Abstract
The systemic host defence mechanisms, especially innate immunity, in venous leg ulcer patients are poorly investigated. The aim of the current study was to measure Candida albicans killing activity and gene expressions of pro‐ and anti‐inflammatory cytokines and innate immune response regulators, TAM receptors and ligands of peripheral blood mononuclear cells separated from 69 venous leg ulcer patients and 42 control probands. Leg ulcer patients were stratified into responder and non‐responder groups on the basis of wound healing properties. No statistical differences were found in Candida killing among controls, responders and non‐responders. Circulating blood mononuclear cells of patients overexpress pro‐inflammatory (IL‐1α, TNFα, CXCL‐8) and anti‐inflammatory (IL‐10) cytokines as well as TAM receptors (Tyro, Axl, MerTK) and their ligands Gas6 and Protein S compared with those of control individuals. IL‐1α is notably overexpressed in venous leg ulcer treatment non‐responders; in contrast, Axl gene expression is robustly stronger among responders. These markers may be considered as candidates for the prediction of treatment response among venous leg ulcer patients.
Keywords: Cytokine, Inflammation, Innate immunity, TAM receptor, Venous leg ulcer
Introduction
High ambulatory venous pressure is the hallmark of chronic venous insufficiency 1, 2 postulating increased iron deposition 3. There is a growing body of evidence that persistent venous congestion is probably insufficient to produce venous‐origin chronic wounds. Tissue damage evokes coagulation cascade and numerous substances in the injured area act as general leukocyte‐ and macrophage‐attractant mediators 1, 2, 4. Failures in defence mechanism and haemostasis seem to be the coplayers of venous leg ulcer (VLU) formation causing a cascade of inflammation and tissue demolition with a concomitant microbial colonisation. The abundant complexity of chronic wound healing sheds light on the significance of innate and adaptive immune responses, especially in the phases of inflammation and proliferation. Innate immunity provides the first defence line against invaders and tissue damage particles encompassing epithelial barriers, phagocytes [macrophages, polymorphonuclear leukocytes (PMNLs)], natural killer cells (NK cells), complement system proteins and the iron homeostasis regulator, innate immunity component lactoferrin 1, 2, 3, 5, 6, 7. Bacterial colonisation amplifies inflammatory response via interaction with effector immune cells, cytokines and extracellular matrix components. Bacteria are known to potentially modulate the local immune response and drive the pro‐inflammatory phenotype of the wound and are supposed to influence the systemic immune surveillance 8. Collaborating cells use a specific pattern of cytokine and chemokine array. Upregulation of antimicrobial peptides and inflammatory cytokines (e.g. IL‐1α, TNFα, CXCL‐8, IL‐10) highlights the persistent inflammation in chronic wound 1, 2, 6, 7, 8, 9, 10, 11. PMNLs clean the wounded area after which the circulating monocytes enter the wound and mature into tissue macrophages and dendritic cells. NK cells are also activated by macrophages to kill intracellular microbes 1, 2. Among these components, macrophages have a fundamental role in the immunological phenomena that occur during wound healing processes 12, 13. Basically, chronic non‐healing wounds are locked in non‐resolving inflammation 6, 7, 10, 14. It might be due to two distinct reasons: (i) eradication of microbes is insufficient, and thus a weak immune response could result in granulomatous‐like reactions 15 and (ii) on the contrary, exaggerated immune response also does not let wounds go towards the proliferation phase. Dysregulation might play a pivotal role in each mechanism. One of the key features in non‐healing wounds appeared to be the constant stimulation of innate immune response 10. Besides effector mechanisms, regulation is assumed to play a crucial role in tailoring innate immune responses. The TAM (Tyro, Axl, MerTK) family of receptor tyrosine kinases and their ligands Gas6 and Protein S (ProS) are a group of proteins with innate immune regulation function 16, 17, 18, 19, 20. Although it is known that they promote apoptosis and phagocytosis, NK‐cell maturation, platelet aggregation, angiogenesis and also downregulate inflammation 16, 17, 18, 19, 20, 21, 22, their role in chronic wound healing has not yet been investigated.
Chronic wound healing studies are mostly devoted to local mechanisms, but we assumed that VLU patients have systemic host defence alterations. According to our working hypothesis, peripheral blood mononuclear cells (PBMCs) from patients with VLUs could possess reduced Candida albicans killing 23 ability and altered gene expression pattern of pro‐ and anti‐inflammatory cytokines/chemokines, TAM receptor family members and their ligands. To test this hypothesis, we (i) quantified the innate immune function of circulating PBMCs with in vitro measurement of Candida killing activity as a general assessment for function and (ii) determined the gene expression of immune effector molecules IL‐1α, TNFα, CXCL‐8 and IL‐10 and receptors Tyro3, Axl and MerTK as well as their ligands Gas6 and ProS in probands with and without VLU. Finally, we sought to examine whether responder and non‐responder probands to VLU therapy could be distinguishable according to Candida killing efficacy, gene expression or microbial colonisation patterns.
Materials and methods
Patients
A total of 69 patients with VLU [33 females and 36 males with a mean age of 66·71 years (range 23–90 years)] and another 42 age‐ and gender‐matched control probands [25 females and 17 males with a mean age of 60·81 years (range 25–86 years)] without VLU were recruited following the approval of the Local Research Ethical Committee of the University of Szeged and a written informed consent at the Wound Care Outpatient Unit of the Department of Dermatology and Allergology, University of Szeged. Exclusion criteria comprised age <18 years, immunocompromised status, immunosuppressant therapy, antibiotic therapy, diabetes mellitus, osteomyelitis, exposed bone in the ulcer bed, ankle‐brachial pressure index <0·8 and clinical signs of severe inflammation (e.g. erysipelas, cellulitis).
Among the VLU patients, duplex ultrasound findings disclosed superficial vein incompetence in 22 cases (31·88%), perforator incompetence in 15 cases (21·73%) and combined (superficial + perforator) in 32 cases (46·37%). Deep venous reflux was detected in 19 cases (27·53%). Mean ulcer area was 23·2 cm2 (2·3–200·85 cm2).
Eligible patients underwent blood sampling for laboratory examinations (HbA1c, C‐reactive protein, white blood cell, kidney and liver function, uric acid, total protein, albumin, iron) and wound bed swabs were collected for microbiological culture.
The VLU patients were further stratified on the basis of their responsiveness to the applied wound care procedure 24. Patients with an ulcer area reduction ≥ 20% in a 4‐week period became responders (n = 25) and those with slower wound healing were enrolled to non‐responder group (n = 44).
Microbiological procedure
Between undressing and cleansing, the ulcer bed was swabbed using a broad Z‐stroke technique with a sterile cotton bud that was placed immediately in sterile transport medium. Tubes with transport media were suspended in 1 ml reduced brain heart infusion (BHI; pH 7·2; Oxoid, Basingstoke Hampshire, UK) broth and after gentle dispersion, these suspensions were plated immediately on selective and non‐selective media. Samples were inoculated onto 5% sheep blood agar, chocolate agar, eosin methylene blue agar (bioMérieux, Marcy‐L'Étoile, France) and Sabouraud agar plates. Endo agar (bioMérieux) was used for selective isolation of Enterobacter species. These plates were incubated in 10% CO2 atmosphere and normal atmosphere for 24 hours at 37°C; Sabouraud plates were incubated for another 5 days at room temperature. Columbia agar base supplemented with 5% blood, haemin and vitamin K1 was used to isolate anaerobes and to determine the colony‐forming units (CFUs) in the case of tissue samples. Because of the possible presence of black‐pigmented anaerobic gram‐negative bacilli (Prevotella spp., Porphyromonas spp.) kanamycin‐vancomycin‐laked blood (KVLB) agar (Oxoid) was applied. Cultures were incubated for 5 days in an anaerobic cabinet (90% N2, 5% H2 and 5% CO2) (Bactron Sheldon Man, Cornelius, OR, USA) at 37°C. Species‐ or genus‐level identifications were achieved by traditional biochemical methods, ATB/VITEK (bioMérieux) kits and/or MALDI‐TOF (Bruker Daltonik, Bremen, Germany) method.
PBMC isolation from blood samples
Citrated blood samples (20 ml) drawn from cubital veins were collected from age‐ and gender‐matched probands with and without VLU. PMBCs were separated using Ficoll‐Paque Plus (GE Healthcare, Santa Clara, CA, USA) density gradient centrifugation as described previously 25. For this, twofold dilutions were made from blood samples with ice‐cold phosphate‐buffered saline (PBS) (Gibco, Paisley, United Kingdom) and layered on Ficoll‐Paque Plus. Samples were centrifuged for 30 minutes at 93 × g and the PBMC layers were collected and washed with PBS. About 25% of the isolated PBMCs were suspended in RPMI‐1640 medium (Gibco) supplemented with 10% heat‐inactivated foetal bovine serum (FBS, Gibco) and 1% penicillin‐streptomycin solution (Gibco) for killing experiments.
C. albicans killing assay
C. albicans growth conditions: C. albicans SC5314 was inoculated into 2 ml of yeast extract, pepton and glucose as dextrose (YPD) [0·5% m/V yeast extract, 1% (m/V) peptone and 1% (m/V) glucose) supplemented with 100 U/ml penicillin–streptomycin solution and was agitated at 37°C in an orbital shaker at × g.
Candida killing assay was performed in 96‐well plates. A total of 50 000 PBMCs in 100 µl of RPMI‐1640 media were plated per well immediately after isolation. C. albicans culture was washed two times with sterile PBS (centrifugation at 2500 g, 5 minutes) and diluted in RPMI‐1640 to 2·5 * 106/ml, and 100 µl of this suspension was added to PBMCs and to wells containing only 100 µl RPMI‐1640 media as control. The cells were incubated for 3 hours at 37°C, 100% relative humidity and 5% CO2 tension. PBMCs were then disrupted by forcing them through a 27G needle with a sterile syringe for five times. The lysate was diluted in sterile PBS, plated onto YPD supplemented with penicillin–streptomycin and incubated for 2 days at 30°C. Colonies were then counted and the killing efficiency was calculated by using the following formula: %killing = [(Average CFUcontrol − Average CFUcoincubated)/Average CFU control] * 100.
Quantitative reverse transcriptase polymerase chain reaction (QRT‐PCR)
Total RNA extraction, cDNA synthesis and QRT‐PCR measurements were performed as described previously 26. Briefly, total RNA was extracted from PBMCs by using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The quality and the quantity of extracted RNAs were determined by NanoDrop (Thermo Scientific, Wilmington, DE, USA), Qubit (Life Technologies, Carlsbad, CA, USA) and Bioanalyzer (Agilent, Santa Clara, CA, USA) measurements. cDNA was synthesised from 100 ng of total RNA by using High Capacity RNA to cDNA Kit (Life Technologies) according to the manufacturer's instructions. The relative abundance of Axl, Tyro3, CXCL8, Gas6, IL‐1α, IL‐10, MerTK, ProS and TNFα was determined by QRT‐PCR by using StepOne Plus Real‐Time PCR System (Life Technologies). Reactions were performed by using TaqMan Gene Expression Master Mix (Life Technologies) for commercially available FAM‐ or VIC‐conjugated TaqMan probes (Life Technologies) in multiple reactions; TaqMan assay IDs are listed in Table 1. As controls, reaction mixtures without cDNA were used. All of the experiments were performed in two technical replicates. The ratio of each mRNA relative to the 18S rRNA was calculated using the 2−ΔΔCt method.
Table 1.
FAM (6‐carboxyfluorescein)‐ or VIC‐conjugated commercially available TaqMan assays used in QRT‐PCR studies
| Target name | Assay number |
|---|---|
| 18S rRNA (FAM conjugated) | Hs99999901 |
| IL‐10 (FAM conjugated) | Hs99999035_m1 |
| MerTK (VIC conjugated) | Hs01031979_m1 |
| Tyro3 (VIC conjugated) | Hs00170723_m1 |
| Axl (FAM conjugated) | Hs00242357 |
| CXCL8 (VIC conjugated) | Hs00174103_m1 |
| TNFα (FAM conjugated) | Hs00174128_m1 |
| Gas6 (FAM conjugated) | Hs00181321_m1 |
| ProS (VIC conjugated) | Hs00165590_m1 |
FAM,; QRT‐PCR, quantitative reverse transcriptase polymerase chain reaction; VIC,.
Statistical analysis
Data show average ± standard error of the mean. The significance of difference between sets of data was determined by one‐way ANOVA following Neuman–Keuls post hoc test, or Student's paired t‐test (comparison of laboratory values), using GraphPad Prism for Windows. A probability (P) value of <0·05 was considered significant.
Sample size calculations, performed with Statistica 9.1 (StatSoft, Tulsa, OK, USA), showed that at least four probands in the VLU and at least four probands in the control group were needed to be included if suspected unstimulated Candida killing activity is 10% in the VLU and 20% in the control groups with a power of 80% and an α error of 0·05 (one‐sided). Importantly, our group sizes greatly exceeded the calculated ones.
Results
Patients with VLU have altered laboratory values
At first, we determined and compared the laboratory values of all 111 persons involved in the study. We found significant differences in the following laboratory values between the patient and control groups: leukocytosis was found in 45·45% of patients and 21·95% of control probands (P = 0·022), low haemoglobin value was observed in 48·52% of patients and 19·51% among control persons (P = 0·039), high urea value was detected in 13·23% of affected and in 0% of unaffected persons (P = 0·013) (data not shown).
Microbial composition of VLUs
The most common microorganisms involved in VLU microbial burden were Staphylococcus aureus (18%), Pseudomonas aeruginosa (17%) and Escherichia coli (9%) (data not shown). Interestingly, there is a notable shift in the proportion of microbes after stratification into responders and non‐responders (Figure 1). Patients with recalcitrant ulcers showed predominantly S. aureus (39%) and P. aeruginosa (22%). In contrast, responders showed a more balanced composition of detected infectious agents; however, P. aeruginosa (16%) and S. aureus (13%) remained the predominant species (Figure 1).
Figure 1.
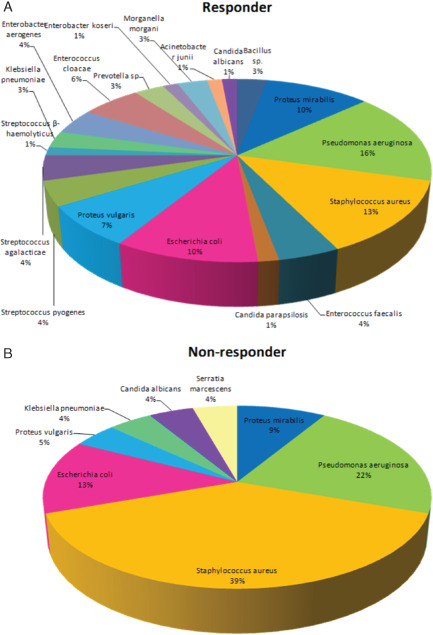
Pseudomonas aeruginosa and Staphylococcus aureus are characteristic for swab cultures obtained from non‐responders. Although more microbial species are present in swab cultures obtained from responders as compared with non‐responders (19 versus 8), the amount of isolated P. aeruginosa and Staphylococcus aureus was elevated as compared with that in responders (22% and 39% versus 16% and 19%, respectively).
PBMCs from patients with VLU exhibit normal Candida killing activity
In order to determine if PBMCs from VLU patients exhibit altered functional properties, we performed in vitro Candida killing assay. Even though PBMCs of patients with VLU had lower Candida killing activity, there were no significant differences among control, responder and non‐responder patient groups (Figure 2).
Figure 2.
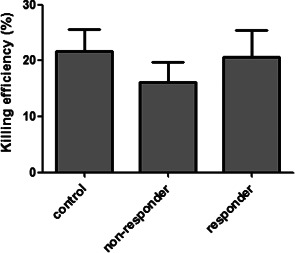
Candida albicans killing by circulating peripheral blood mononuclear cells. Killing efficiencies were evaluated in responder and non‐responder venous leg ulcer patients and control persons. No significant differences were detected in the percentage of dead yeasts in the comparative study. Bars show means ± SEM (one‐way ANOVA following Neuman–Keuls post hoc test; P ≤ 0·05).
Altered gene expression pattern of pro‐ and anti‐inflammatory mediators in responders and non‐responders
The outcome of the immune response depends, in part, on the nature of the pro‐inflammatory proteins released locally by the immune cells. Pro‐ and anti‐inflammatory cytokines and chemokines, such as IL‐1α, TNFα, IL‐10 and CXCL8, secreted by various cell types play a fundamental role in attracting neutrophils and T cells to the site of skin infection. Therefore, we determined the gene expression pattern of the above‐mentioned molecules in PBMCs from control probands and patients with VLU. QRT‐PCR results showed that the expression of all four genes was significantly higher in patients with VLU in comparison to control probands (Figure 3A). Moreover, we detected marked differences when the expression of the above‐mentioned four genes from PBMCs was compared between responder and non‐responder VLU patients. Pro‐inflammatory mediators IL‐1α and CXCL‐8 showed significantly increased expression in non‐responders, with TNFα having similar profile although statistically non‐significant (P = 0·757) (Figure 3B). In contrast, the anti‐inflammatory molecule IL‐10 showed increased expression level in responders (P = 0·248) (Figure 3B).
Figure 3.
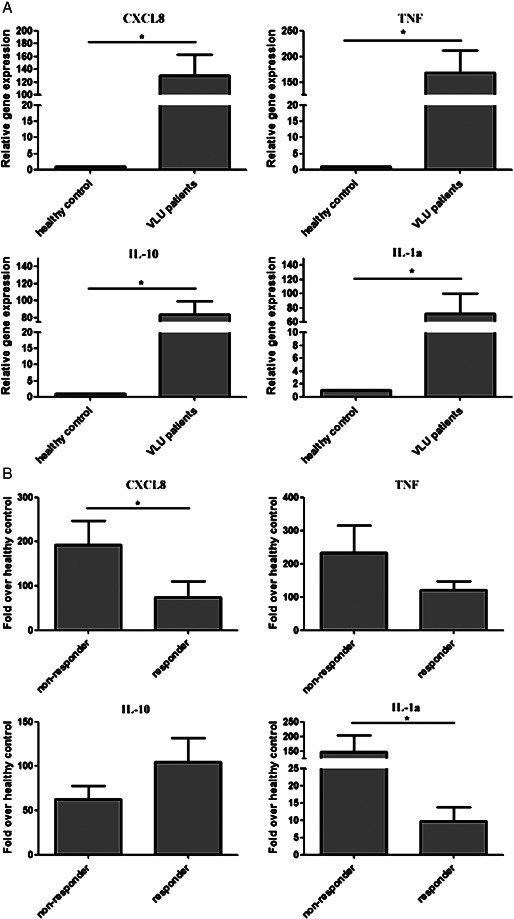
Gene expression pattern of inflammatory chemokines/cytokines: venous leg ulcer (VLU) patients versus control probands (A) and VLU non‐responders versus VLU responders (B). The ratio of each mRNA is relative to 18S rRNA. Bars show means ± SEM. *Significantly different from each other as determined with one‐way ANOVA following Neuman–Keuls post hoc test; P ≤ 0·05.
Patients with VLU exhibit elevated mRNA levels of TAM receptors and their ligands
We determined that elevated cytokine/chemokine levels and microbial superinfection are a hallmark of VLU. We hypothesised malfunctions in the negative regulation of innate immune response and clearance of microbes and apoptotic cells in diseased tissue. Thus, we sought to determine the expression patterns of TAM receptors (Tyro3, Axl, MerTK) and their ligands Gas6 and ProS in patients with VLU and control probands. We determined marked increase in the expression of all five mRNAs in VLU patients (Figure 4A). Interestingly, when we compared the expression of the respective molecules in responders versus non‐responders, we identified Axl as significantly upregulated and Gas6 as significantly downregulated in responders (Figure 4B).
Figure 4.
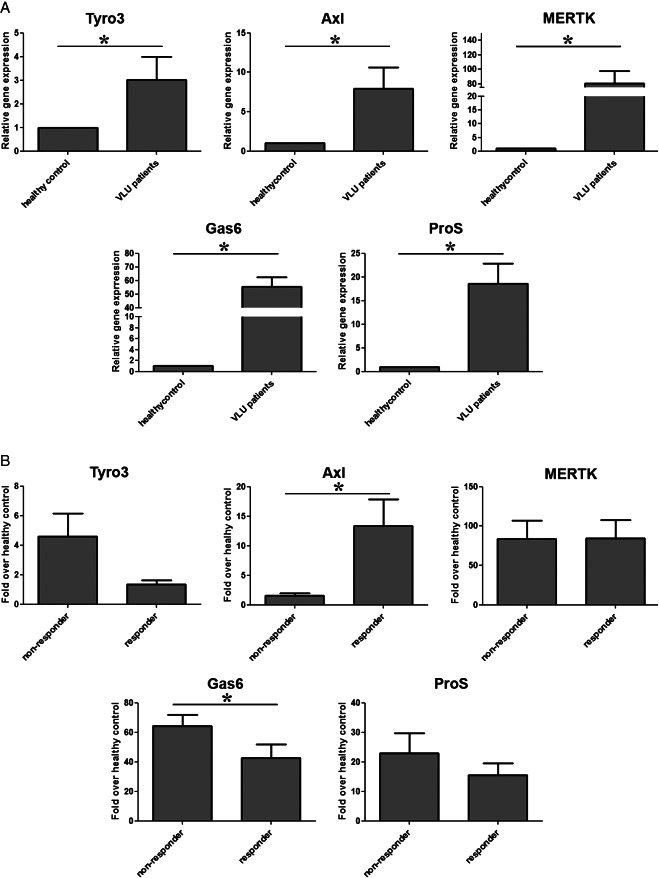
Gene expression pattern of TAM (Tyro, Axl, MerTK) receptors and their ligands Gas6 and ProS: venous leg ulcer (VLU) patients versus control probands (A) and VLU non‐responder versus VLU responders (B). The ratio of each mRNA is relative to 18S rRNA. Bars show means ± SEM. *Significantly different from each other as determined with one‐way ANOVA following Neuman–Keuls post hoc test; P ≤ 0·05.
Discussion
In our study, we evaluated the expression of pro‐ and anti‐inflammatory cytokines/chemokines and the intrinsic inhibitors of the inflammatory response, the TAM receptors and their ligands (Gas6 and ProS) in probands with and without VLU. To explore gene expression with more insight, we also made a comparison within the VLU group on the basis of responsiveness to appropriate wound care, and thus we were able to discriminate between responder and non‐responder VLU patients.
Unlike normal wound healing, VLUs are trapped in non‐resolving, sustained inflammation 5, 6, 8, 14 and long‐standing inflammation enhances the propensity for intensive microbial colonisation. Conversely, infectious agents amplify and even prolong the ongoing inflammatory process. The current host condition influenced by age and nutritional and metabolic status might be a real burden for efficient wound healing 27. In addition, immune function plays a critical role in the modulation of inflammation. Regulation might play a central role in the agitation or silencing of immune mechanisms and this area has deserved an increasing interest in recent times.
All chronic wounds contain microorganisms regardless of whether they are clinically infected or not 5, 28, 29. To assess the correlation between the presence of microorganisms and VLU, we also analysed the microbiota spectrum of chronic wounds. After wound dressing, removal bacterial swabs were collected immediately. Instead of biopsy or other complicated techniques, superficial swabs were carried out, as Gjødsbøl et al. recently compared swab, biopsy and filter padding methods and reported that no differences were detected 30. Microbial cultures showed the dominance of S. aureus and P. aeruginosa, which is in good concordance with a large comparative study on leg ulcer colonising bacteria 31. The proportion of S. aureus from therapy‐resistant ulcers appeared to be markedly lower in our study compared with the results of the latest clinical investigation (39% versus 53%, respectively) whereas P. aeruginosa represented nearly the same prevalence (22% versus 25%, respectively). Our experimental setting clearly showed that therapy‐responder VLUs had a more balanced microbial spectrum with 13% of Staphylococcus‐ and 16% of Pseudomonas‐positive cultures.
The different microbial colonisation patterns of responder and non‐responder chronic wounds might reflect the different inflammatory cytokine responses. Microbes express a wide range of virulence factors, which challenge macrophage immune competence. Phagocytosis could be a crucial mechanism in S. aureus and P. aeruginosa clearance; however, macrophages are capable of mounting an inflammatory response eliciting strong production of IL‐1α, TNFα and CXCL‐8 32, 33, 34, 35.
One of the key features in non‐healing wounds appeared to be the constant stimulation of innate immune response 10 and macrophages have a critical role throughout wound healing. They have a polarised action: there is a pro‐inflammatory subset (M1) with inflammatory cytokine production and bactericidal activity, and the other is an anti‐inflammatory (immunomodulator) (M2) subset linked with wound healing and tissue repair processes. The initial phase requires effector function, followed by tissue formation with vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) production, and finally, the anti‐inflammatory function should be predominant with IL‐1RA, IL‐10 and TGF‐β1 release 13. Taken these data together, a switch from pro‐ to anti‐inflammatory phenotype with adequate timing might be beneficial by promoting wound healing 10.
Because killing and phagocytic activities are reliable measures of innate immune function, we applied a simple method to assess the killing activity of PBMCs isolated from VLU patients. By using the power of in vitro C. albicans killing assay, which serves as a standard model to quantify host response 23, we determined that PBMCs from VLU patients do not harbour gross perturbations in immune function.
Clinical studies in wound healing mostly focus on local alterations of inflammatory cytokine expressions 9; however, a novel study found that infected wounds produced an upregulation of circulating inflammatory cytokine pattern compared with non‐infected ones 8. This is one of the first evidence to prove a remarkable influence of leg ulcers on systemic immune response. The current results show that circulating PMBCs of VLU patients robustly overexpress both pro‐ and anti‐inflammatory cytokines/chemokines compared with those measured in control proband group. Importantly, non‐responder VLU patients show a notable upregulation of IL‐1α and CXCL‐8 gene expression supporting the prolonged pro‐inflammatory status of their leg ulcers and the massive Staphylococus and Pseudomonas colonisation 32, 33, 34, 35. Lower expression of pro‐inflammatory effectors (IL‐1α, CXCL‐8 and TNFα) and higher, although statistically non‐significant, expression of anti‐inflammatory cytokine IL‐10 refer to the silencing and clearance of inflammation in patients with better wound healing prognosis.
TAM family of receptor tyrosine kinases – Tyro3, Axl, MerTK – and their common ligand Gas6, among others, play a central role in the intrinsic inhibition of the inflammatory response to pathogens and regulate phagocytosis of apoptotic cells 18. TAM receptor signaling – prominently through MerTK – is required for the phagocytosis of apoptotic cells by immune cells, such as macrophages and dendritic cells. The majority of these cells express MerTK and Axl but weakly Tyro3. In addition, Axl regulates and stimulates angiogenesis, proliferation and cell migration. Circulating innate immune cells of patients with septicaemia show increased MerTK expression compared with those of healthy individuals or trauma patients. In contrast, Tyro3 and Axl expressions were more pronounced in monocytes from trauma patients 21. Interestingly, the final stage of inflammatory cycle is known to involve TAM signalling 36: as a consequence of TAM activation, the TLR‐driven pro‐inflammatory cytokine/chemokine secretion is markedly downregulated. Our recent study has shown that their expression is also altered in psoriasis 37, another pathophysiological condition with increased cytokine expression. Hence, dysregulation of innate immune surveillance could explain some features of prolonged inflammation in chronic wound healing. Here, we show that both TAM receptors and their ligands show elevated expression in VLU patients as compared with control probands, indicative of the activity of the negative feedback regulation of the inflammation and also reflecting the active phagocytic activity. Within VLU patient group, responders show much higher Axl and significantly lower Gas6 gene expressions. High Axl activity may explain the reduced rate of inflammation and the better response to treatment option. Furthermore, Axl expression of PBMC is strongly stimulated by inflammatory mediators of classical M1 activation. Simultaneously, a modest downregulation of Gas6 ligand gene could be detected 20. Our results are in close agreement with the classical form of Axl upregulation and Gas6 downregulation, which serves the efficient inner control of turbulent inflammatory response.
In fact, the exact mechanism and cooperation among TAM tyrosine kinase family members and Gas6 and ProS ligands remain to be elucidated; however this receptor tyrosine kinase family and its ligands could presumably play a general role in the comprehensive control of inflammation and, in turn, the wound healing process.
To our knowledge, this is the first report where the expression pattern of TAM receptor family members and their ligands was studied in chronic wound healing. Our approach is in good concordance with an earlier editorial paper of International Wound Journal, urging a more insightful focus on the unexplored immune mechanisms involved in chronic wound healing 38. Of note, IL‐1α is robustly overexpressed in patients remaining unresponsive to VLU treatment; in contrast, Axl gene expression is dramatically upregulated among VLU carriers with sufficient healing properties. Hence, these markers could be considered for facilitating the prediction of therapeutical response as a translation of our results into general practice.
Acknowledgements
This study has been fully funded by the Servier‐International Phlebology Union 2011–2013 research fellowship grant. István Nagy was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. The authors wish to thank Angéla Meszes, MD, Judit Vasas and Ildikó Gyurmán, wound care nurses, for their assistance in patient care and sample collection. The authors acknowledge Françoise Pitsch and Jean‐Jerome Guex, MD, for their critical reading of the manuscript.
References
- 1. Simka M. Cellular and molecular mechanisms of venous leg ulcers development – the “puzzle” theory. Int Angiol 2010;29:1–19. [PubMed] [Google Scholar]
- 2. Raffetto JD, Mannello F. Pathophysiology of chronic venous disease. Int Angiol 2014;33:212–21. [PubMed] [Google Scholar]
- 3. Spadaro M, Caorsi C, Ceruti P, Varadhachary A, Forni G, Pericle F, Giovarelli M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J 2008;22:2747–57. [DOI] [PubMed] [Google Scholar]
- 4. Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: immunological aspects. Injury 2006;37(Suppl 1):S5–12. [DOI] [PubMed] [Google Scholar]
- 5. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–25. [DOI] [PubMed] [Google Scholar]
- 6. Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner‐Tuderman L, Krieg T, Shannon JD, Fox JW. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res 2010;9:4758–66. [DOI] [PubMed] [Google Scholar]
- 7. Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy‐Szabo A, Szell M, Dobozy A, Kemeny L. Microbial compounds induce the expression of pro‐inflammatory cytokines, chemokines and human beta‐defensin‐2 in vaginal epithelial cells. Microbes Infect 2005;7:1117–27. [DOI] [PubMed] [Google Scholar]
- 8. Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S. Extracellular matrix assessment of infected venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J 2014. DOI: 10.1111/iwj.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg 2009;49:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pukstad BS, Ryan L, Flo TH, Stenvik J, Moseley R, Harding K, Thomas DW, Espevik T. Non‐healing is associated with persistent stimulation of the innate immune response in chronic venous leg ulcers. J Dermatol Sci 2010;59:115–22. [DOI] [PubMed] [Google Scholar]
- 11. Mannello F, Ligi D, Canale M, Raffetto JD. Omics profiles in chronic venous ulcer wound fluid: innovative applications for translational medicine. Expert Rev Mol Diagn 2014;14:737–62. [DOI] [PubMed] [Google Scholar]
- 12. Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology 2011;216:753–62. [DOI] [PubMed] [Google Scholar]
- 13. Willenborg S, Eming SA. Macrophages – sensors and effectors coordinating skin damage. J Dtsch Dermatol Ges 2014;12:214–21. [DOI] [PubMed] [Google Scholar]
- 14. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–7. [DOI] [PubMed] [Google Scholar]
- 15. Caprilli R, Frieri G. The dyspeptic macrophage 30 years later: an update in the pathogenesis of Crohn's disease. Dig Liver Dis 2009;41:166–8. [DOI] [PubMed] [Google Scholar]
- 16. van der Meer JH, van der Poll T, van't Veer C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood 2014;123:2460–9. [DOI] [PubMed] [Google Scholar]
- 17. Lew ED, Oh J, Burrola PG, Lax I, Zagórska A, Través PG, Schlessinger J, Lemke G. Differential TAM receptor‐ligand‐phospholipid interactions delimit differential TAM bioactivities. Elife 2014;29:3. DOI: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lemke G, Rothlin C. Imunobiology of the TAM receptors. Nat Rev Immunol 2008;8:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemke G, Burstyn‐Cohen T. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci 2010;1209:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zagórska A, Través PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol 2014;15:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guignant C, Venet F, Planel S, Demaret J, Gouel‐Chéron A, Nougier C, Friggeri A, Allaouchiche B, Lepape A, Monneret G. Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Med 2013;39:1556–64. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen KQ, Tsou WI, Kotenko S, Birge RB. TAM receptors in apoptotic cell clearance, autoimmunity, and cancer. Autoimmunity 2013;46:294–7. [DOI] [PubMed] [Google Scholar]
- 23. Du C, Calderone RA. Phagocytosis and killing assays for Candida species. Methods Mol Biol 2009;499:17–26. [DOI] [PubMed] [Google Scholar]
- 24. Geske T, Hachmann E, Effendy I. Wound treatment with ethacridine lactate in venous leg ulcers: a prospective, randomized, placebo‐controlled, single‐blind study. Vasomed 2005;17:99–103. [Google Scholar]
- 25. Nagy I, Filkor K, Németh T, Hamari Z, Vágvölgyi C, Gácser A. In vitro interactions of Candida parapsilosis wild type and lipase deficient mutants with human monocyte derived dendritic cells. BMC Microbiol 2011;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Filkor K, Hegedűs Z, Szász A, Tubak V, Kemény L, Kondorosi É, Nagy I. Genome wide transcriptome analysis of dendritic cells identifies genes with altered expression in psoriasis. PLoS One 2013;8:e73435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol 2005;43:325–30. [DOI] [PubMed] [Google Scholar]
- 29. Carter MJ, Tingley‐Kelley K, Warriner RA III. Silver treatments and silver‐impregnated dressings for the healing of leg wounds and ulcers: a systematic review and meta‐analysis. J Am Acad Dermatol 2010;63:668–79. [DOI] [PubMed] [Google Scholar]
- 30. Gjødsbøl K, Skindersoe ME, Christensen JJ, Karlsmark T, Jørgensen B, Jensen AM, Klein BM, Sonnested MK, Krogfelt KA. No need for biopsies: comparison of three sample techniques for wound microbiota determination. Int Wound J 2012;9:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jockenhöfer F, Chapot V, Stoffels‐Weindorf M, Körber A, Klode J, Buer J, Küpper B, Roesch A, Dissemond J. Bacterial spectrum colonizing chronic leg ulcers: a 10‐year comparison from a German wound care center. J Dtsch Dermatol Ges 2014;12:1121–7. [DOI] [PubMed] [Google Scholar]
- 32. Rabehi L, Irinopoulou T, Cholley B, Haeffner‐Cavaillon N, Carreno MP. Gram‐positive and gram‐negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect Immun 2001;69:4590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cole J, Aberdein J, Jubrail J, Dockrell DH. The role of macrophages in the innate immune response to Streptococcus pneumoniae and Staphylococcus aureus: mechanisms and contrasts. Adv Microb Physiol 2014;65:125–202. [DOI] [PubMed] [Google Scholar]
- 34. Al Moussawi K, Kazmierczak BI. Distinct contributions of interleukin‐1α (IL‐1α) and IL‐1β to innate immune recognition of Pseudomonas aeruginosa in the lung. Infect Immun 2014;82:4204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mukaida N. Pathophysiological roles of interleukin‐8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 2003;284:L566–77. [DOI] [PubMed] [Google Scholar]
- 36. Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 2007;131:1124–36. [DOI] [PubMed] [Google Scholar]
- 37. Szász A, Strifler G, Vörös A, Váczi B, Tubak V, Puskás LG, Belső N, Kemény L, Nagy I. The expression of TAM receptors and their ligand Gas6 is downregulated in psoriasis. J Dermatol Sci 2013;71:215–6. [DOI] [PubMed] [Google Scholar]
- 38. Davis PJ. The double‐edged sword of the immune system – a force for good or evil in the wound? Int Wound J 2009;6:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


