthalmology for the treatment of diseases such as retinitis pigmentosa.
Galvanocautery
Galvanocautery is the use of galvanic current to heat an instrument – knife or needle. Galvanocautery was preferable for treating suppuration of the cornea and non-healing chronic corneal ulcers. Embedded powder grains in the cornea, conjunctiva, or lids were preferentially removed with galvanocautery. Jackson also cited a study in which five patients with retinal detachments underwent treatment with galvanocautery with improved visual outcomes lasting one to three years in four out of five patients
Faradization
Faradization was used to treat patients suffering from asthenopia – idiopathic pain in the eye and orbit. Ocular muscle paralysis was frequently treated with galvanic currents, but if failed, mild faradic currents were tried.
Electrolysis
Electrolysis the process of using high electrical currents for cauterization with a fine needle, was used to treat patients with trachoma and bacterial infections of the eye, trichiasis, small to medium sized angiomata of the lids or orbit, and lacrimal obstruction. Benjamin Eliasoph from the Pathology Institute of Freiburg University in 1922 published his works on using electrolysis with a fine needle in the anterior chamber to ablate iris cysts.17
The electromagnet
The electromagnet was first designed by Nicolaus Meyer of Minden (Germany) in 1842, and it was designed to remove intraocular metal foreign body objects from the globe.18 Many versions of the electromagnet were created such as the Johnson portal magnet, Hirschberg’s electromagnet, and the Haab magnet. The uses of electrolysis and the electromagnet were developed in the early 20th century and are two common treatments still used in contemporary ophthalmology.
Henri Dor
In 1873, Henri Dor was one of the first to perform experiments using electrical stimulation for the treatment of eye diseases, including retinochoroiditis, glaucoma, amblyopia and optic atrophy. His works on electrical stimulation provided inspiration for the development of transcorneal electrical stimulation (TES).9,10
Iontophoresis
In 1908, Robert Wirtz was the first to use iontophoresis to treat ocular diseases. Iontophoresis is the use of electrical current to drive ionically charged medications into the body. Wirtz developed a “cataphoresis electrode” that increased the surface area of the device on the eye promoting increased penetrance of therapeutic agents to the internal parts of the eye (Figure 4). The device handles were made of celluloid, and the current entered one end of the device while the opposite end was covered with the dissolved medication. Wirtz was successful in treating a serpiginous corneal ulcer with 0.5% zinc sulphate and interstitial keratitis with 1% sodium iodide solution.19,20 In 1922, Benjamin Eliasoph and Ludwig Aschoff used a specially designed contact lens with a collared reservoir for medication to control the delivery of medication into the eye using iontophoresis.17
Figure 4.
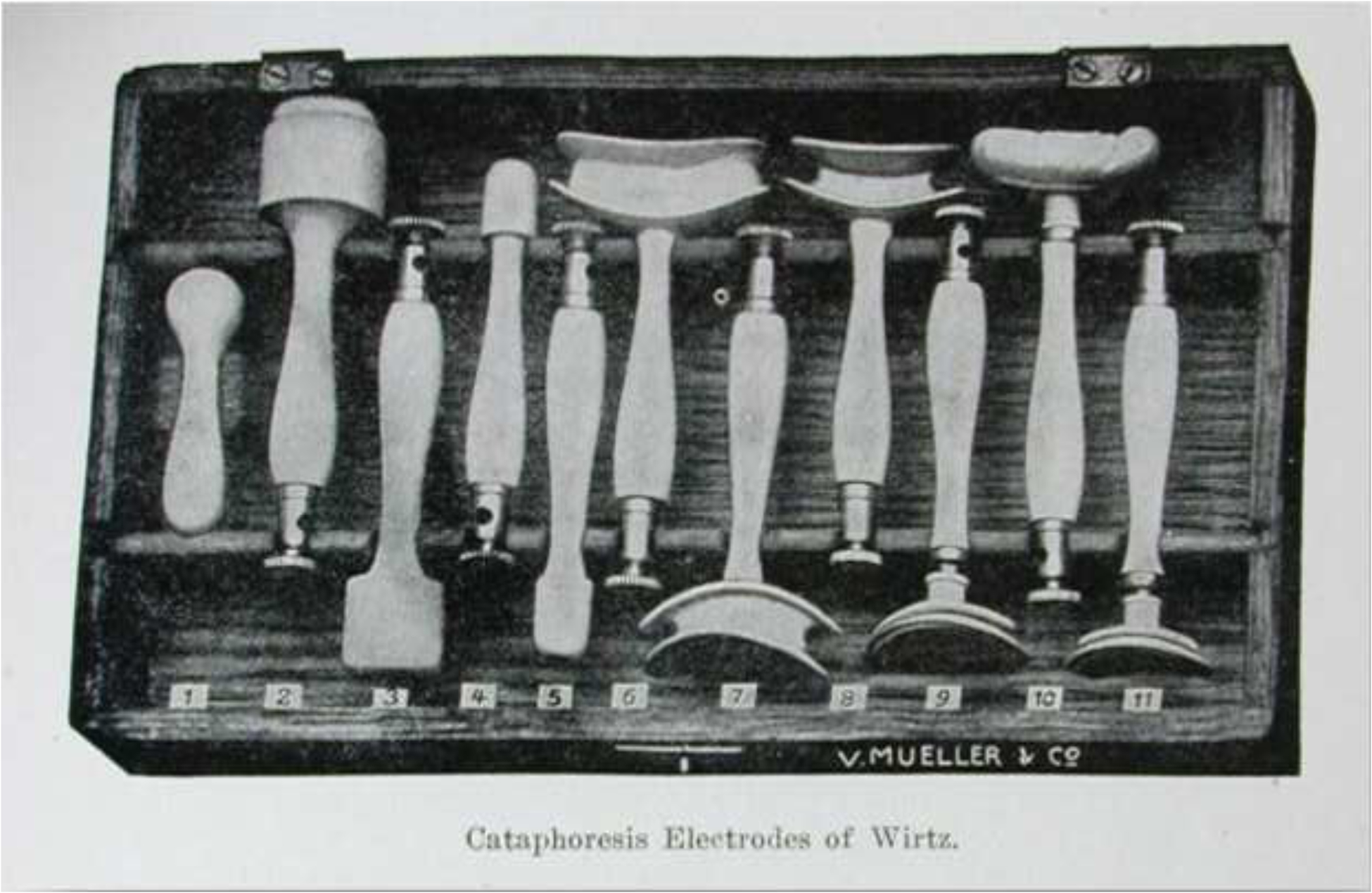
Cataphoresis instruments developed by Robert Wirtz for treating corneal diseases using iontophoresis.
Thermophore
The Shahan thermophore was invented by W.E. Shahan of St. Louis in 1916 as an apparatus to place heat to various lesions of the eye – particularly for the treatment of pneumococcal ulcers (Figure 5). It consisted of a brass tube attached to a thermometer fastened at one end to regulate temperatures with a resistance coil wrapped around the tube. Shahan found that the pneumococcus in the cornea could be destroyed at a temperature of 152 degrees Fahrenheit, and the use of the thermophore did less damage to the cornea than any other means currently in use.21 A case report by M.F. Weymann mentioned the efficacy of using the thermophore on tumors of the cornea, lids, and skin surrounding the eye.22
Figure 5.

The thermophore produced by W.E. Shahan of St. Louis in 1916 used to treat patients with pneumococcal ulcers and tumors of the cornea. (Photo courtesy of the Museum of the American Academy of Ophthalmology)
Contemporary Ophthalmology
Despite the rise of electrotherapeutics in ophthalmology in the late 19th and early 20th centuries, there was a significant decline in its use as a therapeutic modality with the exception of a few very successful applications such as electrocautery and the electromagnet for foreign body removal. It was only in the late 20th and early 21st centuries that new therapeutic modalities emerged. In contemporary ophthalmology, electrotherapy can be divided into four categories: standard employment, non-conventional therapy, cutting edge technology, and therapeutics in development.
Current standard employment
Electrocautery
Electrocautery is the use of electrical current to generate heat at a metal tip to the tissue. There is no current that is being driven into the body through electrocautery as opposed to electrosurgery. Electrocautery is used primarily to treat hemostasis following surgery, to induce scarification for the treatment of painful bullous keratopathy, and for punctal occlusion in the management of dry eye.23,24,25 Studies have demonstrated that electrocautery is efficacious in treating patients with painful bullous keratopathy and poor visual potential. The electrocautery is used to scarify Bowman’s layer to prevent fluid from reaching the surface of the cornea. This substantially reduces the level of discomfort in patients with advanced disease.24,26
Electrolysis
Electrolysis in contemporary ophthalmology employs direct current flow in a unidirectional pattern and produces a strong polarity at each electrode. The strong polarity around the electrodes produces chemical ionization with the formation of acids and the release of metallic ions at the cathode and hydroxides at the negative pole. The use of direct current for electrolysis will allow for the dissolution of tissues caused by the hydroxides, and this results in destruction of the hair root. Electrolysis is applied at the base of the eyelashes for trichiasis.25
Electromagnet –
The use of the electromagnet had been employed to treat patients with intraocular foreign bodies. This technique is widely used around the world but is associated with a 23% risk of vitreous hemorrhage and 10% risk of developing endophthalmitis.18
Iontophoresis
Iontophoresis although still being employed today, has never moved to the forefront of treatment. In contemporary ophthalmology, there are two types of iontophoresis – transcorneal and transscleral iontophoresis.20
Non-conventional therapy
Biofeedback therapy
Biofeedback therapy for the treatment of eye pain has been developed and employed by Malcolm Ing, Clinical Professor and Chair of the Ophthalmology Division at the University of Hawaii, John A. Burns School of Medicine. The Self-Controlled Energy Neuro-Adaptive Regulator (SCENAR) was a medical device first developed in Russia in the 1970s, and Ing was successful in treating a shingles patient with SCENAR for her ocular pain from post-herpetic neuralgia. Ing then introduced SCENAR to his patients informally for two years with reports that pain relief was dramatic in some patients. In 2002, the Food and Drug Administration (FDA) approved SCENAR for use in the United States as a class II biofeedback device.27
Transcorneal Electrical Stimulation (TES)
Transcorneal Electrical Stimulation (TES) is designed as a bipolar contact lens or a microfiber DTL (Dawson, Trick, Litzkow) electrode that is placed on the cornea of patients after local anesthesia.28 The electric current pulses are generated and delivered through a stimulus isolation unit with another inactive electrode placed on the skin around the eye acting as a reference electrode. Two studies in 2006 and 2007 showed that patients with anterior ischemic optic neuropathy (AION) and retinal artery occlusion (RAO) had improved visual outcomes after treatment with TES.29,30 Various clinical trials theorize that the protective effects of TES include vasodilation, neurotrophic activation, anti-apoptosis, anti-glutamate, and anti-inflammatory mechanisms. The retina in Retinitis pigmentosa (RP) is characterized by restriction of the retinal blood circulation due to thinning of the vascular plexus and obliteration of vessels. A study showed that TES induced phosphenes and increased vasodilatory effects which protected RP retinas.10,28,31 TES is also thought to enhance the expression levels of endogenous neurotrophic factors and increase the intrinsic neuronal sensitivity to these factors. TES down-regulates the expression of Bax, tumor necrosis factor (TNF), and glutamate release in degenerative retinas, which ultimately prevents apoptosis. An in-vitro study found that the application of trans-culture electrical stimulation could suppress microglia – cells which release a variety of inflammatory cytokines, reactive oxygen species, and nitrogen intermediates and excitotoxins which are hazardous to photoreceptors.28
Neuromodulation
Neuromodulation uses alternating electrical currents to increase the function of residual photoreceptors through enhanced brain plasticity. The idea of neuromodulation was introduced by Bernhard Sabel, director of the Institute of Medical Psychology at the Otto-von-Guericke University of Magdeburg in Magdeburg, Germany. In 2013, he presented a lecture at the National Eye Institute (NEI) and suggested that alternating currents can increase blood flow to partially damaged areas of the brain which can then increase visual function.32,33 Subsequent studies on repetitive transorbital alternating current stimulation (rtACS) reported that partially blind patients had a 24% improvement in visual fields and that rtACS treatment “is a safe and effective means to partially restoring vision by modulating brain plasticity.”34
Cutting Edge Technology – Visual Prosthetics
Studies from Kreig, Shaw, Button & Putnam in the mid-1900s discussed the possible development of a visual prosthesis. However, the possibility of developing such a device did not become widely accepted until human experiments by Brindley & Lewis were published.35,36 Today there are several prosthetic devices that have been demonstrated to restore visual function in patients that are partially or completely blind due to retinal degenerative diseases such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD). These devices were developed to target potential sites for implementation of the visual prosthetic such as the subretinal space, epiretinal surface, optic nerve, lateral geniculate body, and visual cortex.36
Subretinal prosthetics
Subretinal prosthetics are implants that contain light-sensitive components (photodiode) and an electrode to simulate that of a photoreceptor synapse relaying electrical signals to the inner retina.37 Connecting the implant to the inner retina helps stimulate bipolar cells with local electric currents, and the positioning in the subretinal space allows for retinotopically correct perception in the visual field. The implanted chip moves with the eye, which helps to stabilize the image. In addition, the subretinal space is immunoprivileged and less prone to rejection.38
The retinal implant, Alpha, devised by Eberhart Zrenner in Germany has been in development for over 20 years. It is the only light sensitive subretinal implant that has received commercial approval in Europe. A clinical trial at Oxford testing the Alpha IMS (first-generation) showed four out of six patients with RP reporting improved function for daily living with no adverse events after surgery.37 The second-generation Alpha implant, Alpha AMS, is marketed to have improved longevity with 1600 pixels and received commercial approval (CE mark) in March 2016.37,38 A recent study by Thomas Edwards at the University of Oxford showed that the Alpha AMS improved visual performance of five of six patients with end stage RP for up to 24 months.39
In 2004, Chow et al (Optobionics Inc) developed an artificial silicon retina (ASR) microchip, a 2-mm-diameter silicon-based chip that contains 5000 microelectrode-tipped microphotodiodes. Chow implanted it into the right eyes of six patients with RP. Results showed that visual function improved in all patients with no rejection, infection, or inflammation. Additional results showed improved visual function in retinal areas distant from the implant (Figure 6).40
Figure 6.
The ASR subretinal microchip developed by Optobionics Inc. (Courtesy of and with permission for publication by Alan Chow MD Optobionics Inc.)
The Boston Retinal Project was one of the first research projects formed in the field of retinal prosthetics. The project consists of using an external camera mounted on spectacles, and the images collected are sent to a smart-phone sized controller which processes the images wirelessly to the subretinal implanted device. Other subretinal implants have been developed by Pixium Vision (PRIMA), Yagi & Watanabe (Biohybrid Retinal Implant), Tano, Ikuno & Ohta (Japan Retinal Implant Group), Li & Ren (C-sight: Chinese Project for Sight), Palanker (Biomedical Physics and Ophthalmic Technology), and Pelizzone (Eye Clinic, University Hospital of Geneva).36
Epiretinal Prosthetics
Epiretinal Prosthetics employ a multielectrode array placed on the inner surface of the retina in direct contact with the nerve fiber layer.8 Epiretinal prosthetics are advantageous due to easier surgical implantation compared to subretinal implantation.
Both epiretinal and subretinal implants are produced to stimulate electrically phosphenes, basic visual phenomena received without the perception of light.38
The Argus II epiretinal prosthesis was developed by Second Sight and is the first retinal implant to receive commercial approval in 2011 in Europe and the United States. It was the first device tested in humans to pass safety and efficacy assessments, and it is currently the most widely used prosthesis worldwide.38 The epiretinal implant is attached to an external glass mounted video camera. The signals from the external camera are acquired and are transformed into electrical pulses from a visual processing unit, VPU. These impulses are sent to the retinal ganglion cells and inner retina to elicit phosphenes through video processing.8,36,38,41
Pixium Vision’s Intelligent Retinal Implant System (IRIS-I and II) epiretinal device was first manufactured as a 49-electrode wireless implant and then updated to 150 electrodes with the IRIS II. Like the Argus II, the implant is connected externally to a visual interface (ATIS) image sensor to capture visual information and is processed through a pocket processor to transform the visual data into stimulation commands. The implant then converts the stimulation commands from the pocket processer into electrical signals to activate functional RGCs.38
Suprachoroidal Implant
In 2014, Bionic Vision developed a suprachoroidal prototype implant that generated phosphene activation in all subjects tested with no serious device-related adverse events. The suprachoroidal implant is advantageous in that the insertion of the electrode is minimally invasive in comparison to epiretinal and subretinal implants.42
Optic Nerve Implant
The AV-DONE device (Direct Optic Nerve Japan) and Microsystems-Based Visual Prosthesis for Optic Nerve (MiViP) in Belgium had been tested and developed as an optic nerve prosthesis. The dense packing of RGC axons in the optic nerve may limit the degree of spatial detail of the induced images that could be achieved and has proved restrictive in the development of such prosthetic devices.
Lateral Geniculate Body and Visual Cortex Implants
Whereas retinal implants would not be a viable treatment for glaucoma, lateral geniculate body (LGB) and visual cortex implantation have been suggested as a treatment option.36 The visual cortex provides a larger surface area relative to the retina, and the larger surface area allows for implantation of multiple stimulation electrodes which can provide higher-resolution artificial vision. The cortical implants, however, would require a more challenging surgical procedure, with exposure of the dura mater, and possibly the need to stimulate deep into the calcarine fissure where the foveal projections are buried.42
A surface cortical electrode array, Orion, developed by Second Sight, has been developed. The first implementation of the Orion prosthetic was performed in January 2018 at the University of California, Los Angeles. The cortical implant aims to restore vision to patients who are completely blind from glaucoma, diabetic retinopathy, cancer or trauma.11 Clinical trials for the device began in Germany in 2018.
Cutting Edge Technology – Brain Port
Visual prosthetic devices are limited to those with functional optic nerves and retinal ganglion cells. For those who do not have visual potential, sensory substitution devices are designed to bypass primary visual pathways and provide visual information through nonvisual, afferent pathways. The BrainPort is a device that utilizes the ability of the brain to learn and translate nonvisual stimuli into visual equivalents. It is a nonsurgical and noninvasive device that can be used safely for patients with blindness. The BrainPort is an electrotactile sensory substitution device that consists of a wide-angle sensor mounted in the center of a pair of sunglasses that will send live video to a handheld processor. The processer will sample the video and transform the images as an electrotactile sensation to a tethered, removable resin lollipop called the intra-oral device (IOD). The sensation stimulates a square array of 400 electrodes embedded in the IOD for the tongue to perceive as visual information. The BrainPort has now received both commercial and FDA approval.43,44
Contemporary Investigation: Endogenous Electric Fields in the Eye during Wound Healing
In addition to pursuing therapeutic effects through exogenous electric techniques, experiments have been carried out to understand endogenous electric fields and their roles in ocular biology,15 since electric fields are found endogenously at the cornea, lens, and retinal pigment epithelium (RPE).14 In fact, the eye is one of the organs with the most active naturally occurring electrical activity in human body from the surface of cornea all the way to the visual cortex.
The cornea naturally maintains an electric potential difference.46 Electrical potential differences (PD) exist between the epithelial, stromal, and endothelial layers of the cornea. The transport of ions in the three compartments (tear film, cornea and aqueous humor) generate the PDs and electrical fields (EF). When injuries break the epithelial barrier, endogenous laterally orientated electric fields are established pointing towards the wound center (positive potential to negative potential).47 The PD is maintained in areas with intact epithelium and the difference between wounded and intact areas is termed “wound EF.” The endogenous wound EFs provide a powerful signal to induce directional migration (galvanotaxis or electrotaxis) for wound healing.14,45
Using corneal wounds as a model, it was found that applied electrical fields of physiological strength would override the default EF and other directional cues produced by the wound and, thus, manipulate directional cell migration in wound healing (Figure 7). Genetic and pharmacological studies demonstrated that phosphatidylinositol-3-OH kinase- γ (PI3K γ) and tumor suppressor phosphatase and tensin homolog (PTEN) were the two proteins responsible for directional epithelial migration in wound healing. Manipulating the transepithelial ion transport using pharmaceutical intervention, indeed, regulate wound healing of rat cornea. Solutions of silver nitrate, AgNO3, increase the efflux of chloride ions and influx of sodium ions in the corneal epithelium which amplified the transcorneal potential difference and endogenous wound electric field resulting in faster corneal wound healing. Furosemide was noted to have the opposite effect, which decreased the transcorneal epithelial difference (Figure 8).45
Figure 7.
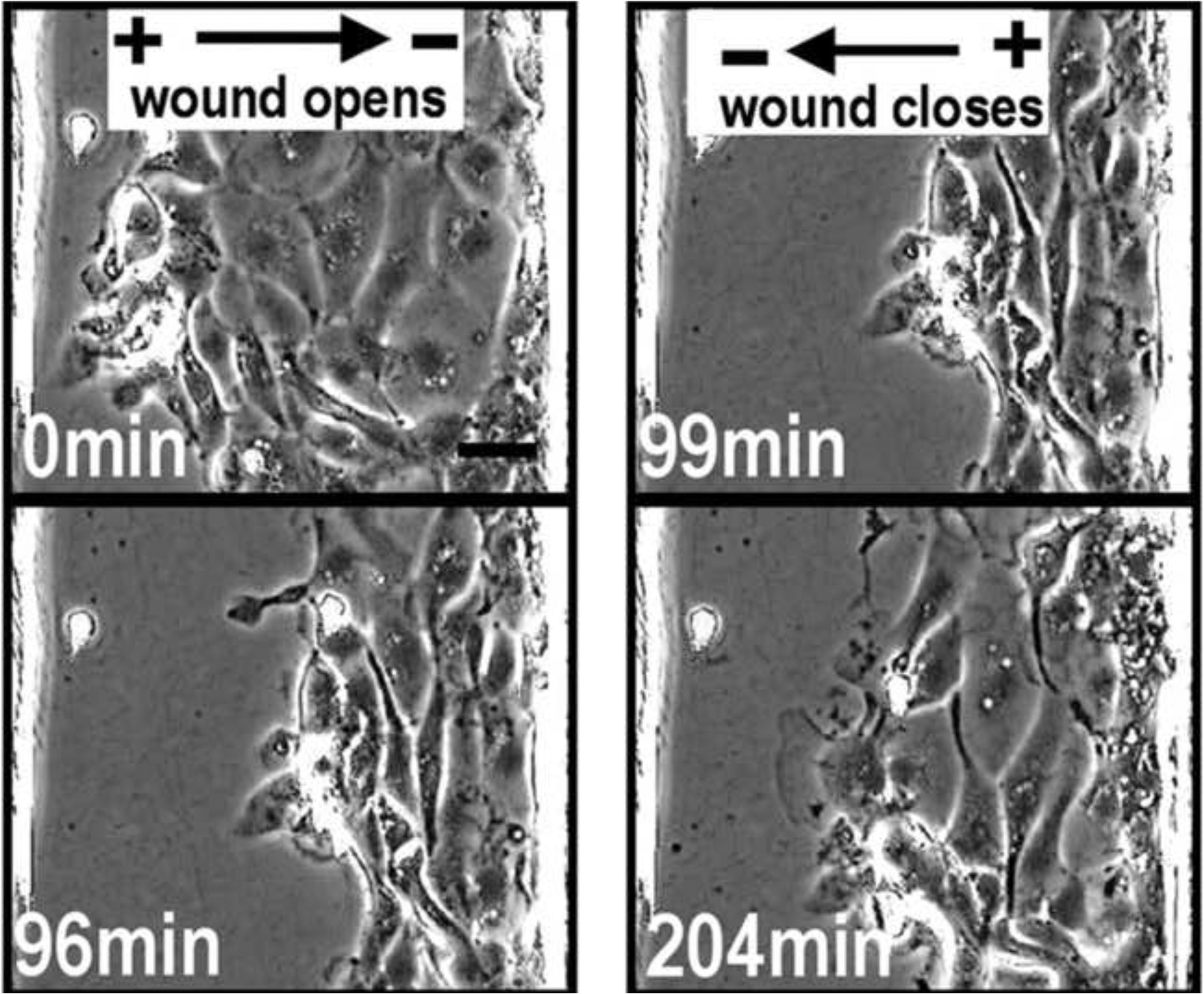
The healing of a corneal wound by an induced electrical current. Electrical fields can both open (left) and close (right) a wound. An electric field of physiological strength is applied with the polarity pointing away from the wound center at 0 minutes and at 96 minutes, the cells move away from the wound, thus opening the wound. The field polarity is then reversed at 99 minutes and the cells now migrate into the wound resulting in wound closure at 204 minutes.
Figure 8.
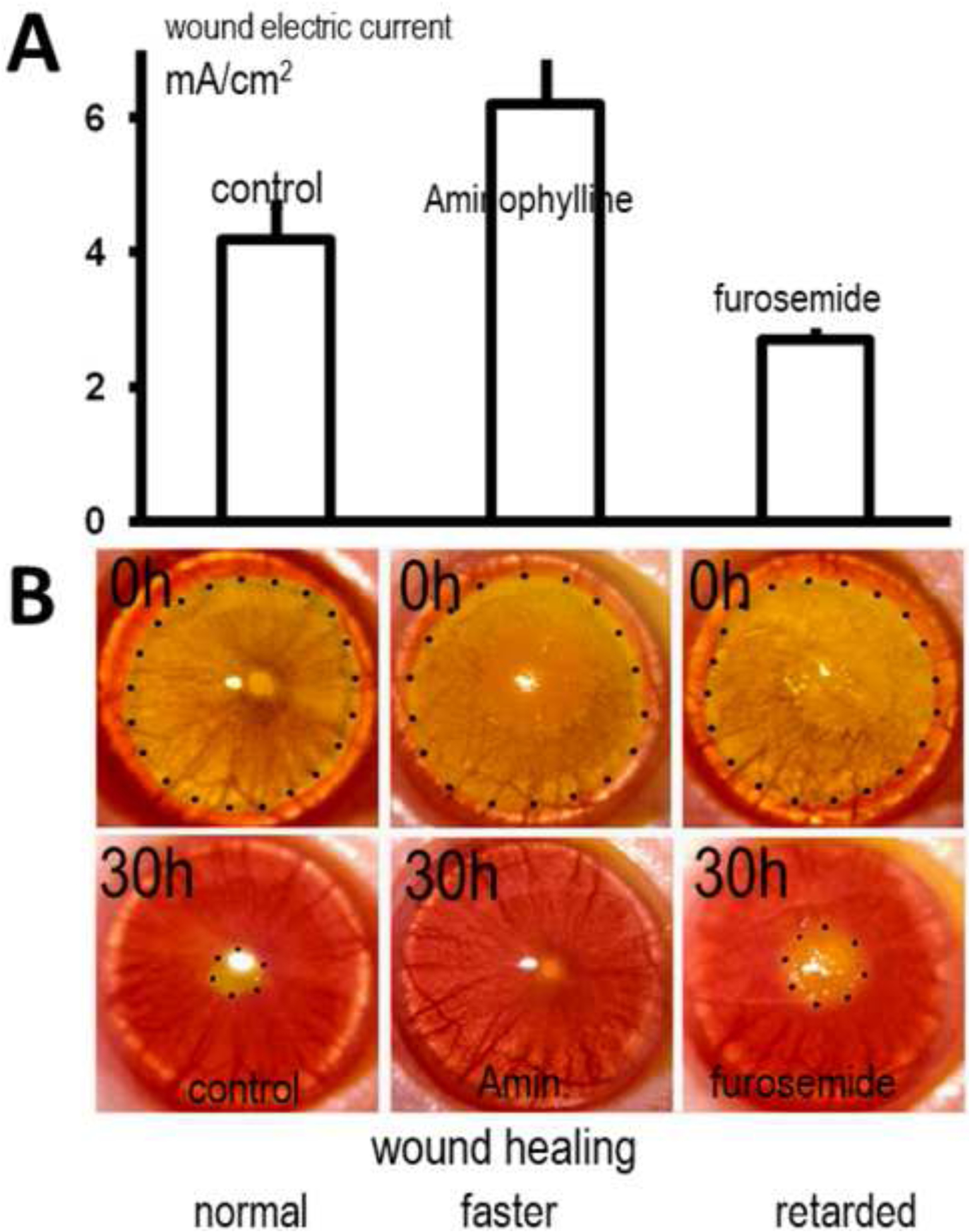
The use of pharmaceutical manipulation and the effects on corneal wound healing. (A) pharmacological manipulation of corneal epithelial transportation of Na+ and Cl− significantly enhances (aminophylline) or decreases (furosemide) endogenous wound electric currents. (B) The healing of circular lesions in the cornea is shown over time. Circular keratectomy was performed on corneas at 0 h. Lesions were labeled yellow with fluorescein and are shown here outlined with dots. Aminophylline was used to increase the wound current which subsequently showed significantly increased wound healing; whereas furosemide that was used to decrease the wound current significantly decreased wound healing. Modified from FASEB J. 2005 Mar;19(3):379–86. Wound healing in rat cornea: the role of electric currents. Reid B1, Song B, McCaig CD, Zhao M. https://www.fasebj.org/doi/10.1096/fj.04-2325com
Reid et al discussed the potential of using pharmaceutical manipulation to increase the EF strength at the cornea. The use of aminophylline or chloride-free solution eye drops enhanced the healing of damaged cornea in patients with reduced wound healing such as in diabetic patients or the elderly. This discovery suggests the potential of bioelectric stimulation without the use of electrodes.14,50
The crystalline lens has a remarkable electric “circulation” with currents flowing out from the equator and entering the anterior and posterior poles.48 Restoring circulation of the currents appears to be involved in lens regeneration.49 The retina exhibits active electrical activitiy, which can be recorded at the front of the eye and serves as an indicator of retinal function and pathology.14 This activity is the basis for contemporary electrophysiologic testing in clinical practice. The manipulation of EFs at the cornea, lens, and RPE brings suggests the potential for new therapeutic modalities in the field of ophthalmology.
Conclusion
Electrotherapy in the field of medicine has been practiced for centuries. The world’s fascination with electricity is justified considering the vital role of electrical impulses in biological life. Electricity as a therapeutic modality in ophthalmology has proven efficacious, albeit in the context of many bogus applications. The development of visual prosthetics and electrotactile devices provides novel advancements for improving visual function in degenerative ocular diseases, and pharmaceutical manipulation of electrical fields brings great potential for improved healing of ocular diseases. This renewed interest in understanding electrobiology gives promise for new applications in many areas of ophthalmology.
Financial Disclosures:
KieuYen Luu: None
Min Zhao: is a board director with no income of Aaken Insites, Inc. Davis, CA. He gave one-time lectures and received honorarium at CooperVision, Inc., Pleasanton, CA.; Unilever, NY. He is a named inventor of US Patent and Patent applications - U.S. Patent Application Serial No. 14/698,747, No. 62/610,992, 62756342. He has the following active research grants NIH EY019101 (PI), AFOSR FA9550-16-1-0052 (UC Davis PI), R21AG060335 (co-PI).
Mark Mannis: None
Other Acknowledgements:
Experimental research in authors’ lab are supported by NIH EY019101, AFOSR FA9550-16-1-0052, an Unrestricted Grant from Research to Prevent Blindness, Inc., Core Grant (P-30 EY012576).
References
- 1.Kellaway P The Part Played By Electric Fish In The Early History Of Bioelectricity And Electrotherapy. Johns Hopkins Univ Press. 2019;20(2):112–137. https://www.jstor.org/stable/44441034. [PubMed] [Google Scholar]
- 2.Finger. Dr Stanley. Alexander Garden, a Linnaean in Colonial America, and the Saga of Five “Electric Eels.” Perspect Biol Med. 2010;53(3):388–406. doi: 10.1353/pbm.0.0163 [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Galvanism - The “unhallowed arts” of Frankenstein. doi: 10.1360/zd-2013-43-6-1064 [DOI]
- 4.Karamanou M, Androutsos G, Lymperi M, Gennimata V, Tsoucalas G. The “torpedo” effect in medicine. Int Marit Health. 2014;65(2):65–67. doi: 10.5603/imh.2014.0015 [DOI] [PubMed] [Google Scholar]
- 5.Bertucci P The shocking bag: Medical electricity in mid-18th-century London. Nuova Voltiana. 2003;5(1748):31–42. http://ppp.unipv.it/Collana/Pages/Libri/Saggi/NuovaVoltiana5_PDF/p__031-042.pdf%5Cnpapers3://publication/uuid/899D62C0-7FBF-46B2-AE41-845FDE0175B3. [Google Scholar]
- 6.Wainsztein RD, Schwartz SG, Pflugrath A, Leffler CT, Peterson E. Ophthalmology in North America: Early Stories (1491–1801). Ophthalmol Eye Dis. 2017;9:117917211772190. doi: 10.1177/1179172117721902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson E Electricity in Diseases of the Eye. In: Electrotherapy. Philadelphia: P. Blackiston’s Son & Co; 1902:227–238. https://books.google.com/books?id=11cSAAAAYAAJ&pg=PR2&lpg=PR2&dq=edward+jackson+electrotherapy+george+jacoby&source=bl&ots=rboD5HYCHn&sig=ACfU3U39xBuDHeNiTpZ3q0_3oEOp5l8bCQ&hl=en&sa=X&ved=2ahUKEwjNk-j_ya_hAhWBFXwKHe0-BGcQ6AEwAHoECAYQAQ#v=onepage&q=edward. [Google Scholar]
- 8.Luo YH-L, Cruz L da. The Argus II Retinal Prosthesis System. Retin Eye Res. 2015:1–19. doi:1037//0033–2909.I26.1.78 [DOI] [PubMed] [Google Scholar]
- 9.Wagner SK, Jolly JK, Pefkianaki M, et al. Transcorneal electrical stimulation for the treatment of retinitis pigmentosa: results from the TESOLAUK trial. BMJ Open Ophthalmol. 2017;2(1):e000096. doi: 10.1136/bmjophth-2017-000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gekeler F A shock to the System: Can transcorneal electrical stimulation help reverse retinal degeneration in retintis pigmentosa? Ongoing clinical trials suggest that it may. The Ophthalmologist. 2013. [Google Scholar]
- 11.Densford F Second Sight touts 1st-in-human Orion cortical implant. February 5, 2018. https://www.massdevice.com/second-sight-touts-1st-human-orion-cortical-implant/.Published2018.
- 12.Reynolds JR. Lectures on the Clinical Uses of Electricity. Second Edi. Leopold Classic Library; 2011. [Google Scholar]
- 13.Finkelstein G Emil du Bois-Reymond vs Ludimar Hermann. Comptes Rendus - Biol. 2006;329(5–6):340–347. doi: 10.1016/j.crvi.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Zhao M, Reid B, Vieira AC, Cao L, Chalmers L, Mannis M. Electrical signaling in control of ocular cell behaviors. Prog Retin Eye Res. 2011;31(1):65–88. doi: 10.1016/j.preteyeres.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colburn JE. The Galvanic Current in the Treatment of Certain Forms of Cataract. JAMA. 1879;VIII. [Google Scholar]
- 16.de Schweinitz GE, Jackson E, Risley SD. The Treatment of Immature Cataract and When to Operate for Cataract. Remarks made in a Conference During the Special Week on Cataract. JAMA. 1894;XXII(4):105–108. [Google Scholar]
- 17.Eliasoph B The Question of Electrolysis in Tissue. J Gen Exp Med. 1922. [Google Scholar]
- 18.Guevara-Villarreal DA, Rodríguez-Valdés PJ. Posterior Segment Intraocular Foreign Body: Extraction Surgical Techniques, Timing, and Indications for Vitrectomy. J Ophthalmol. 2016;2016:1–5. doi: 10.1155/2016/2034509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keeler R, Singh AD, Dua HS. Electric eyes: Wirtz iontophoresis electrodes. Br J Ophthalmol. 2009;93(11):1415. doi: 10.1136/bjo.2009.172825 [DOI] [PubMed] [Google Scholar]
- 20.Shoeibi N, Mahdizadeh M, Shafiee M. Iontophoresis in ophthalmology: A review of the literature. Rev Clin Med. 2014;1(4):183–188. doi: 10.17463/RCM.2014.04.003 [DOI] [Google Scholar]
- 21.Post MH. The role of the Shahan Thermophore in Ophthalmic Therapeutics. Am J Ophthalmol. 1949;32(2):2150220. [DOI] [PubMed] [Google Scholar]
- 22.Weyman MF. The Thermophore - It’s use in eye therapy. Cal West Med. 1927;27(4):333. doi: 10.2307/40322060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosaka E, Tatematsu Y, Tsubota K, et al. Surgical Punctal Occlusion With a High Heat-Energy Releasing Cautery Device for Severe Dry Eye With Recurrent Punctal Plug Extrusion. Am J Ophthalmol. 2011;151(3):483–487.e1. doi: 10.1016/j.ajo.2010.08.045 [DOI] [PubMed] [Google Scholar]
- 24.DeVOE A Electrocautery of Bowman’s Membrane. Arch Ophthalmol. 1966;76. [DOI] [PubMed] [Google Scholar]
- 25.Blankenship MJ. Physical modalities - Electrosurgery, Electrocautery, and Electrolysis. Int Soc Trop Dermatology. 1979;18:443–452. doi: 10.1016/B978-141602443-9.50019-2 [DOI] [PubMed] [Google Scholar]
- 26.Karp CL, Aziz H, Galor A, Shalabi N, Jeng BH. Superficial Epithelial Keratectomy, Cautery, and Amniotic Membrane Transplant for the Treatment of Painful Bullous Keratopathy in Eyes With Poor Visual Potential. Cornea. 2014;33(7):755–759. doi: 10.1097/ico.0000000000000137 [DOI] [PubMed] [Google Scholar]
- 27.Altonn H Best-Case Scaenar. Honolulu Star Bulletin. May 15, 2005:2. [Google Scholar]
- 28.Tao Y, Chen T, Liu B, et al. The transcorneal electrical stimulation as a novel therapeutic strategy against retinal and optic neuropathy: a review of experimental and clinical trials. Int J Ophthalmol. 2016. doi: 10.18240/ijo.2016.06.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujikado T, Matsushita K, Shimojo H, Morimoto T, Okawa Y, Tano Y. Effect of Transcorneal Electrical Stimulation in Patients with Nonarteritic Ischemic Optic Neuropathy or Traumatic Optic Neuropathy. Jpn J Ophthalmol. 2006;50(3):266–273. doi: 10.1007/s10384-005-0304-y [DOI] [PubMed] [Google Scholar]
- 30.Inomata K, Miyake Y, Hanazono G, et al. Transcorneal electrical stimulation of retina to treat longstanding retinal artery occlusion. Graefe’s Arch Clin Exp Ophthalmol. 2007;245(12):1773–1780. doi: 10.1007/s00417-007-0610-9 [DOI] [PubMed] [Google Scholar]
- 31.Gekeler F, Bartz-Schmidt KU. Electrical stimulation - A therapeutic strategy for retinal and optic nerve disease? Graefe’s Arch Clin Exp Ophthalmol. 2012;250(2):161–163. doi: 10.1007/s00417-012-1930-y [DOI] [PubMed] [Google Scholar]
- 32.Wart O Electrical Stimulation: Using Electrical Impulses to Combat Blindness. https://www.medica-tradefair.com/cgi-bin/md_medica/lib/pub/tt.cgi/Electrical_Stimulation_Using_Electrical_Pulses_to_Combat_Blindness.html?oid=80120&lang=2&ticket=g_u_e_s_t. Published 2016. Accessed January 6, 2019.
- 33.Sabel B Bernhard Sabel Speaks on Treating Blindness with Brain Plasticity. In: ; 2013. https://nei.nih.gov/news/special/brain_plasticity. [Google Scholar]
- 34.Gall C, Schmidt S, Schittkowski MP, et al. Alternating current stimulation for vision restoration after optic nerve damage: A randomized clinical trial. PLoS One. 2016;11(6):1–19. doi: 10.1371/journal.pone.0156134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobelle WH, Mladejovsky MG. Phosphenes Produced By Electrical Stimulation of Human Occipital Cortex, and Their Application to the Development of a Prosthesis for the Blind. J Physiol. 1974;243:553–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tombran-Tink J, Barnstable CJ, Rizzo JF III, eds. Visual Prosthesis and Ophthalmic Devices. Totowa, New Jersey: Humana Press Inc; 2007. [Google Scholar]
- 37.MacLaren RE. Electronic retinal implant surgery. Eye. 2017;31(2):191–195. doi: 10.1038/eye.2016.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabel VP, ed. Artificial Vision: A Clinical Guide. Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 39.Edwards TL, Cottriall CL, Xue K, et al. Assessment of the Electronic Retinal Implant Alpha AMS in Restoring Vision to Blind Patients with End-Stage Retinitis Pigmentosa. Ophthalmology. 2018;125(3):432–443. doi: 10.1016/j.ophtha.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sciences C, Chow AY, Chow VY, et al. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004;122(4):460–469. doi: 10.1001/archopht.122.4.460 [DOI] [PubMed] [Google Scholar]
- 41.Bourzac K Bionic Eye Implant Approved for U.S. Patients. [Google Scholar]
- 42.Guymer R, Brandli A, Luu C, Ayton L. Progress in the clinical development and utilization of vision prostheses: an update. Eye Brain. 2016:15. doi: 10.2147/eb.s70822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nau AC, Pintar C, Arnoldussen A, Fisher C. Acquisition of visual perception in blind adults using the BrainPort artificial vision device. Am J Occup Ther. 2015;69(1):1–8. doi: 10.5014/ajot.2015.011809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant P, Spencer L, Arnoldussen A, et al. The Functional Performance of the BrainPort V100 Device in Persons Who Are Profoundly Blind. J Vis Impair Blind. 2016;110(2). [Google Scholar]
- 45.Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature. 2006;442(7101):457–460. doi: 10.1038/nature04925 [DOI] [PubMed] [Google Scholar]
- 46.Klyce SD. Electrical profiles in the corneal epithelium. J Physiol. 1972;226(2):407–429. doi: 10.1113/jphysiol.1972.sp009991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang M, Robinson KR, Vanable JW Jr. Electrical Fields in the Vicinity of Epithelial Isolated Bovine Eye. Exp Eye Res. 1992;54:999–1003. [DOI] [PubMed] [Google Scholar]
- 48.Robinson KR, Patterson JW. Localization of steady currents in the Lens. Curr Eye Res. 1982;2(12):843–847. doi: 10.3109/02713688209020020 [DOI] [PubMed] [Google Scholar]
- 49.Lois N, Reid B, Song B, Zhao M, Forrester J, McCaig C. Electric currents and lens regeneration in the rat. Exp Eye Res. 2010;90(2):316–323. doi: 10.1016/j.exer.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 50.Reid B, Graue-Hernandez EO, Mannis M, Zhao M. Modulating Endogenous Electric Currents in Human Corneal Wounds - A Novel Approach of Bioelectric Stimulation without Electrodes. Cornea. 2011;30(3):338–343. doi: 10.1002/0471142905.hg1504s82.ENU [DOI] [PMC free article] [PubMed] [Google Scholar]


