Abstract
This study aims to determine health‐related quality of life (HRQoL) in patients suffering with venous ulceration and to correlate wound's severity status with HRQoL loss as well as identify the aspects of HRQoL most negatively affected by the presence of venous ulcers. In this observational, cross‐sectional, descriptive, analytical multi‐centre study, data was compiled over a period of 3·5 months. Thrity‐four patients with venous ulceration were recruited. The RESVECH 2·0 scale was used to monitor wounds. The MAID scale was used to measure wound's severity. The Charing Cross Venous Ulcer Questionnaire (CCVUQe) (Spanish version) was used to evaluate quality of life. The mean CCVUQe score was 60·58 ± 16·04. The HRQoL dimension most affected was ‘Emotional state’ (mean score = 77. 67 ± 17·34). The average RESVECH 2.0 score for the wounds was 13·15 ± 5·07. A statistically significant association between total CCVUQ‐e score and total RESVECH 2.0 score was detected [Pearson correlation coefficient r = 0·546 (P ≤ 0·001)]. Venous ulcers affect patients' HRQoL, particularly their emotional status. There is a relationship between the severity of the wound and loss of HRQoL. The presence of non‐viable tissue, poor exudate control and infection all determine loss of HRQoL. New studies are needed to confirm these findings.
Keywords: Health‐related quality of life, Patient‐reported outcomes, Venous leg ulcers, Wound assessment, Wound severity
Introduction
The first Spanish study on leg ulcer prevalence in Spain, conducted by the National Group for the Study and Assessment of Pressure Ulcers and Chronic Wounds (Spanish acronym GNEAUPP), found a prevalence of 0·16% of these lesions in people aged older than 14 years in Spain; 56% of these lesions were venous ulcers 1, 2. Nevertheless, according to the Spanish Conference for Leg Ulcers (CONUEI), between 75% and 80% of leg ulcers are from venous aetiology 3. This concurs with estimations by authors in other countries, who describe percentages between 70% and 90% 4. The estimated prevalence of venous ulceration in Spain is 0·5–0·8%, with an incidence of two to five new cases per 1000 people each year 3. Some authors believe that venous ulceration may affect between 1% and 3% of the world's population 5. We can therefore consider the prevalence of venous ulceration to be high.
The importance of the pathology is determined not only by its high prevalence rate; the financial cost of treating wounds is very high, although insufficiently documented 2. In the UK, the total cost of treating venous ulcer patients in the 2005–2006 period was estimated to be at least £168–198 million 6. Some authors point to an annual cost to health care providers of €6·5 billion 2.
The psychosocial costs associated with venous ulcers are also high. The relationship between venous ulceration and anxiety, depression, social isolation and insomnia, and, accordingly, its impact on patients' lives, is well established 7. It manifests in reduced quality of life for people with venous ulcers. Health‐related quality of life (HRQoL) encompasses physical, functional and emotional aspects as well as social well‐being 8. There is a growing interest in the link between HRQoL and venous ulceration 9.
The importance of studying HRQoL in venous ulcer patients lies in the fact that the data obtained from measuring HRQoL can help to inform clinical decision making and defining health policies for the treatment of patients with venous ulcers 8, 9.
However, measuring HRQoL is frequently problematic, requiring, as it does, tools that allow for reliable and objective measurement 10. In the case of venous ulcers, investigators have used a range of generic and specific instruments 9.
Another aspect that has not yet been resolved is determining which characteristics inherent to venous ulceration are the most decisive in loss of HRQoL. Authors have identified pain 9, 11, 12, 13, 14, surface area 15, 16, discharge 17 and odour 17, 18 as key factors in declining HRQoL in people with venous ulcers.
In general, studies conducted in this field, to date, suffer from a sparseness of validated wound‐monitoring systems, which would allow for cross comparison with other studies 9.
From this perspective, the aims of this study are to determine HRQoL in a sample of venous ulcer patients using a specific instrument for measuring HRQoL in subjects with venous ulcers and to explore the possible existence of a relationship between the severity status of the wounds and loss of HRQoL. Other aims are to identify the areas of HRQoL that deteriorate to a greater extent in venous ulcer patients and to compile information about which wound characteristics most negatively impact HRQoL.
Methods
Observational, cross‐sectional, analytical, multi‐centre study
Data were collected in the Ingenio and Miller Bajo health centres and the Functional Wounds Unit of Cueva Torres health centre, all dependants of the Primary Care Department of Gran Canaria (Spain). Ingenio health centre is located in the town of Ingenio, in the southern part of the island of Gran Canaria. Miller Bajo and Cueva Torres health centres are located in Las Palmas de Gran Canaria metropolitan area, Gran Canaria. The physical environment of the study consisted of the nursing rooms of the Miller Bajo and Ingenio health centres and the Functional Wounds Unit of the Cueva Torres health centre. Data were collected over a period of 3 months and 15 days.
At the Miller Bajo and Ingenio centres, patients were recruited in the course of normal medical and nursing consultations and were referred directly to the researchers by health centre staff. At the Cueva Torres centre, patients were recruited through the Primary Care Department Functional Wounds Unit, which receives patients from all over Gran Canaria Island through an inter‐consultation‐referral system.
Regulatory authorisation was sought from the Gran Canaria Primary Healthcare Department and obtained prior to the start the study. Subjects were given an informed consent document before their data were taken. Names were not used during any part of the study, and the data collected could not be associated or identified with specific individuals.
Accidental non‐probability sampling was performed on all venous ulcer patients assigned to the selected centres. Because of the connotations and aims of this study and the impossibility of getting together a suitable sample size in the established data collection period using probability sampling, we opted for this sampling method as it gave the researchers feasible access to the study population. Nevertheless, a post‐hoc statistical power analysis will be carried out based on the relationship between the severity status of the wounds and loss of HRQoL.
Inclusion criteria
People with venous leg ulcers.
Exclusion criteria
Being under age.
Patients with cognitive impairment. Patients referred for inclusion in the study but suspected of possible cognitive impairment during the data collection period underwent cognitive testing (Lobo mini‐mental test) 19, and subjects testing positively for possible or confirmed cognitive impairment were excluded.
Investigators responsible for data collection at the three centres had received specific training in the treatment of chronic wounds. The data collection process lasted approximately 30 min for each subject.
The data collection process consisted of several phases
An interview
This phase consisted of guided questioning, using a form specifically designed to collect information on certain variables. To evaluate the variables to be included in the study, we conducted a comprehensive review of papers previously published on quality of life in people with venous ulceration in order to detect variables that could be studied. Accordingly, no pre‐testing was considered necessary.
A physical examination
Each subject's wounds were examined individually. Examination consisted of:
Manual palpation of the posterior tibial and dorsalis pedis pulse of the wounded leg in order to rule out possible arterial involvement.
Visual examination of the legs to detect clinical signs and symptoms of chronic venous insufficiency.
Staging the severity of the wound using the MAID Score 20.
Monitoring the wound using RESVECH 2.0 21.
Questioning using the Charing Cross Venous Ulcer Questionnaire (CCVUQe) 22, Spanish version 23.
The variables included in the study were: sex, age, marital status, educational level, progress of the ulcer over time (from the moment of its appearance), recurrence of the wound, use of compression therapy, MAID score, RESVECH 2.0 score and CCVUQe score.
The following operative definitions were used for these variables:
Venous ulcers
Leg wounds complying with the following criteria were classified as venous ulcers:
Wounds with the typical signs and symptoms of this type of wound, including location in the distal third of the leg and an oval, round or irregular shape 3, 24.
The coexistence of chronic venous insufficiency‐related skin disorders, such as ochre dermatitis, white atrophy, hyperkeratosis, lipodermatosclerosis, varicose veins, eczema and oedema 3, 24.
In all cases, the presence of a palpable tibial or pedis pulse was required 3, 25. All of the subjects were classified as stage C6 of the CEAP classification for peripheral venous disease 3, 25.
Pulses
Pulse was considered to be present when at least the posterior tibial or pedis pulse could be palpated in the wounded leg.
Educational level
The following criteria were established to assess subjects' educational level:
No studies: This category encompassed individuals who had not been to school and/or could not read or write.
Primary level: Subjects who had received basic schooling till the age of 13 and who could read and write.
Secondary level: Individuals who had completed further studies equivalent to high school or vocational training (to any level).
University level: Individuals who had completed any type of university studies.
Compression therapy
Compression therapy included all techniques used to apply pressure to the extremity, reduce swelling and favour venous return, regardless of the technique used (bandage, compression stocking or intermittent pneumatic compression) 26. While a classification has been proposed for the level of pressure applied (light: <20 mm Hg, moderate: ≥20–40 mm Hg, strong: ≥40–60 mm Hg and very strong: >60 mm Hg) 26, in this study, no distinction was made regarding level of pressure.
Recurrence
The term ‘recurrent wounds’ was applied to wounds that had formerly healed completely but that had reopened by the time of assessment, independent of the severity of the wound at the time of assessment and making no distinction with regards to the period of time between healing and recurrence. The term ‘new wounds’ was applied to lesions that had not previously emerged.
Severity and HRQoL tools applied
The RESVECH 2.0 scale was used to monitor wounds 21. Based on the results of a PhD thesis 21, the scale has content validity by means of a modified Delphi consensus method and achieves a Content Validity Index over 0.9. Also, reliability was evaluated by internal consistency with an alpha Cronbach's index of 0,72 (based on typified elements); Pearson's correlation coefficient was used to test the items with the final score and, finally, demonstrated responsiveness by means of a repeated measures analysis of variance with time as factor. This model was analysed in general terms and by aetiologies, healing and the worst and best scores at start point (based on quartiles). So, it can be used to assess characteristics and evaluate the healing process in all types of chronic wounds, including of the legs 21. It assesses six parameters: wound size, depth of the affected tissues, status of the edges, type of tissue in the wound bed, level of exudate and inflammation‐infection in the wound (presence of biofilm). The maximum score of 35 points indicates the worst possible wound status. The working definitions for each of the components of the test have been published 27.
In Addition, the MAID scale 20 was used to measure the severity of chronic leg wounds. The MAID scale assesses four easily observable clinical parameters and requires no sophisticated technical equipment 20. The parameters are pedal pulse (absence of a pulse is scored as 1), wound size (wounds > 4 cm2 are scored as 1), wound persisting more or less than 130 days (a duration of more than 130 days scores as 1) and multiple or single wounds (having multiple wounds scores as 1). Thus, scores may vary from 0 (best possible status) to 4 (worst possible status). According to the developers of the scale, it offers a valid diagnostic tool to anticipate the healing probability of leg ulcers 20. Because of the design of our study, the wounds we assessed could never score maximum points 4 on the MAID scale as we required all wounds to have a palpable pedal pulse.
To assess HRQoL, we used a specific tool to measure quality of life with venous ulceration, the CCVUQe 23, specifically, the recently validated Spanish language version of the questionnaire 23. CCVUQe gives scores of 0–100, where a higher score indicates worse quality of life. It is considered an ideal tool to measure HRQoL in people with venous ulcers as it offers excellent psychometric properties as well as being simple and short (the questionnaire takes no longer than 10 min) 9, 23.
SPSS 18.0 software was used for statistical data analysis and for the descriptive statistics of the study variables. The chi‐square test, the Mann–Whitney U‐test, Pearson's linear correlation coefficient and Spearman's correlation coefficient were used to analyse possible statistical dependence between pairs of variables, establishing a significance of α = 0·05.
Results
Characteristics of the sample
The sample was composed of a total of 34 patients from Ingenio Health Centre (n = 14), Miller Bajo Health Centre (n = 6) and the Cueva Torres Chronic Wounds Unit (n = 14); there were 15 women (44·12%) and 19 men (55·88%). The mean age of the subjects was 72·06 ± 11·93 years; the youngest age reported was 40 years and the oldest was 87 years. Regarding marital status, 20 of the subjects were married (58·82%), 7 were widowed (20·59%), 4 were unmarried (11·76%) and 3 were separated (8·82%). Twenty‐one individuals had completed primary studies (61·76%), three had secondary studies (8·82%), three had university studies (8·82%) and seven had no formal studies (20·59%). Eighteen subjects (52·94%) were receiving compression therapy, compared with 16 (47·06%) who were not.
Characteristics of the wounds
Sixteen of the wounds were newly occurring (47·06%), while 18 (52·94%) were recurrences of previous wounds.
Four wounds (11·76%) scored 0 on the MAID scale; 8 wounds (23·53%) scored 1; 15 wounds (44·12%) scored 2; and 7 (20·59%) scored 3 points. The mean MAID scale score was 1·74 ± 0·971. The parameter that contributed mostly to a higher MAID score was duration of the wound followed by surface area (Table 1).
Table 1.
Distribution of MAID score items
| MAID score n (%) | |
|---|---|
| Existence of pedal pulses | |
| Yes | 34 (100·0) |
| No | 0 (0·0) |
| Duration of injury | |
| Less than 130 days | 9 (26·5) |
| More than 130 days | 25 (73·5) |
| Area of injury | |
| Less than 4 cm2 | 16 (47·1) |
| More than 4 cm2 | 18 (52·9) |
| Number of injuries | |
| Single injury | 18 (52·9) |
| Multiple injuries | 16 (47·1) |
The mean RESVECH 2.0 score for the wounds was 13·15 ± 5·07. The lowest score was 4 points, and the highest was 25 points. The characteristics of the wounds with reference to RESVECH 2.0 values (wound size, depth of the affected tissues, status of the edges, type of tissue in the wound bed, level of exudate and infection–inflammation) are given in Table 2.
Table 2.
Distribution of RESVECH 2.0 items
| RESVECH 2.0 n (%) | |
|---|---|
| Depth/tissues involved | |
| Dermis–epidermis involved | 26 (76·5) |
| Subcutaneous tissue involved | 8 (23·5) |
| Edges | |
| Not distinguishable (not wound edges) | 1 (2·9) |
| Diffuse | 5 (14·7) |
| Delimited | 20 (58·8) |
| Damaged | 6 (17·6) |
| Thickened (‘aged’, ‘reverted’) | 2 (5·9) |
| Wound dimensions | |
| <4 cm2 | 16 (47·1) |
| 4 ‐ < 16 cm2 | 15 (44·1) |
| 16 ‐ < 36 cm2 | 2 (5·9) |
| 64 ‐ < 100 cm2 | 1 (2·9) |
| Type of tissue in the wound bed | |
| Epithelial tissue | 2 (5·9) |
| Granulation tissue | 7 (20·6) |
| Necrotic tissue and/or slough in the bed | 25 (73·5) |
| Exudate | |
| Moist | 15 (44·1) |
| Wet | 10 (29·4) |
| Saturated | 5 (14·7) |
| Dry | 2 (5·9) |
| Leaking exudate | 2 (5·9) |
| Infection–inflammation (Biofilm signs) | |
| Increasingly painful | 21 (61·8) |
| Erythema around the wound | 13 (38·2) |
| Oedema around the wound | 13 (38·2) |
| Rising temperature | 5 (14·7) |
| Increasing exudate | 14 (41·2) |
| Purulent exudate | 4 (11·8) |
| Tissue is friable or bleeds easily | 8 (23·5) |
| Wound stationary, no progress | 25 (73·5) |
| Tissue compatible with biofilm | 12 (35·3) |
| Odour | 10 (29·4) |
| Hipergranulation | 8 (23·5) |
| Wound increasingly larger | 10 (29·4) |
| Satellite lesions | 11 (32·4) |
| Pale tissue | 3 (8·8) |
The RESVECH 2.0 score concerning infection–inflammation varied widely. The mean score in this section was 4·59 ± 3·569 points. The lowest score was 0, and the highest was 12 (out of a possible maximum of 14 for this section). Six wounds (17·65%) scored 0, showing no signs of potential infection at the time of assessment, compared with three wounds (8·82%), which scored 10, and one wound (2·94%) which scored 12, all with obvious signs of infection.
Analysing the presence of infection–inflammation in the wounds, the most frequent sign was ‘Non‐progressing wound’, reported in 25 cases (73·5%), followed by ‘Increasing pain’, reported in 21 cases (61·8%). Other signs reported with similar frequency were ‘Increasing discharge’ (41·2%), ‘Perilesional erythema’ (38·2%), ‘Perilesional edema’ (38·2%), ‘Biofilm‐compatible tissue’ (35·3%), ‘Satellite wounds’ (32·4%), ‘Odour’ (29·4%) and ‘Increased wound size’ (29·4%). Less frequently occurring signs included ‘Friable tissue that bleeds easily’ (23·5%), ‘Hypergranulation’ (23·5%) and ‘Purulent exudate’ (11·8%). The least‐frequently reported sign was ‘Pallid tissue’, found in only three cases (8·8%).
Analysis of HRQoL
The mean CCVUQe score obtained by individuals in our sample was 60·58 ± 16·04. Scores ranged from a low of 20 to a high of 84.
The aspects scored in the questionnaire were social interaction, domestic activities, cosmesis and emotional status. In our study, the aspect that most affected HRQoL was emotional status (mean score 77·67 ± 17·34) followed by cosmesis (63·91 ± 16·80). Social interaction scored 54·91 ± 19·04. The aspect that least impacted HRQoL was domestic activities, with a score of 47·47 ± 22·42.
Inferential analysis
We analysed the association between the variables and perceived HRQoL, using a range of statistical tests according to the type of variable in question. We detected no statistically significant dependence between total CCVUQe score and gender, marital status, educational level, recurrence of the wound, use of compression therapy or centre where the data was gathered.
We found no statistically significant dependence between total CCVUQe score and severity of the wound (r = 0·22) according to the MAID scale. However, we found a statistically significant association between total CCVUQe score and total RESVECH 2.0 score (Pearson correlation coefficient, r = 0·546; P ≤ 0·001). This indicates that a higher RESVECH 2.0 score correlates with greater loss of quality of life (Figure 1).
Figure 1.
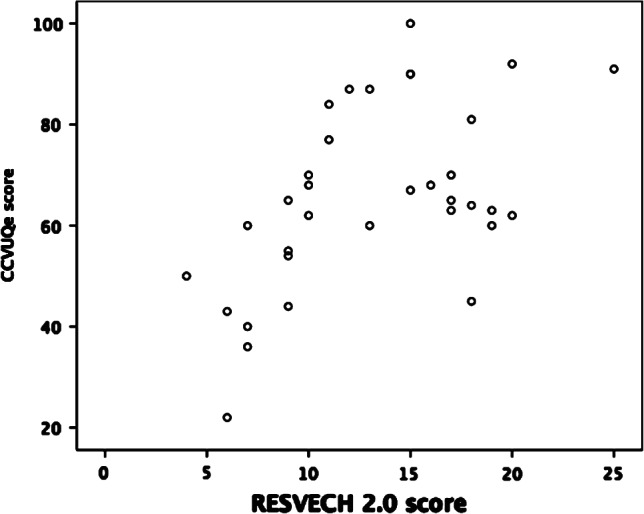
Correlation between CCVUQe and RESVECH 2.0 total scores.
Also, analysing the possible relationship between total CCVUQe score and the first five components of RESVECH 2.0, Spearman's coefficient indicates a statistically significant association between overall quality of life score and the type of tissue found in the wound bed (Spearman's Rho = 0·543; P ≤ 0·001) and exudate (Spearman's Rho = 0·485; P = 0·004). The correlation between overall quality of life and these two parameters may be observed in the respective box diagrams (Figures 2 and 3).
Figure 2.
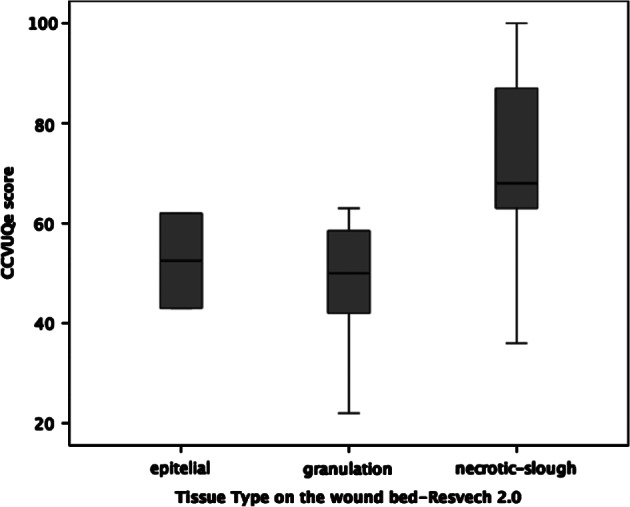
Box diagram of CCVUQe versus tissue type on wound bed.
Figure 3.
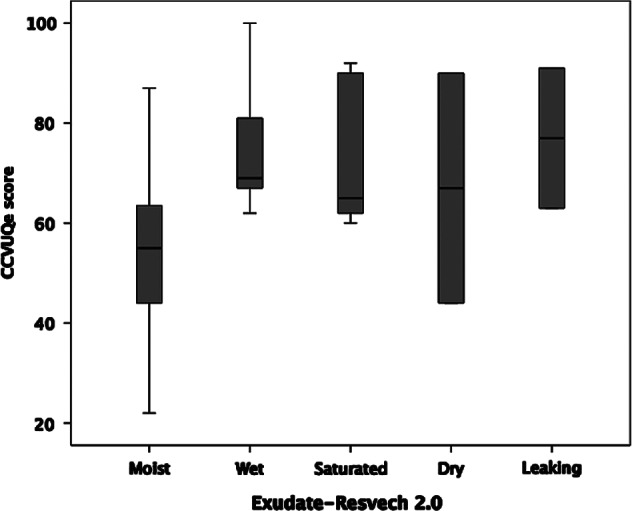
Box diagram of CCVUQe versus exudate.
We also found a statistically significant association between the total CCVUQe score and the sixth item on the RESVECH 2.0 scale, infection–inflammation (Pearson's correlation coefficient, r = 0·485; P = 0·004). This indicates that a higher likelihood of infection may negatively impact quality of life (Figure 4).
Figure 4.
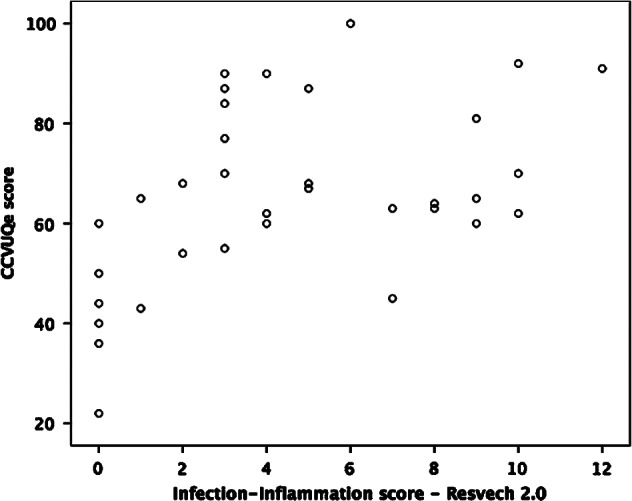
Correlation between CCVUQe and infection–inflammation sub‐score from RESVECH 2.0.
Discussion
One of the possible limitations of this study is its small sample size, 34 patients, recruited using non‐probability sampling. Non‐representativity is a frequent problem when non‐probability sampling methods are used. Nevertheless, the inclusion of subjects from the Primary Care Department Functional Wounds Unit largely palliates this problem as the unit, a specialised reference unit for the treatment of patients with chronic wounds within the scope of primary health care, serves venous ulcer patients from all over the island. However, as we mentioned in the Methods section, a post‐hoc statistical power analysis was made using the value of Pearson's correlation coefficient (r = 0·543) between total CCVUQe score and total RESVECH 2.0 score. We have used G*Power 3.1 28, a flexible statistical power analysis programme for the social, behavioural and biomedical sciences. Using a bivariate normal model to compute, we achieved a power value of 96%.
Although certain studies on HRQoL in venous ulcer patients have larger sample sizes, the design of many of them facilitates the inclusion of patients with wounds that are not venous ulcers 29, 30. This increases sample size but may introduce significant bias in the evaluation of results 9. Clear examples of this are the study by Guarnera et al., with a sample of 381 patients, of which only 52% were venous ulcers 16, and the one by Franks and Moffat, with a sample of 758 and a venous ulcer percentage of 66% 12. Accordingly, taking into account that Iglesias et al., in order to recruit a sample of 387 patients with exclusively venous ulcers, had to recruit in nine regions of the UK 31, we believe that a sample size of 34 in the environment where this study was conducted should be evaluated positively, although, as we have already said, this possible limitation should not be ruled out.
Another element to be taken into account is not having used the Ankle‐Brachial Pressure index (ABPI) when screening wounds to rule out possible ischaemic aetiology. It is worth noting that certain studies fail to provide information regarding the aetiology of the wounds presented by their sample subjects as in the case of Furtado et al. 13 and Jull et al. 32 In our case, we included only lesions of venous aetiology, excluding possible ischaemic involvement (hence, the inclusion only of wounds with a palpable pulse). We accept that screening using the ABPI would have been better 19, but we did not have the necessary technical means available to us when conducting the study.
One striking aspect is the gender as the majority subjects were male (55·88%), when most studies on this subject have a female majority 9, although there are exceptions, such as Wong et al. 33 Some studies report that female venous ulcer patients have poorer quality of life compared to men 16, although we found no significant relationship between gender and perceived quality of life.
Certain studies, including this one, explore the relationship between quality of life and level of education. We found no statistically significant relationship here, concurring with other published studies. Heinen et al. 30 and Yamada and Santos 34, respectively, reported that 65% and 62% of their samples had educational levels they classified as low. In our study, 20·59% had no formal studies, and 61·76% had only primary education. We may therefore assert that, although no direct relationship between educational level and perceived quality of life has been established, individuals with lower educational levels do appear to be more prone to suffering from venous ulcers. This may be related to the fact, already pointed out by certain authors, that venous ulcers mainly affect people with lower incomes and in lower social strata 35.
Regarding marital status, which we could relate with cohabitation and family support, our findings were not so consistent with those of other studies. Compared with the 20·59% of widows/widowers in our sample, Franks et al. report percentages of over 40% 36; compared to our 58·82% of married people, the same authors report percentages of less than 30% 36.
The shortage of studies on quality of life in venous ulcer patients in Spain means that we cannot contrast our findings against studies for which the family aspect may be extrapolated, although the authors who validated the Spanish version of the CCVUQ obtained percentages quite similar to ours in a sample of 65 patients (48·3% were married, 38·3% widowed, 8·3% divorced and 5% single; 73·3% of their cases had either no studies or primary‐level studies) 37. It would be interesting to explore these variables in future studies as they may indicate a discordance in the study location regarding the family cohabitation model adopted by subjects and how it influences perceived quality of life. This aspect has already been pointed out by certain authors 33.
One complex aspect of venous ulcer treatment is compression, and it appears that the use of compression may be related to lower recurrence rates 38. While the consensus is that compression is an essential aspect of venous ulcer therapy 39, there is currently little evidence available regarding which type of compression is most effective 26, 40. Accordingly, when designing our study, we did not distinguish the type of compression used, although the ‘use of compression therapy’ variable was included to see if we could detect a significant relationship between the use of compression and quality of life; as it happens, we did not. In Spain, compression therapy is as yet insufficiently implemented as treatment for venous ulcers 1.
Another point to note is the scarcity of studies reflecting perceived quality of life as a secondary measurement of the effectiveness of compression therapy as most use other values (usually pain) that, although related to quality of life, should be considered indirect measurements 40. Therefore, as O'Meara et al. report, the inclusion of quality of life measurements using suitable measuring tools (such as specific questionnaires like the one used in this study) is one of the shortfalls to be addressed by investigators approaching this subject in the future 40.
In general, when evaluating studies on HRQoL and venous ulcers, we found that although great importance was given to the quality of life measuring system, whether a generic or a specific system was used, the fundamental aspect of wound characterisation had been neglected. A recent review of the subject referred specifically to this aspect 9. This has negative repercussions when assessing which characteristics inherent to venous ulceration have a greater impact on quality of life as it makes it difficult to compare wounds across studies.
This happens because most studies either fail to use validated monitoring systems or use systems that are not valid for venous ulceration. Ferreira et al. used the PUSH (Pressure Ulcer Scale for Healing) system in their validation of the Cardiff Wound Impact Schedule (CWIS) 41. However, while this is valid for pressure ulcers, its use for venous ulcers is, to say the least, controversial 21. Accordingly, implementing specific validated monitoring systems for each wound type is crucial in order to faithfully reflect the status of the chronic wound being studied. We opted to monitor wounds using two systems specifically validated for venous ulcers.
The first is the MAID score, a specific scale to measure the severity of leg wounds 20. This scale should be understood as a gauge of the probability of healing. Its descriptive capacity is limited, but it has in its favour the fact that it is very easy to use, making it very practical at the clinical level as well as an effective tool to aid decision making 20. In our study, although we observed a certain tendency for quality of life to decrease as scores rose, the relationship was not statistically significant. In addition, our study design excluded wounds scoring 4 on the scale as palpable pulse was a criterion for inclusion on the study. This limitation should be taken into account when using this scale to evaluate venous lesions. As the creators of this scale stress, adequate and standardised wound care is an indispensable prerequisite for it to be a valid diagnostic tool 20.
The second system we used was RESVECH 2.0 21. We believe that this system is destined to be one of the most useful tools for the monitoring of chronic wounds, in both research and in clinical practice 27. RESVECH 2.0 assesses six parameters: wound size, depth of the affected tissues, status of the edges, type of tissue in the wound bed, level of exudate and inflammation‐infection of the wound (presence of biofilm). This system gives a total score that establishes wound severity, making it possible to compare wounds very descriptively 21.
Our study aimed to give equal importance to two aspects (measuring quality of life and characterising wounds) as we considered it important to establish whether there was a relationship between severity of the lesions and loss of quality of life. In view of our results, the link appears to be clear as we found a statistically significant association between the total CCVUQe score and total RESVECH 2.0 score, indicating that a higher RESVECH 2.0 score correlates with greater loss of quality of life. While the relationship between loss of quality of life and venous ulceration is well established in the literature 9, 42, the relationship between quality of life and severity of the lesion has not as yet been clearly established. Accordingly, our findings demand further research in this direction. We consider this to be quite important as the RESVECH 2.0 scores of the wounds in our study were not particularly high (13·15 ± 5·07). Higher scores may be expected to go hand‐in‐hand with greater HRQoL loss.
The most frequently occurring wound in our study was a recurrent venous ulcer progressing over more than 4 months, no greater than 16 cm2 in size, affecting epidermal and/or dermal tissue, with defined edges, slough or necrotic tissue in the wound bed and optimum moisture. With regard to infection, most wounds had several symptoms/signs compatible with critical colonisation/infection 43 or possibly established biofilm. Most ulcers were painful and healed very slowly.
Bearing in mind the difficulty of comparing across studies, for the reasons already given, we may, however, affirm that most of the wounds we studied were similar in size to those included in other studies 29, 30, 31, 33, 36. Only Jankunas et al. 15, 44 with a mean surface area of 50 cm2, and Guarnera et al. 16 where 30% of wounds were greater than 30 cm2 in size, reported significantly larger surface areas. The importance of surface area resides in the fact that certain authors found a statistically significant relationship between area and pain 15, 16 and between area and social isolation 12. Analysing area as an independent parameter, we observed no such relationship. In this, we concur with Hareendran et al., who found no direct association between wound size and quality of life 11.
However, there is an association between exudate and quality of life score. This parameter was not evaluated in depth in the studies reviewed, once again highlighting deficiencies in wound monitoring. This is particularly significant when we take into account that this parameter has already been signalled as a direct cause of loss of quality of life by many authors, including Jones et al. 17 and Green and Jester 42. Although measuring exudate can be problematic 45, exudate monitoring systems need to be used in future studies on venous ulcers. We consider that RESVECH 2.0 may be a useful tool in this regard.
Another variable identified as directly responsible for loss of quality of life was the presence of non‐viable tissue in the wound bed (slough, necrotic tissue). The importance of controlling non‐viable tissue in the wound bed as a priority strategy towards the definitive healing of chronic wounds has already been pointed out 46. However, other investigators did not report this aspect as having a negative impact on HRQoL in venous ulcer patients. Our findings show that controlling non‐viable tissue is also essential to ensure that quality of life is impacted as little as possible. Accordingly, choosing an effective debridement method is a fundamental aspect of the treatment of venous ulcers.
We also detected a statistically significant association between perceived loss of quality of life and the presence of infection–inflammation in wounds. This is understandable as infection is closely linked to other areas such as pain, exudate and odour 43. Nevertheless, as the EWMA position document ‘Identifying Criteria for Wound Infection’ points out, detecting these signs and symptoms is not always easy 42. The document warns of the importance of signs ‘of a subtle nature’ when diagnosing and making clinical decisions 43. Moreover, chronic wound types may present with different clinical signs of infection 43. The RESVECH 2.0 monitoring system aims to respond to this problem, integrating a large variety of these subtle signs and giving great weight to this variable in the overall score.
None of the studies reviewed for our study observed a direct relationship between infection and loss of quality of life in venous ulcer patients. Although it may appear logical to infer a relationship between infection–inflammation, presence of biofilm and loss of quality of life, the manner in which infection affects quality of life has not been sufficiently well explained. Infected wounds are more painful and also slower to heal. Infection may also be responsible for relapses. We believe that future studies should try to clarify this relationship.
What our findings do highlight is that venous ulcers tend to recur and are slow to heal, concurring with many other studies published to date 12, 13, 16, 47. We observed that 47·06% of wounds were newly emerging, while 52·94% were recurrences; 38·02% of wounds in our sample had progressed over more than 12 months. Although no direct relationship between recurrence and quality of life has been established, we did observe a certain tendency of recurrent wounds to impact more negatively on quality of life. This has also been reported by other authors 12, 16.
We did not monitor pain using a specific system, although certain authors have identified this aspect as the factor that most determines loss of quality of life in venous ulcer patients 9. CCVUQ question 1 evaluates this aspect, so we understand that, in theory, the evaluation of pain as a negative impact on quality of life is already included when the questionnaire is applied.
Apart from the cultural adaptation and validation of CCVUQe 23, this is the first study conducted in Spain to assess HRQoL in venous ulcer patients using the specific CCVUQe questionnaire. As it happens, very few studies conducted in this country evaluate quality of life in these patients 36, 48. Our study shows that venous ulcer patients suffer a decline in quality of life, closely related to the general status of their wounds: the more serious the ulcer, the greater the loss of quality of life. Accordingly, overall wound status directly impacts physical and emotional status as well as social well‐being.
One very striking aspect of our findings is the fact that what impacted quality of life most heavily were emotional factors, rather than others such as difficulty in performing certain tasks or influence on social environment. This concurs with what we observed during the CCVUQe cultural adaptation and validation process 36. Worrying that their ulcers are not healing and time spent thinking about their wounds are constant concerns of these patients. This makes it necessary to rethink the therapeutic model offered to venous ulcer patients, towards one that addresses not only clinical aspects but also other frequently forgotten elements, such as psychological and emotional support 8. The impact of wounds on physical appearance (cosmesis) is another important aspect to be taken into account when treating these patients.
Also key in this regard is entrusting the care of these patients to professionals trained specifically for the purpose. In fact, Franks and Moffatt found that patients treated in nurse‐led specialised leg ulcer clinics experienced better HRQoL than patients treated by non‐expert professionals 12.
Conclusions
Venous ulcers are high‐prevalence chronic lesions. They are usually painful, recurrent and slow to heal, notably impacting quality of life. However, we believe that further study is required as although there is much information available about HRQoL in people with venous ulcers, reflecting the huge interest in the subject among the scientific community, there is little evidence available on how to improve patients' quality of life. New strategies need to be developed to treat these patients, including plans not only to mitigate the effects of the wounds but also to prevent recurrence and the emergence of new wounds.
Treatment plans should also place special importance on wound monitoring. Using a validated monitoring system will allow professionals to properly assess the parameters of the wound and to apply specific therapeutic measures for each of their aspects. Proper exudate control, debridement of non‐viable tissue and lowering the bacterial load are essential not only to achieve healing but also to prevent loss of quality of life. Accordingly, wound monitoring should be a key element in the treatment of venous ulcer patients and needs to be performed using validated systems that allow for the comparison of results.
Another important factor when treating venous ulcer patients is their emotional well‐being. In addition to purely physical signs and symptoms, patients suffer other manifestations, such as body image disorders, social isolation, decreased willpower, impotence, loss of self‐confidence, depression, despair, frustration, dissatisfaction, low self‐esteem, feelings of dirtiness, anxiety, anger, discrimination and rejection by their partners. Evaluating and addressing these factors is just as important as the technical aspects of ulcer care. Emotional and psychological support is essential for these patients.
Acknowledgements
We would like to acknowledge all the patients who have contributed to this work. We also appreciate the help of the ‘Servicio de Traducción de la Universidad de Alicante’ (Translation Service at the University of Alicante) on the translation of the paper into English.
References
- 1. Soldevilla J, Torra JE, Verdu J, Rueda J, Martinez F, Roche E. Epidemiology of chronic wounds in Spain: results of the First National Studies on Pressure and Leg Ulcer Prevalence. Wounds 2006;18:213–26. [Google Scholar]
- 2. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care 2009;18:154–61. [DOI] [PubMed] [Google Scholar]
- 3. Verdú J, Marine‐lo J, Armans E, Carreño P, March JR, Martín V, Soldevilla J. Documento de Consenso CONUEI. Conferencia Nacional de Consenso sobre Úlceras de la Extremidad Inferior‐CONUEI. Spain: EdiKaMed, SL, 2009. ISBN 978‐84‐7877‐555‐2. [Google Scholar]
- 4. Jones KR. Why do chronic venous leg ulcers not heal? J Nurs Care Qual 2009;24:116–24. [DOI] [PubMed] [Google Scholar]
- 5. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–6. [DOI] [PubMed] [Google Scholar]
- 6. Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5. [PubMed] [Google Scholar]
- 7. European Wound Management Association (EWMA) . Position document: hard‐to‐heal wound: an holistic approach. London: MEP Ltd, 2008. [Google Scholar]
- 8. Wounds International . Optimising wellbeing in people living with a wound. An expert working group review. London: Wounds International, 2012. URL http://www.woundsinternational.com. [Google Scholar]
- 9. González‐Consuegra RV, Verdú Soriano J. Quality of life in people with venous ulcers: integrative review. J Adv Nurs 2011;67:926–44. [DOI] [PubMed] [Google Scholar]
- 10. Urzúa A. Calidad de vida relacionada con la salud: Elementos conceptuales. Rev Med Chile 2010;138:358–65. [PubMed] [Google Scholar]
- 11. Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, Symonds T. Measuring the impact of venous leg ulcers on quality of life. J Wound Care 2005;14:53–7. [DOI] [PubMed] [Google Scholar]
- 12. Franks PJ, Moffatt CJ. Do clinical and social factors predict quality of life in leg ulceration? Int J Low Extrem Wounds 2006;5:236–43. [DOI] [PubMed] [Google Scholar]
- 13. Furtado K, Pina E, Moffatt CJ, Franks PJ. Leg ulceration in Portugal: quality of life. Int Wound J 2008;5:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones J, Barr W, Robinson J, Carlisle C. Depression in patients with chronic venous ulceration. Br J Nurs 2006;15:S17–23. [DOI] [PubMed] [Google Scholar]
- 15. Jankūnas V, Rimdeika R, Jasenas M, Samsanavicius D. Changes in patient's quality of life comparing conservative and surgical treatment of venous leg ulcers. Medicina (Kaunas) 2004;40:731–9. [PubMed] [Google Scholar]
- 16. Guarnera G, Tinelli G, Abeni D, Di Pietro C, Sampogna F, Tabolli S. Pain and quality of life in patients with vascular leg ulcers: an Italian multicentre study. J Wound Care 2007;16:347–51. [DOI] [PubMed] [Google Scholar]
- 17. Jones JE, Robinson J, Barr W, Carlisle C. Impact of exudate and odour from chronic venous leg ulceration. Nurs Stand 2008;22:53–4, 56, 58 passim. [DOI] [PubMed] [Google Scholar]
- 18. Douglas V. Living with a chronic leg ulcer: an insight into patients' experiences and feelings. J Wound Care 2001;10:355–60. [DOI] [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 20. Beckert S, Pietsch AM, Küper M, Wicke C, Witte M, Königsrainer A, Coerper S. M.A.I.D.: a prognostic score estimating probability of healing in chronic lower extremity wounds. Ann Surg 2009;249:677–81. [DOI] [PubMed] [Google Scholar]
- 21. Restrepo Medrano, JC . Verdú Soriano, J . Instrumentos de monitorización clínica y medida de la cicatrización en úlceras por presión (UPP) y úlceras de la extremidad inferior (UEI). Desarrollo y validación de un índice de medida, 2010. Doctoral thesis [online]. URL http://www.gneaupp.es/app/adm/publicaciones/archivos/40_pdf.pdf [accessed on 23 January 2012].
- 22. Smith JJ, Guest MG, Greenhalgh RM, Davies AH. Measuring the quality of life in patients with venous ulcers. J Vasc Surg 2000;31:642–9. [DOI] [PubMed] [Google Scholar]
- 23. González‐Consuegra RV, Verdú Soriano J. Proceso de adaptación al castellano del Charing Cross Venous Ulcer Questionnaire (CCVUQ) para medir la calidad de vida relacionada con la salud en pacientes con úlceras venosas. Gerokomos 2010;21:80–7. [Google Scholar]
- 24. Registered Nurses Association of Ontario . Assessment and management of venous leg ulcers. Toronto: Registered Nurses Association of Ontario, 2004. [Google Scholar]
- 25. Gillespie DL, Writing Group III of the Pacific Vascular Symposium 6 , Kistner B, Glass C, Bailey B, Chopra A, Ennis B, Marston B, Masuda E, Moneta G, Nelzen O, Raffetto J, Raju S, Vedantham S, Wright D, Falanga V. Venous ulcer diagnosis, treatment, and prevention of recurrences. J Vasc Surg 2010;52(5Suppl):8S–14. [DOI] [PubMed] [Google Scholar]
- 26. World Union of Wound Healing Societies (WUWHS) . Principles of best practice: compression in venous leg ulcers. A consensus document. London: MEP Ltd, 2008. [Google Scholar]
- 27. Restrepo Medrano JC, Verdú Soriano J. Development of a wound healing index for chronic wounds. EWMA Journal 2012;12:39–44. [Google Scholar]
- 28. Faul F, Erdfelder E, Lang A‐G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioural, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 29. Franks PJ, McCullagh L, Moffatt CJ. Assessing quality of life in patients with chronic leg ulceration using the Medical Outcomes Short Form‐36 questionnaire. Ostomy Wound Manage 2003;49:26–37. [PubMed] [Google Scholar]
- 30. Heinen MM, Persoon G, van Kerkhof P, Otero M, van Achterberg T. Ulcer‐related problems and health care needs in patients with venous leg ulceration: a descriptive, cross‐sectional study. Int J Nurs Stud 2007;44:1296–303. [DOI] [PubMed] [Google Scholar]
- 31. Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and disease specific instruments. Qual Life Res 2005;14:1705–18. [DOI] [PubMed] [Google Scholar]
- 32. Jull A, Walker N, Hackett M, Jones M, Rodgers A, Birchall N, Norton R, MacMahon S. Leg ulceration and perceived health: a population based case‐control study. Age Ageing 2004;33:236–41. [DOI] [PubMed] [Google Scholar]
- 33. Wong IK, Lee DT, Thompson DR. Translation and validation of the Chinese version of the Charing Cross Venous Ulcer Questionnaire. J Clin Nurs 2006;15:356–7. [DOI] [PubMed] [Google Scholar]
- 34. Yamada BFA, Santos VLCG. Quality of life of individuals with chronic venous ulcers. Wounds 2005;17:178–89. [Google Scholar]
- 35. Abbade LP, Lastória S, de Almeida Rollo H, Stolf HO. A sociodemographic, clinical study of patients with venous ulcer. Int J Dermatol 2005;44:989–92. [DOI] [PubMed] [Google Scholar]
- 36. Franks PJ, Moffatt CJ, Doherty DC, Smithdale R, Martin R. Longer‐term changes in quality of life in chronic leg ulceration. Wound Repair Regen 2006;14:536–41. [DOI] [PubMed] [Google Scholar]
- 37. González‐Consuegra, RV . Verdú Soriano, J . Calidad de vida y cicatrización en pacientes con úlceras de etiología venosa. Cross‐cultural adaptation and validation of the Charing Cross Venous Ulcer Questionnaire (CCVVQQ) and PUSH (Pressure Ulcer Scale for Healing), 2011. Doctoral thesis [online]. URL http://www.gneaupp.es/app/publicaciones/default.asp?id=6 [accessed on 30 December 2015].
- 38. Nelson EA, Bell‐Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2012;8:CD002303. [DOI] [PubMed] [Google Scholar]
- 39. European Wound Management Association (EWMA) . Position document: understanding compression therapy. London: MEP Ltd, 2003. [Google Scholar]
- 40. O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2009:21:CD000265. doi: 10.1002/14651858.CD000265.pub2. [DOI] [PubMed] [Google Scholar]
- 41. Ferreira P, Miguéns C, Gouveia J, Furtado K. Medicao da qualidade de vida de docentes como feridas crônicas: a Escala de cicatrização da úlcera de Pressão e o Esquema Cardiff de Impacto da Ferida. Nursing 2007;221:32–41. [Google Scholar]
- 42. Green J, Jester R. Health‐related quality of life and chronic venous leg ulceration: Part 2. Br J Community Nurs 2010;15:S4–6, S8, S10, passim. [DOI] [PubMed] [Google Scholar]
- 43. European Wound Management Association (EWMA) . Position document: identifying criteria for wound infection. London: MEP Ltd, 2005. [Google Scholar]
- 44. Jankunas V, Bagdonas R, Samsanavicius D, Rimdeika R. The influence of surgical treatment for chronic leg ulcers on the quality dynamics of the patient's life. Acta Chir Belg 2007;107:386–96. [DOI] [PubMed] [Google Scholar]
- 45. World Union of Wound Healing Societies (WUWHS) . Principles of best practice: wound exudate and the role of dressings. A consensus document. London: MEP Ltd, 2007. [Google Scholar]
- 46. Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA. Maintenance debridement in the treatment of difficult‐to‐heal chronic wounds. Recommendations of an expert panel. Ostomy Wound Manage 2008;Suppl:2–13; quiz 14–5. [PubMed] [Google Scholar]
- 47. Hareendran R, Doll H, Wild DJ, Moffatt CJ, Musgrove E, Wheatley C, Franks PJ. The venous leg ulcer quality of life (VLU‐QoL) questionnaire: development and psychometric validation. Wound Repair Regen 2007;15:465–73. [DOI] [PubMed] [Google Scholar]
- 48. González‐Consuegra RV, Verdú Soriano J. Calidad de vida relacionada con heridas crónicas. Gerokomos 2010;21:131–9. [Google Scholar]


