Abstract
Anti‐infectives used to treat chronic exuding wounds are diluted by wound exudates, absorbed into dressings, metabolised by proteases and destroyed by pH. In order to mimic such effects of exudates, the efficacy of six topical wound agents was assessed undiluted and at 10% concentrations, including povidone‐iodine ointment and a silver‐impregnated wound dressing, to remove biofilms of Pseudomonas aeruginosa, multi‐species biofilms of Candida albicans and methicillin‐resistant Staphylococcus aureus (MRSA) in vitro in a Centers for Disease Control and Prevention (CDC) reactor. Povidone‐iodine was also diluted to 3·3% and 33·3% of the commercial concentrations. Viable microorganisms in each preparation were quantified by colony count. No viable P. aeruginosa biofilm material was recovered after 4 and 24 hours of treatment with povidone‐iodine ointment at the 100% and 10% concentrations. No C. albicans/MRSA biofilm material was recovered after 4 and 24 hours of treatment with povidone‐iodine ointment at the 100% concentration. In general, following dilution, povidone‐iodine ointment appeared to exhibit greater biofilm removal than the other agents tested. Further research involving different microorganisms in vitro and in vivo over a longer period of time will help elucidate the full potential of povidone‐iodine ointment and liposomal hydrogel.
Keywords: Biofilm, Candida albicans, Methicillin‐resistant Staphylococcus aureus (MRSA), Povidone‐iodine ointment (PVP‐I), Pseudomonas aeruginosa
Introduction
Effective wound treatment requires the establishment of a microenvironment in which the levels of pathogenic microorganisms are reduced to a level that can be appropriately managed by the host's immune system, allowing for progression from the inflammatory phase of wound healing. Appropriate termination of the inflammatory phase stimulates the progressive stages of wound repair, such as re‐epithelialisation, and allows for the wound to heal in a timely manner 1, 2, 3. Wound healing is delayed by infection, which begins when pathogenic bacteria colonise the wound, usually within 6 hours following a superficial lesion to the epithelia 2. This colonisation typically involves aerobic or facultative gram‐negative bacteria such as Pseudomonas aeruginosa (P. aeruginosa), gram‐positive bacteria such as Staphylococcus aureus (S. aureus) and β‐haemolytic streptococci, which cause extensive deterioration and damage to the wound 4, 5. Most bacteria live in biofilms, bacterial communities enveloped in a protective extracellular polysaccharide matrix, which facilitate attachment to surfaces, survival and proliferation 6, 7. Microbial biofilms within chronic wounds can be difficult to eradicate with currently available anti‐infectives and anti‐infective regimes.
The role of biofilms in chronic wounds has received much interest. Chronic wounds contain damaged tissue and proteins, which can allow bacteria to attach and proliferate into biofilms. Well‐known chronic wound pathogens, including methicillin‐resistant S. aureus (MRSA) and P. aeruginosa, are typical biofilm producers. Bacteria encased in biofilms are 50‐1000 times more resistant to conventional antibiotic treatment because of their resolute physical and physiological decrease in susceptibility to antimicrobial agents including antibiotics 8. Appropriate antimicrobial management and wound dressings are required to control microbiological colonisation and prevent infection 9. Dressings containing silver have been shown to help prevent biofilm formation, but not all have been effective because of the variability in silver concentration, silver format and delivery mechanisms 10.
Iodine has been shown to disrupt S. aureus biofilms 11, 12. Iodine has been used for over 150 years to prevent infection in the treatment of wounds. Povidone‐iodine has been used as a topical treatment since the 1950s 13 because of its broad antimicrobial spectrum and highly effective treatment in a variety of wounds 14, and to date, no resistance has been demonstrated 15. Interest in the use of iodine for biofilms in chronic wounds has grown over the last two decades. Studies have shown povidone‐iodine and cadexomer iodine to exhibit antibacterial activity, particularly against the Pseudomonas species and S. aureus that are prevalent in biofilms, while simultaneously facilitating healing 16, 17, 18. Povidone‐iodine has many characteristics that support its place at the forefront of treating epithelial trauma. It exhibits a pro‐oxidant effect in healing 19, is better tolerated than silver sulphadiazine or chlorhexidine on histological appraisal of wound healing 20 and will enhance angiogenesis 21. It also supports healing by a distinct anti‐inflammatory effect on cytokines, T lymphocytes and macrophages and scavenging reactive oxygen species 22, 23. Povidone‐iodine also may inhibit excessive protease levels in chronic, non‐healing wounds and, when present, is known to further delay wound closure 24. As with many wound healing treatments that face the challenge of being antimicrobial whilst supporting healthy wound healing, there is some discussion within the literature regarding povidone‐iodine. Some in vitro data have indicated 10% povidone‐iodine to be toxic to cells involved in wound repair, such as fibroblasts and keratinocytes 25; however, this was not observed clinically, for example, in a sensitive wound 26. Furthermore, a 3% liposomal povidone‐iodine hydrogel has been shown to promote optimal wound healing 27, 28.
Topical agents including povidone‐iodine will often be diluted or deactivated by wound exudates, absorbed into dressings, metabolised by proteases and destroyed by pH. These agents should not only be tested at marketed concentrations but also after dilution to examine their limitations. Antibacterial agents that demonstrate a significant reduction in activity following dilution should be considered with caution for the treatment of chronic exuding wounds. The aim of this study was to assess the efficacy of diluted povidone‐iodine ointment PVP‐I ointment (PVP‐I) versus six diluted topical wound agents and a silver‐impregnated wound dressing to remove biofilms of P. aeruginosa and multi‐species biofilms of Candida albicans (C. albicans) and MRSA in vitro.
Methods
Test microorganisms
Three microorganisms were tested: P. aeruginosa (NCIMB 10434), MRSA (308) and C. albicans (ATCC MYA 2876).
Agents tested
The agents used are listed in Table 1. They were tested at both 100% and 10% concentrations, with the exception of PVP‐I, which was additionally tested at 3·3% and 33%. Phosphate‐buffered saline (PBS) was used as a control.
Table 1.
Test agents and agent concentrations used in the study
| Agent name | Agent format | Active agent | Concentration tested (%) |
|---|---|---|---|
| Mupirocin | Ointment | 2% mupirocin | 10 and 100 |
| Fusidic acid | Cream/ointment | 2% fusidic Acid | 10 and 100 |
| Bacitracin, neomycin, and polymyxin B | Ointment | Bacitracin polymyxin B neomycin | 10 and 100 |
| Polyhexanide/betaine | Hydrogel | 0·1% Polyhexanide/betaine PVP‐iodine | 10 and 100 |
| Octenidine dihydrochloride | Hydrogel | Octenidine | 10 and 100 |
| Povidone‐iodine ointment (PVP‐I) | Ointment | 10% PVP‐iodine | 3·3, 10, 33 and 100 |
| PVP‐I liposomal hydrogel | Hydrogel | 3% PVP‐iodine | 10 and 100 |
| Chlorhexidine gluconate | Liquid | 0·1% chlorhexidine | 10 and 100 |
| Silver dressing | Dressing | Silver nanocrystals | 100 |
| Phosphate‐buffered saline (PBS) | Liquid | N/A | 100 |
Preparation of inoculum and biofilm formation
The test method used throughout this study was a modification of the international standard E2871 29, a test method designed to evaluate disinfectant efficacy of biofilms grows in a Centers for Disease Control and Prevention (CDC) reactor. Modifications were made to the test method in order to assess a range of microorganisms and the various test agents described. Twenty‐four‐hour cultures of P. aeruginosa and MRSA were harvested from tryptone soya agar (TSA) plates and re‐suspended in 20 ml of tryptone soya broth (TSB) and 20 ml of RPMI medium, respectively; a 24‐hour culture of C. albicans was harvested from a Sabouraud dextrose agar plate using a sterile swab and re‐suspended in 20 ml of RPMI medium. P. aeruginosa and MRSA suspensions were diluted to give OD590s of 0·13 ± 0·03 (∼108 cfu/ml) and 0·1 ± 0·02 (∼108 cfu/ml), respectively; C. albicans was diluted to give an OD590 of 0·14 ± 0·02 (∼108 cfu/ml). The microbial suspensions were further diluted in their respective buffers (each containing 1% foetal bovine serum) and used to inoculate the single‐species CDC reactors containing polycarbonate test coupons. The initial concentration for each inoculum was ∼5 × 107 cfu/ml. The CDC reactor was incubated for 48 hours at 37°C, with shaking at 50 rpm in order to encourage biofilm growth.
Mixed‐species reactor
The MRSA and C. albicans suspensions described above were mixed 1:1 and used to inoculate the mixed‐species CDC reactors. The CDC reactor was incubated for 48 hours at 30°C, with shaking at 50 rpm in order to encourage multi‐species biofilm growth.
Test coupons were rinsed thrice in PBS to remove planktonic isolates. The remaining viable organisms were then recovered from the coupons following 15 minutes of sonication and quantified by serial dilution. Multiple rinse steps removed the majority of planktonic organisms in order to ensure that the study was carried out on attached microorganisms only. Throughout this paper, the term ‘biofilm’ refers to bacteria that have attached to a surface so that they are not easily removed by rinsing. Irreversible attachment is the second stage in biofilm formation and is used in international test methods E2647‐13 30, E2799‐12 31 and E2871‐13 29 as a repeatable indicator of biofilm formation. These methods do not attempt to measure metabolic reduction or to quantify the glycocalyx.
Biofilm treatment
Agents were used to treat 48‐hour P. aeruginosa, MRSA and C. albicans biofilms. All agents were applied to encase the biofilm within the treatment. All agents except the control, chlorhexidine gluconate and the nanocrystalline silver dressing were applied as gel formulations (1 g aliquots of each agent were weighed into a petri dish, a coupon was placed onto the agent and then covered by another 1 g aliquot); chlorhexidine gluconate and PBS controls were used as 2 ml aliquots, and the silver dressing was cut into 2 cm2 pieces. The silver dressing was activated prior to testing by the addition of 250 µl of sterile distilled water (P. aeruginosa) and PBS (MRSA and C. albicans) to each 2 cm2 sample. The test agents were diluted in PBS to obtain the 3·3%, 10% and 33% samples and used as 2 ml aliquots. The agents were left in contact with the biofilm for either 4 or 24 hours at 37°C (P. aeruginosa) and 30°C (MRSA and C. albicans). While the test agent formats varied, all agents were applied as per manufacturer's instructions and then diluted as described above. All samples were tested in triplicate.
Statistical methods
Data were reported as log reductions, and, where appropriate, a two‐tailed Student's t‐test was used to determine significant results.
Results
Pseudomonas aeruginosa biofilms
No viable P. aeruginosa was recovered after 4 and 24 hours of treatment with PVP‐I at the 100% and 10% concentrations (Figures 1 and 2), displaying a >6‐log reduction versus the PBS control at 24 hours. Similar results were seen with PVP‐I liposomal hydrogel (PVP‐ILH), polyhexanide/betaine (PHB), bacitracin, neomycin, polymyxin B (BNP), octenidine dihydrochloride and chlorhexidine gluconate, where no viable P. aeruginosa was recovered at both 100% and 10% concentrations after 4 and 24 hours. Furthermore, no viable P. aeruginosa was recovered following PVP‐I treatment at the 3·3% and 33% concentrations. Mupirocin treatment resulted in no recovery of viable bacteria following 4 and 24 hours at the 100% concentration, but its efficacy was negated at the 10% concentration because no difference was observed in the colony count versus the PBS control at both time points. Fusidic acid showed no recovery of biofilm bacteria following 24 hours of treatment at the 100% concentration, but its efficacy also was negated at the 10% concentration because no difference was observed versus the PBS control at both 4 and 24 hours. Treatment with the silver dressing did not reduce bacterial recovery after 4 hours of treatment, with a 1‐log reduction versus the PBS control, but there was a significant 3‐log reduction versus the PBS control at 24 hours.
Figure 1.
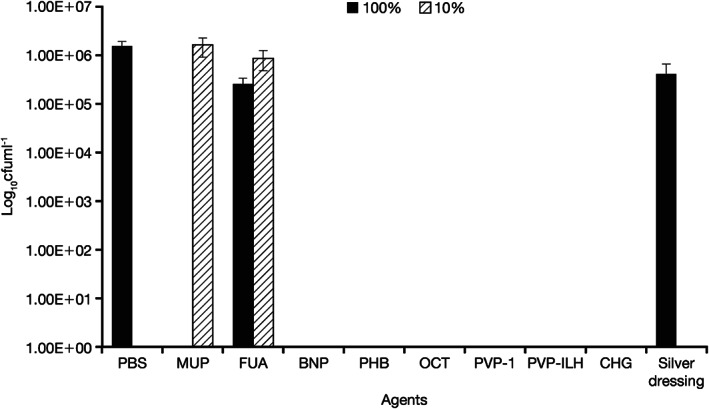
Quantity of viable Pseudomonas aeruginosa recovered from coupons following 4‐hour treatment with all agents, at the commercial concentrations (100%) and at 10% of the commercial concentration. Silver dressing was tested at 100% only. MUP, mupirocin; FUA, fusidic acid; BNP, bacitracin, neomycin, and polymyxin B; PHB, polyhexanide/betaine; OCT, octenidine dihydrochloride; PVP‐I, povidone‐iodine ointment; PVP‐ILH, PVP‐I liposomal hydrogel; CHG, chlorhexidine gluconate; PBS, phosphate buffer solution.
Figure 2.
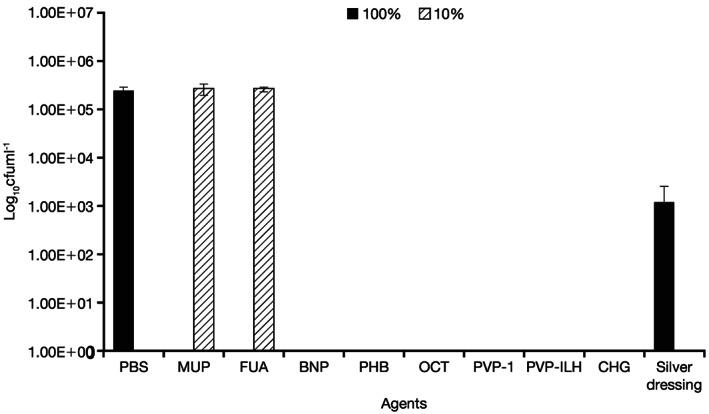
Quantity of viable Pseudomonas aeruginosa recovered from coupons after 24‐hour treatment with all agents, at the commercial concentrations (100%) and at 10% of the commercial concentration. Silver dressing was tested at 100% only. MUP, mupirocin; FUA, fusidic acid; BNP, bacitracin, neomycin, and polymyxin B; PHB, polyhexanide/betaine; OCT, octenidine dihydrochloride; PVP‐I, povidone‐iodine ointment; PVP‐ILH, PVP‐I liposomal hydrogel; CHG, chlorhexidine gluconate; PBS, phosphate buffer solution.
Candida albicans/MRSA biofilms
No C. albicans/MRSA biofilm material was recovered after 4 and 24 hours of treatment with PVP‐I at the 100% concentration (Figures 3 and 4). Treatment with PVP‐I showed a >5‐log reduction versus the PBS control. Similar results were seen with PVP‐ILH, BNP, PHB, octenidine dihydrochloride and chlorhexidine gluconate, where no viable C. albicans/MRSA was recovered at the 100% concentration after 4 and 24 hours. Mupirocin and fusidic acid were considerably less effective in removing viable cultures from the multi‐species biofilm at 4 and 24 hours at 100% concentrations.
Figure 3.
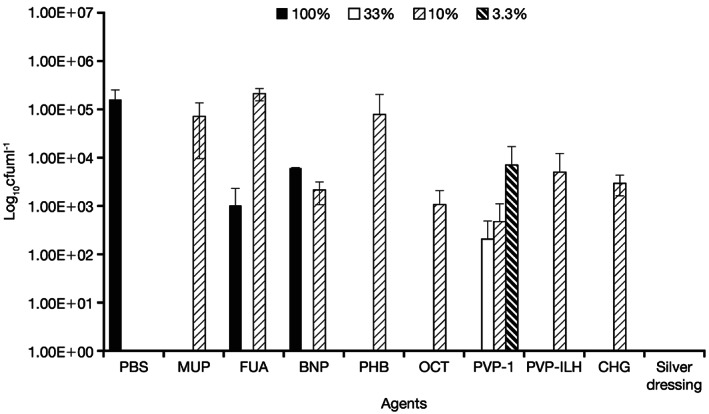
Quantity of viable Candida albicans/MRSA recovered from coupons following 4‐hour treatment with all agents, at the commercial concentrations (100%) and at 10% of the commercial concentration. Silver dressing was tested at 100% only. The 33% and 3·3% concentrations of PVP‐I are also presented. MRSA, methicillin‐resistant Staphylococcus aureus; MUP, mupirocin; FUA, fusidic acid; BNP, bacitracin, neomycin, and polymyxin B; PHB, polyhexanide/betaine; OCT, octenidine dihydrochloride; PVP‐I, povidone‐iodine ointment; PVP‐ILH, PVP‐I liposomal hydrogel; CHG, chlorhexidine gluconate; PBS, phosphate buffer solution.
Figure 4.
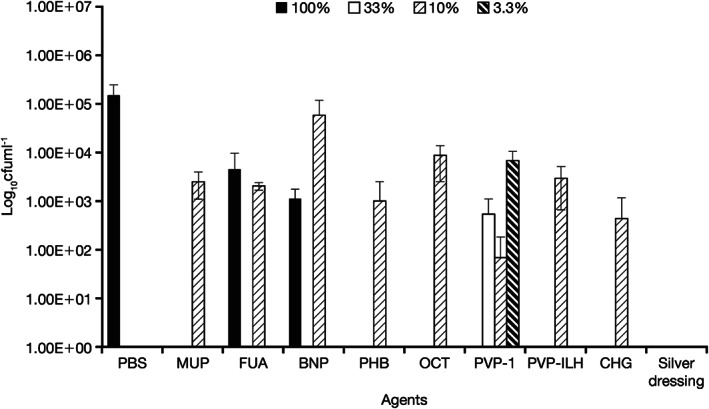
Quantity of viable Candida albicans/MRSA recovered from coupons after 24‐hour treatment with all agents, at the commercial concentrations (100%) and at 10% of the commercial concentration. Silver dressing was tested at 100% only. The 33% and 3·3% concentrations of PVP‐I are also presented. MRSA, methicillin‐resistant Staphylococcus aureus; MUP, mupirocin; FUA, fusidic acid; BNP, bacitracin, neomycin, and polymyxin B; PHB, polyhexanide/betaine; OCT, octenidine dihydrochloride; PVP‐I, povidone‐iodine ointment; PVP‐ILH, PVP‐I liposomal hydrogel; CG, chlorhexidine gluconate; PBS, phosphate buffer solution.
At the 10% concentration, the efficacy of PVP‐I was reduced at 4 hours, but after 24 hours, negligible viable biofilm material was detected. At the 10% concentration, PVP‐ILH showed a >1‐log reduction in mixed biofilm material at both 4 and 24 hours, similar to findings with the other topical preparations. Compared with 10% PVP‐I, mupirocin, fusidic acid, BNP, PHB, octenidine dihydrochloride and chlorhexidine gluconate at 10% concentrations were up to 3 logs less effective in removing viable cultures from the multi‐species biofilm at 4 and 24 hours.
Comparison of PVP‐I across concentrations
No viable P. aeruginosa biofilm was found at either time point across all concentrations for PVP‐I. However, this was not the case for the multi‐species C. albicans/MRSA biofilm. At 4 hours (Figure 5), the antibacterial activity of PVP‐I was the lowest at the 33% concentration and at the 3·3% concentration was <2‐log lower versus the PBS control. At 24 hours (Figure 6), the antibacterial activity of PVP‐I was the lowest at the 10% concentration and at the 3·3% concentration was >1‐log lower versus the PBS control. Furthermore, the antibacterial activity of 10% PVP‐I was greater than the 3% concentration after 24 hours.
Figure 5.
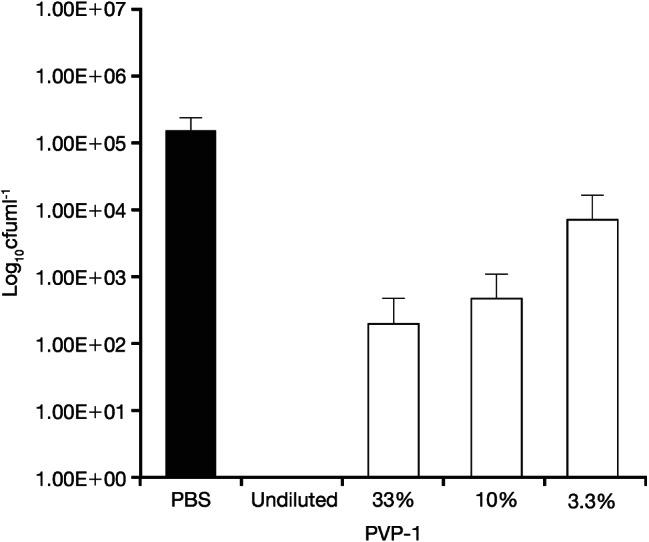
Quantity of viable Candida albicans/MRSA recovered from coupons after 4‐hour treatment with PVP‐I at standard concentration and different percentage concentrations. PVP‐I, povidone‐iodine ointment.
Figure 6.
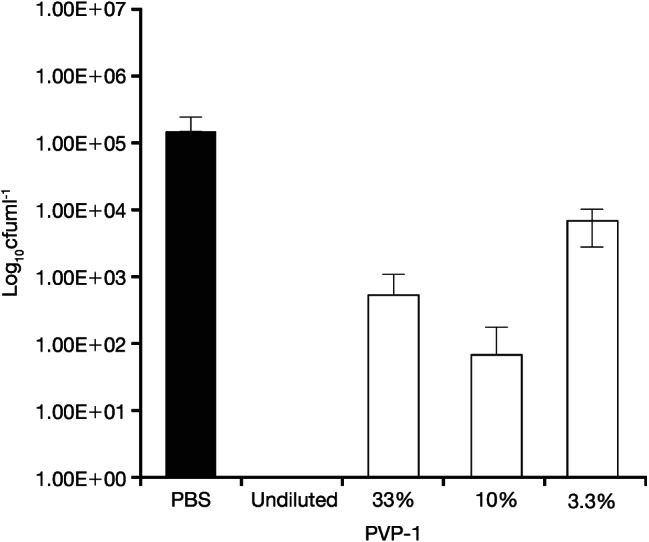
Quantity of viable Candida albicans/MRSA recovered from coupons after 24‐hour treatment with PVP‐I at standard concentration and different percentage concentrations. PVP‐I: povidone‐iodine ointment.
Discussion
Many anti‐infectives adequately eradicate bacterial biofilms from wounds at their original concentrations. However, efficacy is challenged following dilution, for example, by chronic wound exudates, especially with prolonged increased moisture in the wound, heightened risks of an infection and compromised efficacy of the diluted antimicrobial product. This study was performed to assess the efficacy of PVP‐I against biofilm formation in vitro, at its original concentration and in diluted form, versus six other anti‐infectives and a silver‐impregnated wound dressing. Most of the agents tested were effective against biofilm formation at their original concentrations (except mupirocin, fusidic acid and the silver dressing). However, following dilution, PVP‐I was shown to be equivalent to, and in many cases better than, its competitors in the eradication of bacterial biofilm over 24 hours. This highlighted its potential to be used as treatment of choice of highly exuding chronic biofilm‐infected wounds.
PVP‐I was highly efficacious when tested against P. aeruginosa, C. albicans and MRSA at both 4 and 24 hours. Extrapolating these data into the clinical setting indicates that a single application need only be applied in a 24‐hour period; however, this would require further clinical investigation for confirmation. This would not only support the efficient treatment of infected wounds with PVP‐I but also demonstrate the potential improvement in the quality of life of the patients being treated. All agents were less effective against C. albicans/MRSA biofilms compared to P. aeruginosa biofilms, probably because of the physical and physiological superiority of the multi‐species biofilms. In vivo, bacteria and fungi can coexist in wounds to form multi‐species biofilms. The efficacy of PVP‐ILH in wound healing of colonised wounds has already been reported 27. The PVP‐ILH formulation showed a faster wound‐healing time with a better quality of wound healing 27, 28. In our study, PVP‐ILH worked effectively at all concentrations across both time points. However, PVP‐I was again more effective at removing both biofilms after 24 hours following dilution. Furthermore, at the 10% concentration, PVP‐I showed better biofilm removal than PVP‐ILH. The lower efficacy in this in vitro model may differentiate the 3% PVP‐I formulation for wounds with a low burden of pathogens and the 10% PVP‐I formulation for wounds with clinical signs of infection.
Fusidic acid is a topically applied bacteriostatic antibiotic; however, its use has been controversial based on many studies that have reported the development of resistance to S. aureus, thereby negating its use against MRSA 32, 33, 34, 35, 36, 37, 38. After 4 hours, 100% fusidic acid exhibited the least anti‐biofilm efficacy among the agents tested, showing similar bacterial biofilm recovery to the PBS control. Efficacy improved to complete the elimination of bacteria material after 24 hours, demonstrating the slow antimicrobial onset of action compared to some of the other agents. At the 10% concentration, again, the results were similar to the PBS control at both time points.
The efficacy of BNP topical against P. aeruginosa has previously been reported 39, 40, and again, it was highly effective in our study against P. aeruginosa and C. albicans/MRSA biofilms at its original concentration at both time points. However, dilution to 10% negated its activity against the multi‐species biofilm at both time points, highlighting its limitations in the treatment of chronic exuding wounds. Furthermore, resistance to BNP has also been reported in MRSA 41.
The efficacy and tolerability of PHB in bacterial burden control has previously been demonstrated 39, 42. In our study, PHB was shown to be highly efficacious at its original concentration and at the 10% concentration against P. aeruginosa at both time points. However, dilution to 10% negated its activity against the multi‐species biofilm at both time points, highlighting its potential shortcomings in the treatment of exuding wounds.
Mupirocin is an antibiotic particularly effective against gram‐positive bacteria, including MRSA 43; however, it must be used with caution because prolonged use may lead to bacterial resistance 44. At the 10% concentration, mupirocin exhibited similar results to the PBS control at both time points against both biofilms, lessening the potency for treatment of chronic exuding wounds.
Chlorhexidine gluconate is a widely used antiseptic and has previously been compared to PVP‐I in prevention of infection following a caesarean section, where it was associated with lower rates of bacterial growth after 18 hours 45. In our study, chlorhexidine gluconate again displayed efficacy against both biofilms over both time points. However, at the 10% concentration, PVP‐I again showed superior biofilm‐removal capacity compared with chlorhexidine gluconate. This supports the clinical observation made in venous leg ulcers where PVP‐I was also more efficacious than chlorhexidine gluconate 20. Chlorhexidine gluconate showed excellent activity against both biofilms at both time points at its original concentration and following 10% dilution. However, compared with PVP‐I at the 10% concentration, it was <1‐log less effective at removing the P. aeruginosa biofilm.
Octenidine dihydrochloride has been highly successful in vivo, significantly lowering bacterial colonisation of skin graft donor sites 46. However, the octenidine group required more time to full wound closure and was statistically not significant. In a similar wound, PVP‐I showed a reduction in bacterial count with a better trend for wound closure 26.
The nanocrystalline silver dressing in our study could only be used at its 100% concentration. Nanocrystalline silver dressings have been shown to prevent biofilm formation. However, not all silver dressings are the same because of the different forms of silver used within the dressings 10. Although the nanocrystalline silver dressing was more efficacious against the multi‐species biofilm of C. albicans and MRSA at both time points, its lack of efficacy against P. aeruginosa highlighted its shortcomings in broad spectrum antibacterial activity.
The ultimate goal of using anti‐infective agents in wound care is to control and balance the bacterial bioburden in the wound bed. Bioburden is the amount and types of bacteria in a wound. In this in vitro study, PVP‐I lowered the bioburden of bacteria and biofilm formation in the wound bed across all concentrations at all time points. Translating this efficacy in vivo, the potency of PVP‐I becomes ever more prominent, reducing bacterial biofilm formation while correcting other non‐bacterial wound‐healing parameters. However, although low‐grade contaminated wounds are considered a requisite for optimal wound healing, it has been suggested that sub‐infective levels of bacteria accelerate healing in acute wounds by heightening the immune response with an excess of inflammation and increase of protease production but with risk of damage to the dermal matrix architecture 47. In order to be confident that a sufficiently low pathogen count conducive for wound healing can be achieved in vivo, a significant reduction must be observed in vitro. It should then be considered that the additional variations observed within an in vivo scenario may further decrease the efficacy of any wound care product.
The success of diluted PVP‐I over 24 hours against aerobic microorganisms is an important advantage in its potential to being the anti‐infective agent of choice in the treatment of chronic exuding wounds. Interestingly, the 10% PVP‐I performed better than the 3% concentration against P. aeruginosa after 24 hours. This may not have been a significant result; however, the experiment must be repeated to elucidate whether this outcome was an anomaly or a phenomenon of a lower concentration performing better than those higher. Future research could focus on testing PVP‐I in vitro, ex vivo and in vivo versus biofilms from both aerobic and anaerobic microorganisms. Anaerobic microorganisms make up a large proportion of the microbial population of chronic wounds 48, especially wounds close to orifices that contain microbes consistent with those found at those sites 49. Furthermore, adding bovine serum albumin or sheep erythrocytes in vitro or to an ex vivo pig skin biofilm model will further assess the efficacy of PVP‐I in the treatment of chronic exuding wounds. Another study evaluating the effect of single applications of original and diluted preparations of PVP‐I beyond the 24 hours studied here, for example, 36 and 48 hours, would be of interest in terms of cost benefits to health care authorities and quality of life benefits to patients. Any guideline on biofilm testing should consider probing various exposure times and concentrations in order to asses potential limits of a product, as already implemented in suspension testing 50. Assessing the dilution ratio of products clinically is not a practical option as wounds vary significantly in their size, structure, location and the amount and type of exudate produced. As such, this study and studies such as this are critical to aid the understanding of the potential changes that can occur in the efficacy of a product following dilution by wound exudates.
A limitation of this study may be that a single test method has been utilised to compare a range of product types (i.e. ointments, hydrogels, liquids, dressings). In theory, it would have been possible to directly compare key active agents alone; however, the method by which a product is delivered can vastly alter its efficacy, and thus, the authors aimed to compare clinically relevant treatments in their commercially available formats. Clinically, a wound biofilm may be treated with a range of product types at varying concentrations and for varying durations. This study strictly controlled the formation of the test biofilm and treatment conditions so that the only variables were the test products and the dilution ratios.
In conclusion, PVP‐I exhibited superior bacterial biofilm‐removal capacity following dilution compared to the other agents tested, especially in light of recent literature 51. Repetition of these results for PVP‐I in future research involving different microorganisms in vitro and in vivo over longer periods of time will elucidate the potential of PVP‐I to become the agent of choice to be used in the treatment of chronic exuding wounds. Our data further suggests that any guideline for the testing biofilms should incorporate testing at various concentrations and incubation times to examine the limits of the tested products. This may generate valuable information for health care practitioners and will provide better insight on existing clinical data. Moreover, the potential economic viability of PVP‐I must not be overlooked because a single application of PVP‐I offering anti‐infective activity over a period of 24 hours would reduce the need to reapply PVP‐I, reducing the burden on health care professionals performing the application. Additional parameters further complicate the situation encountered in clinical practice, but a product that delivers a rapid and sustained response is predicted to drive appropriate wound healing and minimise recontamination of the wound bed. A treatment that offers prolonged antimicrobial activity and requires minimal clinician training would ultimately decrease treatment costs whilst supporting appropriate wound healing.
Author Contribution
Matthias Johannes Hoekstra received no revenue for support of this study. He mediated in obtaining a grant by the Burns Research Institute (foundation BRI), Beverwijk, the Netherlands to perform this study. He has been member of the scientific board of Mundipharma Research GmbH & Co KG.
Samantha Westgate is the CEO of Perfectus Biomed Ltd, UK, an independent microbiological services provider.
Stefan Mueller is an employee of Mundipharma Research GmbH & Co KG.
Acknowledgements
This study was funded by Mundipharma Research GmbH & Co KG. Medical writing support was provided by Bilal Bham of Scripsi Scriptum Ltd, UK and funded by Mundipharma Research GmbH & Co KG.
References
- 1. Crovetti G, Martinelli G, Issi M, Barone M, Guizzard M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004;30:145–51. [DOI] [PubMed] [Google Scholar]
- 2. Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis 2004;32:88–94. [DOI] [PubMed] [Google Scholar]
- 3. Bryant RA, Nix DP. Acute and chronic wounds: current management concepts, 4th edn. Mosby, Elsevier, 2011. [Google Scholar]
- 4. Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage 1999;45:23–7, 29–40; quiz 41–2. [PubMed] [Google Scholar]
- 5. Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage 2003;49(Suppl. 7A):1–7. [PubMed] [Google Scholar]
- 6. Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am 1978;238:86–95. [DOI] [PubMed] [Google Scholar]
- 7. Cooper R, Okhiria O. Biofilms, wound infection and the issue of control. Wounds UK 2006;2:48–56. [Google Scholar]
- 8. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 1999;37:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramer A et al. Polypragmasia in the therapy of infected wounds ‐ conclusions drawn from the perspectives of low temperature plasma technology for plasma wound therapy. GMS Krankenhhyg Interdiszip 2008;3:Doc13. [PMC free article] [PubMed] [Google Scholar]
- 10. Driffield K, Woodmansey E., Floyd H, The use of silver‐containing dressings to prevent biofilm formation by single and mixed bacterial flora. Poster presentation #285, EMWA, 2008.
- 11. Kunisada T, Yamada K, Oda S, et al. Investigation on the efficacy of povidone‐iodine against antiseptic‐resistant species. Dermatology 1997;195(2 Suppl):14–8. [DOI] [PubMed] [Google Scholar]
- 12. Akiyama H, Oono T, Saito M. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol 2004;31:529–34. [DOI] [PubMed] [Google Scholar]
- 13. Garnes AL, Davidson E, Taylor LE, et al. Clinical evaluation of povidone‐iodine aerosol spray in surgical practice. Am J Surg 1959;97:49–53. [DOI] [PubMed] [Google Scholar]
- 14. Fleischer W, Reimer K. Povidone‐iodine in antisepsis – state of the art. Dermatology 1997;195(2 Suppl):3–9. [DOI] [PubMed] [Google Scholar]
- 15. Lanker KB, Widmer HR, Frey F. Nondevelopment of resistance by bacteria during hospital use of povidone‐iodine. Dermatology 1997;195(Suppl. 2):10–3. [DOI] [PubMed] [Google Scholar]
- 16. Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds 2003;15:149–66. [Google Scholar]
- 17. Mertz PM, Oliveira‐Gandia MF, Davis SC. The evaluation of a cadexomer iodine wound dressing on methicillin resistant Staphylococcus aureus (MRSA) in acute wounds. Dermatol Surg 1999;25:89–93. [DOI] [PubMed] [Google Scholar]
- 18. Danielsen L, Cherry GW, Harding K, et al. Cadexomer iodine in ulcers colonised by Pseudomonas aeruginosa . J Wound Care 1997;6:169–72. [DOI] [PubMed] [Google Scholar]
- 19. Cooper RA. Iodine revisited. Int Wound J 2007;4:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fumal I, Braham C, Paquet P, et al. The beneficial toxicity paradox of antimicrobials in leg ulcer healing impaired by a polymicrobial flora: a proof‐of‐concept study. Dermatology 2002;204(Suppl. 1):70–4. [DOI] [PubMed] [Google Scholar]
- 21. Bennett LL, Rosenblum RS, Perlov C, et al. An in vivo comparison of topical agents on wound repair. Plast Reconstr Surg 2001;108:675–87. [DOI] [PubMed] [Google Scholar]
- 22. Beukelman CJ, van den Berg AJ, Hoekstra MJ, et al. Anti‐inflammatory properties of a liposomal hydrogel with povidone‐iodine (Repithel) for wound healing in vitro. Burns 2008;34:845–55. [DOI] [PubMed] [Google Scholar]
- 23. Konig B, Reimer K, Fleischer W, et al. Effects of Betaisodona on parameters of host defense. Dermatology 1997;195(2 Suppl):42–8. [DOI] [PubMed] [Google Scholar]
- 24. Eming SA, Smola‐Hess S, Kurschat P, et al. A novel property of povidon‐iodine: inhibition of excessive protease levels in chronic non‐healing wounds. J Invest Dermatol 2006;126:2731–3. [DOI] [PubMed] [Google Scholar]
- 25. Damour O, Hua SZ, Lasne F, et al. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns 1992;18:479–85. [DOI] [PubMed] [Google Scholar]
- 26. Vehmeyer‐Heeman M, Van den Kerckhove E, Gorissen K, et al. Povidone‐iodine ointment: no effect of split skin graft healing time. Burns 2005;31:489–94. [DOI] [PubMed] [Google Scholar]
- 27. Vogt PM, Hauser J, Rossbach O, et al. Polyvinyl pyrrolidone‐iodine liposome hydrogel improves epithelialization by combining moisture and antisepis. A new concept in wound therapy. Wound Repair Regen 2001;9:116–22. [DOI] [PubMed] [Google Scholar]
- 28. Vogt PM, Reimer K, Hauser J, et al. PVP‐iodine in hydrosomes and hydrogel – a novel concept in wound therapy leads to enhanced epithelialization and reduced loss of skin grafts. Burns 2006;32:698–705. [DOI] [PubMed] [Google Scholar]
- 29. ASTM International . ASTM E2871‐13: Standard test method for evaluating disinfectant efficacy against Pseudomonas aeruginosa biofilm grown in CDC biofilm reactor using the single tube method, West Conshohocken, PA, USA, 2013.
- 30. ASTM International . ASTM E2647‐13: Standard test method for quantification of Pseudomonas aeruginosa biofilm grown using drip flow biofilm reactor with low shear and continuous flow, West Conshohocken, PA, USA, 2013.
- 31. ASTM International . ASTM E2799‐12: Standard test method for testing disinfectant efficacy against Pseudomonas aeruginosa biofilm using the MBEC assay, West Conshohocken, PA, USA, 2012.
- 32. Dobie D, Gray J. Fusidic acid resistance in Staphylococcus aureus . Arch Dis Child 2004;89:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown EM, Thomas P. Fusidic acid resistance in Staphylococcus aureus isolates. Lancet 2002;359:803. [DOI] [PubMed] [Google Scholar]
- 34. Brown EM, Wise R. Fusidic acid cream for impetigo. Fusidic acid should be used with restraint. BMJ 2002;324:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Owen SE, Cheesbrough JS. Fusidic acid cream for impetigo. Findings cannot be extrapolated. BMJ 2002;324:1394. [PubMed] [Google Scholar]
- 36. Zadik P, Young N. Fusidic acid cream for impetigo. Resistance trends must be monitored. BMJ 2002;324:1394. [PubMed] [Google Scholar]
- 37. Stoddart B, Collyns T, Denton M. Fusidic acid cream for impetigo. Problem may be clinically important. BMJ 2002;324:1394. [PubMed] [Google Scholar]
- 38. Weston VC, Boswell TC, Finch RG, et al. Fusidic acid cream for impetigo. Emergence of resistance to fusidic acid limits its use. BMJ 2002;324:1394. [PubMed] [Google Scholar]
- 39. Romanelli M, Dini V, Barbanera S, et al. Evaluation of the efficacy and tolerability of a solution containing propyl betaine and polihexanide for wound irrigation. Skin Pharmacol Physiol 2010;23(Suppl):41–4. [DOI] [PubMed] [Google Scholar]
- 40. Minnich KE, Stolarick R, Wilkins RG, et al. The effect of a wound care solution containing polyhexanide and betaine on bacterial counts: results of an in vitro study. Ostomy Wound Manage 2012;58:32–6. [PubMed] [Google Scholar]
- 41. Suzuki M, Yamada K, Nagao M, et al. Antimicrobial ointments and methicillin‐resistant Staphylococcus aureus USA300. Emerg Infect Dis 2011;17:1917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andriessen AE, Eberlein T. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds 2008;20:171–5. [PubMed] [Google Scholar]
- 43. Hughes J, Mellows G. Inhibition of isoleucyl‐transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J 1978;176:305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis 2009;49:935–41. [DOI] [PubMed] [Google Scholar]
- 45. Hadiati DR, Hakimi M, Nurdiati DS, et al. Skin preparation for preventing infection following caesarean section. Cochrane Database Syst Rev 2014;9:CD007462. [DOI] [PubMed] [Google Scholar]
- 46. Eisenbeiß W, Siemers F, Amtsberg G, et al. Prospective, double‐blinded, randomised controlled trial assessing the effect of an Octenidine‐based hydrogel on bacterial colonisation and epithelialization of skin graft wounds in burn patients. Int J Burns Trauma 2012;2:71–9. [PMC free article] [PubMed] [Google Scholar]
- 47. Laato M, Niinikoski J, Lundberg C, et al. Inflammatory reaction and blood flow in experimental wounds inoculated with Staphylococcus aureus . Eur Surg Res 1988;20:33–8. [DOI] [PubMed] [Google Scholar]
- 48. Wall IB, Davies CE, Hill KE, et al. Potential role of anaerobic cocci in impaired human wound healing. Wound Repair Regen 2002;10:346–53. [DOI] [PubMed] [Google Scholar]
- 49. Bowler PG. The 10(5) bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Manage 2003;49:44–53. [PubMed] [Google Scholar]
- 50. Beuth . Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas – Test method and requirements (phase 2, step 1) English version of DIN EN 1276:2010‐01. DIN EN 1276, 2010.
- 51. Percival SL, Finnegan S, Donelli G, et al. Antiseptics for treating infected wounds: efficacy on biofilms and effect of pH. Crit Rev Microbiol 2016;42:293–309. [DOI] [PubMed] [Google Scholar]


