Abstract
According to previous research, adjunctive negative pressure wound therapy (NPWT) can help manage infected wounds when applied along with appropriate debridement and antibiotic therapy as deemed clinically relevant. NPWT not only removes fluid, and reduces oedema, but also promotes perfusion around the wounds. In addition, NPWT may lead to improved graft fixation when used as a bolster, especially in patients who are less compliant or have poor graft fixation that result from using traditional methods. NPWT is a good choice to bolster skin grafts in young, active and less‐compliant patients. We propose an enhanced segmental compartment‐covered technique, which uses NPWT adjunctively as first‐line wound treatment to help manage postoperative infection. Moreover, NPWT promotes granulation tissue formation to prepare the wound bed for subsequent skin graft and may be used as a bolster over the graft, which helps to attain skin graft viability.
Keywords: Burns, Negative pressure wound therapy, Skin grafts, Wound healing
Introduction
Burn patients need immediate care for dehydration, inhalation injury, infection control and nutritional support. In addition, the most important way to increase survival rate is to remove dead tissues and perform escharotomy, followed by skin grafting. During this process, burn patients remain immunosuppressed, hypermetabolic and sensitive to infection and pain. The challenges in caring for patients with extensive burns are managing wound exudate in the early stage and providing postoperative care after skin graft. Optimally, a dressing should be able to protect the wound from external contaminants, prevent wound trauma or damage and reduce patient discomfort caused by dressing changes.
Using negative pressure wound therapy (NPWT) may resolve some of the problems, such as wound contamination associated with extensive exudate produced in the early stage of burn injury. Previous research shows that NPWT promotes perfusion around the wound and also removes exudate and infectious materials 1, 2, 3, 4. Furthermore, when used as a bolster, NPWT has been reported to help promote graft take and may reduce the risk of repeated skin grafting 5, 6, 7, 8. Finally, using NPWT for wounds in burn patients may reduce the time needed for nursing care. We propose an enhanced segmental compartment‐covered technique using NPWT to manage burn patients.
Materials and methods
In 2015, we admitted five patients with extensive burns (three males, two females, average age = 23.6 ± 0.55 years) to the burn intensive care unit (ICU). The patients were injured by a dust explosion (a large amount of colour powder released into the air had ignited). The total body surface area (TBSA) affected was 60–90%. Patients received standard burn care, which included debridement, wound bed preparation with NPWT (V.A.C.® Therapy, KCI, an ACELITY Company, San Antonio, TX) and autologous split‐thickness skin grafts (STSGs) prepared using the Meek technique 9 for graft expansion and bolstered with NPWT (Figures 1 and 2).
Figure 1.
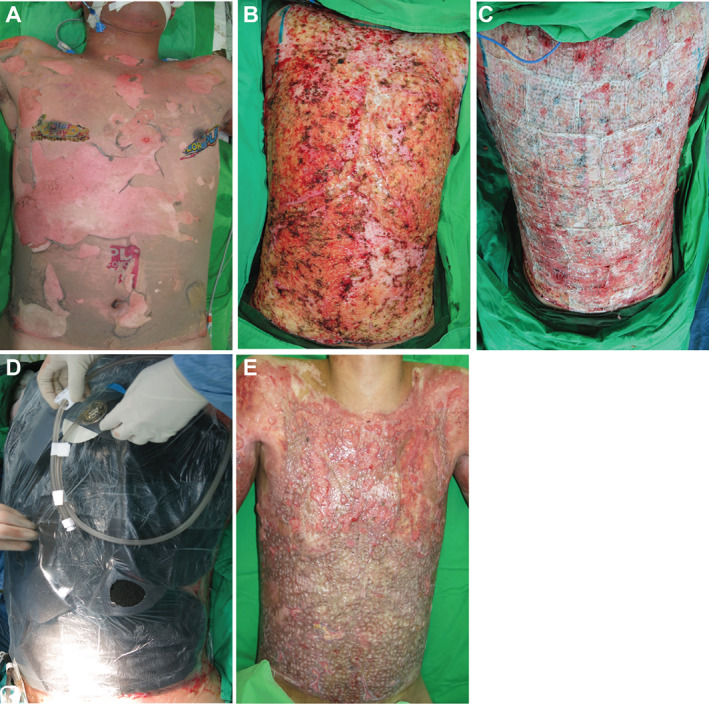
Patient with 90% total burn surface area on the trunk. (A) Trunk burns at presentation; (B) trunk following the first round of surgical debridement; (C) application of Meek technique skin grafts with an expansion ratio of 1:4; (D) application of negative pressure wound therapy (NPWT); (E) trunk after 12 weeks of NPWT.
Figure 2.
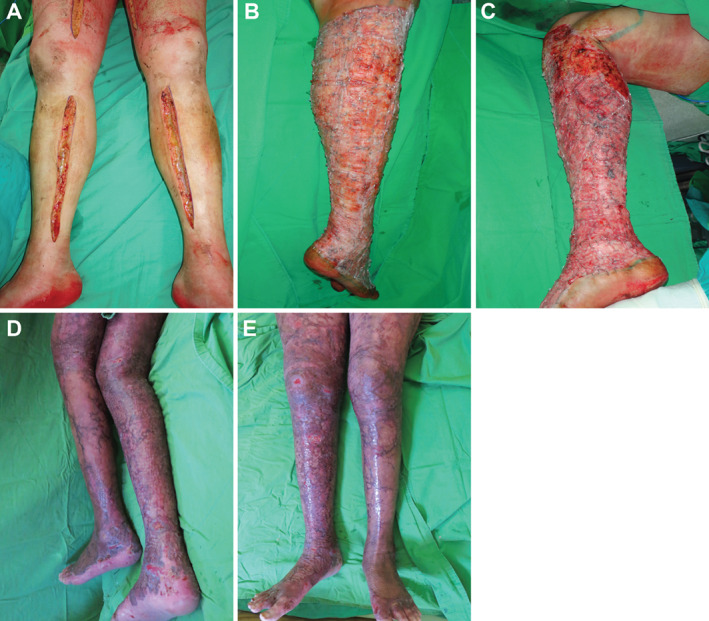
Patient presenting with lower extremity burns. (A) Burns at presentation; (B) right leg following two rounds of surgical debridement; (C) left leg following three rounds of surgical debridement; (D) back of legs following 12 weeks of negative pressure wound therapy (NPWT); (E) front of legs following 12 weeks of NPWT.
NPWT was initiated using a segmental compartment‐cover technique. This technique involved cutting the foam (V.A.C. GranuFoam™ Dressing, KCI, an ACELITY Company) into different shapes and thickness to ensure coverage for all wound sites. A y‐connector (V.A.C.® Y‐Connector; KCI, an ACELITY Company) was also used to ensure that negative pressure was well distributed across all wound sites. The drape covered as much of the patient's peripheral skin as possible. Care had to be taken such that foam was never placed over intact skin without a protective layer. NPWT was set at −125 mmHg with continuous treatment. A non‐adherent dressing (Mepitel® Non‐Adherent Silicone Dressing, Mölnlycke Health Care, Gothenburg, Sweden, or 3M™ Tegaderm™ Non‐Adherent Wound Contact Layer, 3M, Saint Paul, MN) was used as a protective layer when there was exposed bone and tendon. After NPWT application, patients were sent to the ICU. If patients were unable to tolerate treatment because of pain, the negative pressure was decreased by −25 mmHg, with the minimum negative pressure set at −75 mmHg. Dressings were changed twice a week. Once healthy granulation tissue was observed, STSGs were applied. A non‐adherent dressing was used as a protective layer over the STSGs, followed by NPWT using the segmental compartment‐cover technique.
Results
After several rounds of debridement and application of NPWT, healthy granulation tissue was observed. The patients then received skin grafts bolstered with NPWT. All five patients survived and were discharged successfully without requiring re‐grafting. The dressings and drape were able to strengthen the fixation of peripheral areas, and there were no reports of leakage.
Discussion
Patients with extensive burns may have bone and ligament denudation, which is a challenging issue in burn care. Using NPWT may help promote the growth of granulation tissue, which can increase the success rate of skin graft take, especially for those who do not have enough autologous skin. Following surgical debridement, each of our burn patients received NPWT to promote granulation tissue formation and, following skin grafting, to bolster the grafts and promote graft take. NPWT was applied using our method of segmental compartment‐cover technique. All patients survived and were successfully discharged from care without complications.
The segmental compartment‐cover technique to apply the NPWT dressings made it easier to monitor and record wound exudate while allowing the patients to be transferred easily to hospital beds and rehabilitate more comfortably. The segmental compartment‐cover technique was used during both NPWT applications: after surgical debridement and skin grafting. To our knowledge, this is the first publication of this particular NPWT application technique.
The goal of NPWT after debridement was to promote granulation tissue formation and prepare the wound bed for skin grafts. Sahin and colleagues also used NPWT prior to skin grafting in patients (n = 4) with severe burns (TBSA up to 60%) 10. In these patients, healthy granulation tissue was observed in all wounds prior to skin grafting. These results are similar to those observed in our patients, as all five patients developed healthy granulation tissue. It is thought that reducing oedema, removing infectious materials and promoting perfusion and granulation tissue formation in the early stages of burn care may reduce progressive burn injury 1, 7, 11. In addition, this wound bed preparation may decrease the necessity for free flap surgery in patients.
NPWT was used as a bolster following skin grafting in order to help secure the graft to the wound bed and promote graft take. The application of NPWT in this manner has been documented in other studies 12, 13. In one study, patients received STSGs and received either standard dressings (Vaseline or dry gauze) or NPWT for 4 days. Graft take was significantly higher in patients who received NPWT (96.67% versus 87.53%, P < 0.001) 12. In the Waltzman and Bell retrospective study, an average of 99.5% ± 1.5% graft take was observed in burn patients receiving NPWT as a bolster to STSGs 13. Graft take was not recorded in our patients; however, all patients were successfully discharged without requiring re‐grafting, indicating that graft take was sufficient for wound healing.
The use of NPWT in burn patients may help reduce time needed and frequency of dressing changes. This, in turn, has the potential to reduce postoperative infection rates by providing protection from external contaminants and pain in patients. Fewer dressing changes decrease the time the burns are exposed to open air, potentially providing patients with better pain control. However, the efficacy of NPWT relies on appropriate dressing application. Dressing applications with a high number of leaks can affect patient comfort and efficient removal of exudate and infectious materials. Other advanced NPWT treatments, such as NPWT with instillation, may also be beneficial for use in burn patients after the burns have been surgically debrided and prior to skin grafting with the goal of wound cleansing and promotion of granulation tissue formation. However, more evidence of use in severe burns is needed before NPWT with instillation can become an adjunctive part of standard burn care.
Using these techniques during the first week of burn care, health care workers can reduce their workload from changing burn dressings three times a day to every 48–72 hours. As a result, reducing medical expenditures may also have the potential to reduce health care costs. A study by Hop et al. examined costs of various types of grafts with and without NPWT in burn patients 14. In that study, the authors did not find significant differences in the mean total cost per patient between patients with dermal substitutes with or without NPWT and those with STSGs with or without NPWT. However, the patients reported by Hop et al. had lower percentage of TBSA (≤15%) compared with our patients (TBSA 60–90%). More comparative cost studies should be performed using a broader range of TBSA as higher percentages of TBSA can dramatically alter the required patient care.
Our experiences in using NPWT to treat burn patients have been successful, especially those with burns on the hand and other joint locations such as the elbow, knee or ankle. Initial skin grafts in the early stages of healing enable patients to start rehabilitation earlier and, therefore, restore them to their daily life sooner. In these five patients with extensive burns, NPWT was safe and effective.
Acknowledgements
Dr Teng served as a consultant to KCI, an ACELITY Company, and presented as a faculty member at an ACELITY symposium parallel to the 2016 World Union of Wound Healing Societies (WUWHS) conference. This article is a part of an ACELITY‐funded supplement based on the 2016 WUWHS ACELITY symposium presentations. ACELITY provided editorial assistance.
References
- 1. Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 2004;30:253–8. [DOI] [PubMed] [Google Scholar]
- 2. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 3. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 4. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC) device: a review. Am J Clin Dermatol 2005;6:185–94. [DOI] [PubMed] [Google Scholar]
- 5. Blackburn JH, Boemi L, Hall WW, Jeffords K, Hauck RM, Banducci DR, Graham WP. Negative‐pressure dressings as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 6. Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002;137:930–4. [DOI] [PubMed] [Google Scholar]
- 7. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 8. Stone P, Prigozen J, Hofeldt M, Hass S, DeLuca J, Flaherty S. Bolster versus negative pressure wound therapy for securing split‐thickness skin grafts in trauma patients. Wounds 2004;16:219–23. [Google Scholar]
- 9. Lumenta DB, Kamolz LP, Frey M. Adult burn patients with more than 60% TBSA involved‐Meek and other techniques to overcome restricted skin harvest availability – the Viennese concept. J Burn Care Res 2009;30:231–42. [DOI] [PubMed] [Google Scholar]
- 10. Sahin I, Eski M, Acikel C, Kapaj R, Alhan D, Isik S. The role of negative pressure wound therapy in the treatment of fourth‐degree burns. Trends and new horizons. Ann Burns Fire Disasters 2012;25:92–7. [PMC free article] [PubMed] [Google Scholar]
- 11. Banwell PE. Topical negative pressure therapy: advances in burn wound management. Ostomy Wound Manage 2004;50:9S–14. [PubMed] [Google Scholar]
- 12. Petkar KS, Dhanraj P, Kingsly PM, Sreekar H, Lakshmanarao A, Lamba S, Shetty R, Zachariah JR. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split‐thickness skin grafts in burned patients. Burns 2011;37:925–9. [DOI] [PubMed] [Google Scholar]
- 13. Waltzman JT, Bell DE. Vacuum‐assisted closure device as a split‐thickness skin graft bolster in the burn population. J Burn Care Res 2014;35:e338–42. [DOI] [PubMed] [Google Scholar]
- 14. Hop MJ, Bloemen MC, van Baar ME, Nieuwenhuis MK, van Zuijlen PP, Polinder S, Middelkoop E. Cost study of dermal substitutes and topical negative pressure in the surgical treatment of burns. Burns 2014;40:388–96. [DOI] [PubMed] [Google Scholar]


