ABSTRACT
We report a 53‐year‐old female patient presenting with a refractory venous leg ulcer and unremarkable findings in the doppler Ultrasound venous mapping of the leg veins. Further comprehensive diagnostics demonstrated an underlying May–Thurner syndrome. After resolution of the primary mechanical obstruction, rapid wound healing in the following 3 weeks was documented. Iliac vein compression syndrome, commonly known as May–Thurner syndrome, is a distinguishable anatomical variant that results from an external compression over the left iliac vein exerted by the overriding adjacent right common iliac artery. It is mostly seen among young, healthy female patients and can easily be under‐diagnosed. Lower extremities duplex ultrasonography remains the gold standard in diagnosing venous insufficiency, but it should not solely depend on it. Instead, clinicians should consider other possibilities, assessing the patency within the truncal veins, which in turn might contribute to the venous insufficiency along the lower limbs. An active early diagnostic approach can prevent significant overall morbidity and help patients to ease back into their daily‐life activities. Therefore, it is recommended that all patients with suspected venous insufficiency and normal lower limbs duplex findings should undergo further evaluation of the truncal venous system pattern. May–Thurner syndrome, along with other causes of iliac veins compression, should be considered in the differential diagnosis in unclear persistent cases of unilateral venous symptoms.
Keywords: Chronic wound, Iliac vein compression syndrome, Iliocaval compression syndrome, May–Thurner syndrome, Venous leg ulcers
Case report
A 53‐year‐old Caucasian female with a medical history of multiple sclerosis presented to our wound care unit for evaluation of a progressive chronic ulcer on her left lower leg. According to the patient, the ulcer was of 3 months duration, accompanied with moderate dull pain and swelling, exacerbated by prolonged standing. In a detailed anamnesis, the patient added that she underwent sclerotherapy for spider veins on the same affected limb 3 months earlier. Since then, she developed a non‐healing wound. She applied different moist wound dressings in the period before she sought an expert consultation. With respect to her multiple sclerosis, her current medications were Prostanoids therapy, in addition to hydrocortisone, taken only at acute relapses.
Clinical examination
On clinical inspection and palpation at the first visit, there was a leg ulcer that measured approximately 5·6 cm2 in diameter, with a pain score of 7 out of 10 points on the visual analogue scale (VAS). The ulcer was located on the left distal lower limb just below the medial malleolus. The ulcer bed was covered with fibrin as well as a small amount of necrotic tissue and was surrounded by sharply demarcated red inflamed borders (Figure 1). Besides a moderate perimalleolar oedema of the left lower leg, no other manifestations of chronic venous insufficiency (CVI) were noted. Arteria dorsalis pedis and posterior tibial pulses were palpable.
Figure 1.
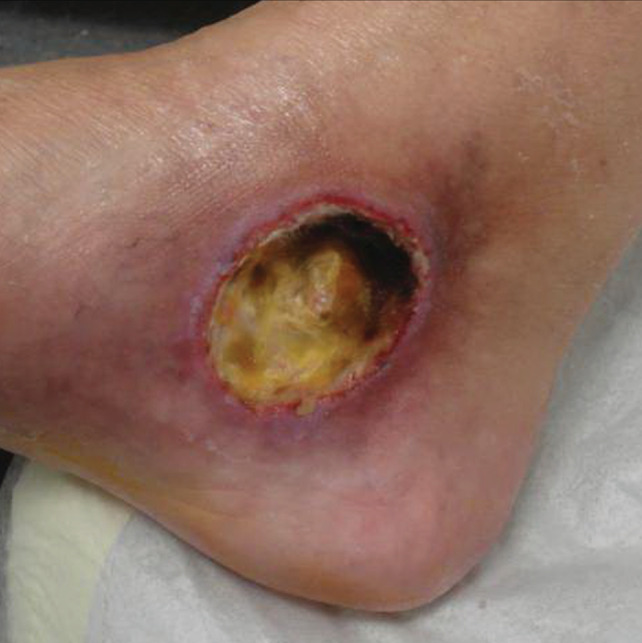
Non‐healing venous leg ulcer, measuring 5·6 cm2 in diameter of 3 months duration.
[Correction added on 26 April 2017: The lesion was mistakenly mentioned on the right leg. It should be on the left and this has been corrected in this current version.]
Differential diagnosis
A variety of pathologies can be considered in the differential diagnosis of chronic leg ulcers 1. However, from our clinical perspective and based on the patient's anamnesis of the presenting complaint, we considered amongst others venous leg ulcer, pyoderma gangrenosum or vasculitis in the differential.
Diagnostics
For the purpose of excluding inflammatory aetiologies or coexisting conditions, we run an extensive workup, including complete blood count, inflammatory markers, comprehensive metabolic panel, coagulation profile, urine and serum protein electrophoresis, anti‐nuclear antibodies and anti‐neutrophil cytoplasmatic antibodies, which was within normal limits. On histopathological examination of a biopsy, signs of dense neutrophilic inflammatory infiltrates along with vasculitis were identified. Bacterial swab cultures found Staphylococcus aureus. In a lower extremities colour duplex ultrasound, no signs of chronic venous insufficiency (CVI) were found. The resting ankle brachial pressure index (ABPI) was 0·98. As part of the evaluation for any potential space‐occupying lesions compromising the venous outflow, images using computerised tomography were obtained, showing no signs of masses in both the abdominal and the thorax cavities.
Therapy
The patient received prednisone initially for 4 days to suppress the painful inflammation caused by a secondary vasculitis. Non‐steroidal anti‐inflammatory drugs and opioids have been used to control the pain. Moreover, the local therapy regimen included moist wound care dressings, supported by consequent compression therapy consisting of daily applied short‐stretch bandages.
Follow‐up
During the follow‐up visits, an increase in the circumference of the ulcer bed surface by almost 3 cm within 4 months after the initial presentation was noted (Figure 2). The outcomes after 9 months of conventional therapy were not satisfactory, lacking significant improvement in the patient's pain and the healing progress of the ulcer. In addition to that, our patient developed a bluish discolouration of the left big toe in the process. At that stage, assessment using contrast venography for further clarification of her condition was advised, showing a severe degree of stenosis within the left common iliac vein. According to the observed radiological findings, the patient was proven to have the typical anatomical features of May–Thurner syndrome (MTS) along with a left ovarian vein reflux as part of an accompanied pelvic congestion syndrome. Therefore, an intravascular ultrasound‐guided stent implantation as the treatment of choice was preformed (Figures 2 and 3) followed by coil embolisation and sclerotherapy of the left ovarian vein. As a result, venous system patency was effectively restored. No complications after the procedure were reported.
Figure 2.
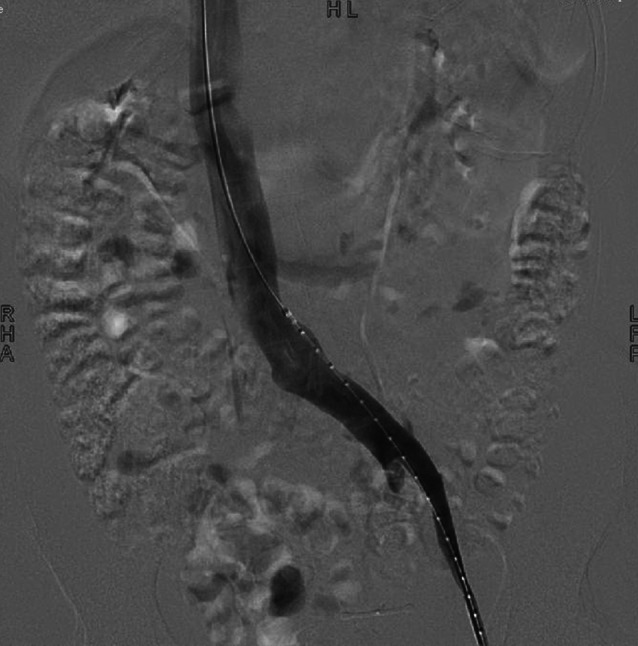
Contrast venogram shows the contrast flow across the confluence of the left common iliac vein into the IVC.
Figure 3.
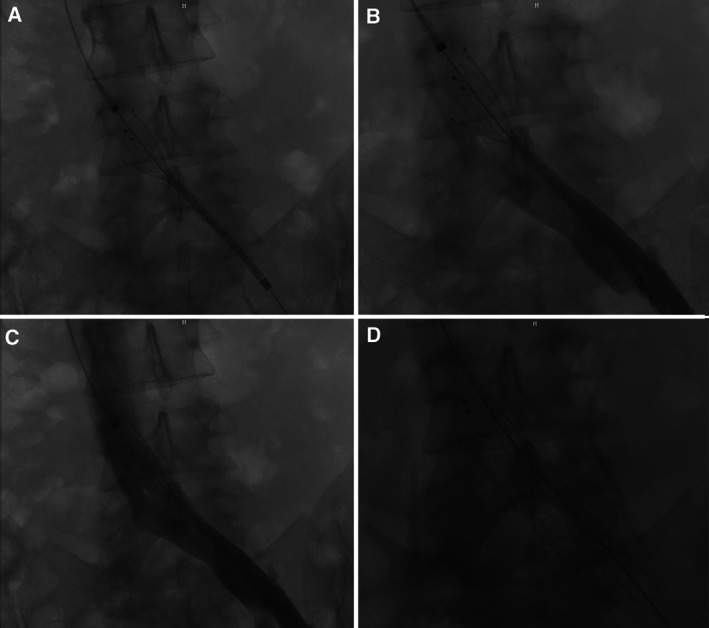
Images showing the preformed intravascular ultrasound (IVUS)‐guided percutaneous transluminal angioplasty (PTA) with stenting implantation.
Subsequently, patient's standard care measures continued in the same manner used before the intervention. At the patient's follow‐up appointment 3 weeks after the executed stent placement therapy, significant outcomes were documented. The ulcer measured 0·6 cm2 in diameter; which represents an approximately 90% decrease in wound surface area. Complete reepithelialisation of the ulcer by secondary intention was reported by our team within 8 weeks.
Discussion
Non‐healing leg ulcers create a significant socioeconomic burden on health systems both as providers and the patients themselves, impacting the quality of their daily life 2, 3, 4, 5. Reported estimations of the mean cost of ulcer treatment in Germany in recent years were exceeding 9000 Euros per year 6. Currently, there are a lot of advances in the treatment strategies used; however, these different methods would not be effective for all the cases unless the underlying cause is identified and managed. In fact, these different investigations and treatment plans should be tailored to each case individually in a scheme‐based approach. Ascertaining the nature of the underlying pathology plays a critical role in defining the entity of the wound in order to provide the optimal treatment accordingly to insure a progressive healing rate and prevent further evitable complications.
The Bonner venous study carried out in Germany suggested that approximately 0·7% of the adult population suffering from venous leg ulcers 7. Our group demonstrated that in Germany, the majority of chronic leg ulcers, accounting for almost 65%, were because of an underlying CVI 8. In the European region, it has been reported that 1% of the Western Europe population is affected with leg venous ulcers, supported by similar statistics in the south of Europe 9, 10, 11, 12. Summing up these data, we can conclude that in Europe, venous insufficiency is the most common cause of chronic leg ulcers. In daily practice, venous ulcers exhibit prolonged therapy duration and poor overall prognosis with high recurrence rate. Refractory venous ulcers need intensive care for a relatively long period of time. Different authors reported that more than 50% of the cases may actually require more than 1 year of therapy 13. Our patient follow‐up continued for the full 9 months, showing no signs of healing, which indeed supports the last statement. In our case, it was evident through contrast venography that the patient suffered from a severe degree of compression of the left common iliac vein, affecting the efficiency of the venous pathway, which in turn clinically manifested as a refractory venous leg ulcer. The fact that the located stenosis within the left common iliac vein was in an area higher up in the pelvis region justifies why we were unable to detect the source of her underlying venous incompetence at the beginning of the presentation through the lower extremities colour duplex ultrasound.
The phenomenon in which an extrinsic compression applied on the left common iliac vein by the contra‐lateral overriding right iliac artery compromising the venous outflow in the lower limbs is known as MTS, also known as iliac vein compression syndrome 14. Towards the end of the 18th century, Virchow, in an attempt to explain the high incidence of left‐sided deep venous thrombosis (DVT), initially referred to the proposed anatomical variant 15. In 1908, McMurrich postulated these strictures, taking a closer look into their pathology without emphasising their clinical features 16. The picture became quite clear thanks to the work of May and Thurner in 1957. They were able to describe the syndrome anatomically by conducting an anatomical dissection study on 430 cadavers, where it yielded significant results. It was found that approximately 22% of the cadavers exhibited an anatomical variant at the level of the left common iliac vein 14. The clinical features of the syndrome were later detailed by Cockett and Thomas in 1965 17. MTS is believed to be an acquired syndrome because of the lack of supportive histological findings and the absence of any anatomical congenital signs 14, 18. The left common iliac vein follows a transverse course unlike the right common iliac vein vertical ascendant path, which predisposes it to the compression effect of the overlying right common iliac artery against the lumbar spine. As a result of the compression exerted on the left common iliac vein and the chronic adjacent arterial pulsations intra‐luminal changes can occur, because of the accumulation of elastin and collagen occur, leading to the formation of obstructive lesions known as ‘spurs’, hindering the venous outflow to the lower extremities 19, 20. MTS is predominantly seen among young to middle‐aged female patients presenting with unilateral chronic venous outflow obstruction symptoms 19, 21. The actual incidence of MTS is unknown 22. Clinical presentations of the syndrome can vary from complete symptom‐free cases, which represent the majority of patients, to more acute presentations of DVT or its debilitating complications. On the other hand, others might only present with symptoms and signs of chronic venous outflow obstruction, like in our patient's case. It was estimated that almost 25% of healthy asymptomatic individuals can have >50% compression within the left iliac vein 23, which again confirms that many cases can go under‐diagnosed and are incidentally detected. It is believed that the development of symptoms occurs mainly following specific provoking triggers in asymptomatic patients, for instance prolonged immobilisation, operative procedures 24or pregnancy, causing significant haemodynamic instability in the venous flow and presenting in either acute or chronic forms. MTS can be easily under‐diagnosed in the presence of other more common risk factors. It is true that doppler ultrasound remains the mainstay gold standard method in diagnosing lower limbs venous insufficiency 25, 26, 27. However, as venous incompetence is not restricted only to the lower limbs level and the fact that the iliac veins are deeply seated within the pelvic cavity, the sensitivity of the duplex ultrasound study in visualising any insufficiency within these veins above the inguinal line will be limited 28. Further investigations using other modalities like computed contrast venography or magnetic resonance venography can assist in precisely clarifying the anatomical pattern and spotting any variants 29. Unexplained cases of unilateral venous symptoms, especially among young or middle‐aged group of females, should be dealt with care within a well‐integrated multidisciplinary team, aiming to alleviate the mechanical obstructed venous outflow and restoring in‐line patency to the venous system.
Conclusion
Our case report highlights an important aspect in the clinical assessment of patients with CVI, particularly when it manifests as a refractory venous leg ulcer. Given the socioeconomic impact and the effects of these ulcers on patients' quality of life, an active approach and an inclusion of unusual causes of CVI, such as MTS, in the clinical evaluation algorithm is warranted. Therefore, it is recommended that all cases of suspected CVI that demonstrated normal lower extremities venous duplex sonographic findings should undergo further comprehensive evaluation of the truncal venous system using other modalities.
[Correction added on 26 April 2017, after online publication: The author made corrections on the texts.]
References
- 1. Jockenhöfer F, Gollnick H, Herberger K, Isbary G, Renner R, Stücker M, Valesky E, Wollina U, Weichenthal M, Karrer S, Kuepper B, Roesch A, Dissemond J. Aetiology, comorbidities and cofactors of chronic leg ulcers ‐ Retrospective evaluation of 1,000 patients from 10 specialised dermatological wound care centers in Germany. Int Wound J 2016;13:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruckley CV. Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology 1997;48:67–9. [DOI] [PubMed] [Google Scholar]
- 3. Green J, Jester R, Mcklinley R, Pooler A. The impact of chronic venous leg ulcers: a systematic review. J Wound Care 2014;23:601–12. [DOI] [PubMed] [Google Scholar]
- 4. Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, Symonds T. Measuring the impact of venous leg ulcer on quality of life. J Wound Care 2005;14:53–7. [DOI] [PubMed] [Google Scholar]
- 5. Dissemond J, Bültemann A, Gerber V, Jäger B, Münter C, Kröger K. Definitionen für die Wundbehandlung. Hautarzt 2016;67:265–6. [DOI] [PubMed] [Google Scholar]
- 6. PurwinsS HK, Debus ES, Rustenbach SJ, Pelzer P, Rabe E, Schäfer E, Stadler R, Augustin M. Cost‐of‐illness of chronic leg ulcers in Germany. Int Wound J 2010;7:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rabe E, Pannier‐Fischer F, Bromen K, Schuldt K, Stang A, Poncar C, Wittenhorst M, Bock E, Weber S, Jöckel KH. Bonner Venenstudie der deutschen Gesellschaft für Phlebologie. Epidemiologische Untersuchung zur Frage der Häufigkeit und Ausprägung von chronischen Venenkrankheiten in der städtischen und ländlichen Wohnbevölkerung. Phlebologie 2003;32:1–14. [Google Scholar]
- 8. Körber A, Klode J, Al‐Benna S, Wax C, Schadendorf D, Steinstraesser L, Dissemond J. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Dtsch Dermatol Ges 2011;9:116–21. [DOI] [PubMed] [Google Scholar]
- 9. Dale JJ, Callam MJ, Ruckley CV, Harper DR, Berry PN. Chronic ulcers of the leg: a study of prevalence in a Scottish community. Health Bull 1983;41:310–4. [PubMed] [Google Scholar]
- 10. Baker SR, Stacey MC, Jopp‐McKay AG, Hoskin SE, Thompson PJ. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864–7. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien JF, Grace PA, Perry IJ, Burke PE. Prevalence and aetiology of leg ulcers in Ireland. Ir J Med Sci 2000;169:110–2. [DOI] [PubMed] [Google Scholar]
- 12. Apollonio A, Antignani PL, Di Salvo M, Failla G, Guamera G, Mosti G, Ricci E, SUV Study Group . A large Italian observational multicentre study on vascular ulcers of the lower limbs (StudioUlcereVascolari). Int Wound J 2016;13:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: a dual case‐control study. J Vasc Surg 1995;22:622–8. [DOI] [PubMed] [Google Scholar]
- 14. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419–27. [DOI] [PubMed] [Google Scholar]
- 15. Virchow R. Über die Erweiterung kleiner Gefasse. Arch Path Anat 1851;3:427. [Google Scholar]
- 16. McMurrich JP. The occurrence of congenital adhesions in the common iliac veins and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908;135:342–6. [Google Scholar]
- 17. Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg 1965;52:816–21. [DOI] [PubMed] [Google Scholar]
- 18. Ehrich WE, Krumbhaar EB. A frequent obstructive anomaly of the mouth of the leftcommon iliac vein. Am Heart J 1943;26:18–31. [Google Scholar]
- 19. Oguzkurt L, Ozkan U, Tercan F, Koc Z. Ultrasonographic diagnosis of iliac vein compression (May‐Thurner) syndrome. Diagn Interv Radiol 2007;13:152–5. [PubMed] [Google Scholar]
- 20. Baron HC, Sharms J, Wayne M. Iliac vein compression syndrome: a new method of treatment. Am Surg 2000;66:653–5. [PubMed] [Google Scholar]
- 21. Butros SR, Liu R, Oliveira GR, Ganguli S, Kalva S. Venous compression syndromes: clinical features, imaging findings and management. Br J Radiol 2013;86:20130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasirajan K, Gray B, Ouriel K. Percutaneous Angiojetthrombectomy in the management of extensive deep vein thrombosis. J Vasc Interv Radiol 2001;12:179–85. [DOI] [PubMed] [Google Scholar]
- 23. Oguzkurt L, Ozkan U, Ulusan S, Koc Z, Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2008;19:366–70. [DOI] [PubMed] [Google Scholar]
- 24. Im S, Lim SH, Chun HJ, Ko YJ, Yang BW, Kim HW. Leg edema with deep venous thrombosis‐like symptoms as an unusual complication of occult bladder distension and right May‐Thurner syndrome in a stroke patient: a case report. Arch Phys Med Rehabil 2009;90:886–90. [DOI] [PubMed] [Google Scholar]
- 25. Campbell WB, Halim AS, Aertssen A, Ridler BM, Thompson JF, Niblett PG. The place of duplex scanning for varicose veins and common venous problems. Ann R Coll Surg Engl 1996;78:490–3. [PMC free article] [PubMed] [Google Scholar]
- 26. Androulakis AE, Giannoukas AD, Labropoulos N, Katsamouris A, Nicolaides AN. The impact of duplex scanning on vascular practice. Int Angiol 1996;15:283–90. [PubMed] [Google Scholar]
- 27. Grabs AJ, Wakely MC, Nyamekye I, Ghauri AS, Poskitt KR. Colour duplex ultrasonography in the rational management of chronic venous leg ulcers. Br J Surg 1996;83:1380–2. [DOI] [PubMed] [Google Scholar]
- 28. Shebel ND, Whalen CC. Diagnosis and management of iliac vein compression syndrome. J Vasc Nurs 2005;23:10–7. [DOI] [PubMed] [Google Scholar]
- 29. Kalu S, Shah P, Natarajan A, Nwankwo N, Mustafa U, Hussain N. May‐Thurner syndrome: a case report and review of the literature. Case Rep Vasc Med 2013;2013:740182. [DOI] [PMC free article] [PubMed] [Google Scholar]


