Abstract
The objective of this work was to evaluate the safety and effectiveness of a next‐generation antimicrobial wound dressing (NGAD; AQUACEL ® Ag+ Extra™ dressing) designed to manage exudate, infection and biofilm. Clinicians were requested to evaluate the NGAD within their standard protocol of care for up to 4 weeks, or as long as deemed clinically appropriate, in challenging wounds that were considered to be impeded by suspected biofilm or infection. Baseline information and post‐evaluation dressing safety and effectiveness data were recorded using standardised evaluation forms. This data included wound exudate levels, wound bed appearance including suspected biofilm, wound progression, skin health and dressing usage. A total of 112 wounds from 111 patients were included in the evaluations, with a median duration of 12 months, and biofilm was suspected in over half of all wounds (54%). After the introduction of the NGAD, exudate levels had shifted from predominantly high or moderate to low or moderate levels, while biofilm suspicion fell from 54% to 27% of wounds. Wound bed coverage by tissue type was generally shifted from sloughy or suspected biofilm towards predominantly granulation tissue after the inclusion of the NGAD. Stagnant (65%) and deteriorating wounds (27%) were shifted to improved (65%) or healed wounds (13%), while skin health was also reported to have improved in 63% of wounds. High levels of clinician satisfaction with the dressing effectiveness and change frequency were accompanied by a low number of dressing‐related adverse events (n = 3; 2·7%) and other negative observations or comments. This clinical user evaluation supports the growing body of evidence that the anti‐biofilm technology in the NGAD results in a safe and effective dressing for the management of a variety of challenging wound types.
Keywords: AQUACEL® Ag+ Extra™, Biofilm, Exudate, Infection, Safety
Introduction
Wound dressings have an important role to play in any clinical protocol of care, particularly with respect to exudate and infection management. In recent years, biofilm has emerged as another important local impediment to wound healing, and although biofilm‐based wound care (involving debridement, cleansing and antimicrobial dressings) is increasingly utilised by some clinicians 1, 2, dressings designed to combat wound biofilm have been lacking. Most antimicrobial dressings and antibiotics available today pre‐date the recognition of biofilm as a problem in wound care 3, 4, 5, 6.
Biofilm‐protected microorganisms are notoriously difficult to combat, and antimicrobial therapy frequently fails, leading to recurrent infections 7. Consequently, the consideration of anti‐biofilm agents in combination with an antimicrobial agent (antibiotic or antiseptic) is a likely important future strategy in enhancing microbial susceptibility in biofilm‐associated infections. This approach has recently been introduced in wound care with a wound dressing that contains both anti‐biofilm (biofilm destabilising excipients) and antimicrobial agents (ionic silver) 8. This next‐generation antimicrobial dressing (NGAD AQUACEL® Ag+ Extra™ dressing) was the culmination of 5 years of research and development and was designed to manage wound biofilm, infection and exudate 4. The effectiveness of the NGAD has been demonstrated both in vitro 9, 10 and in vivo, where complete wound healing was consistently observed in a considerable proportion of patients 11, 12.
The objective of the current clinical user evaluation was to conduct post‐market clinical surveillance on the safety and effectiveness of the NGAD in challenging, non‐healing wounds that were considered to be impeded by suspected biofilm, as judged by direct or indirect clinical indicators of biofilm 13, 14 or infection 15.
Methods
Patient inclusion
The dressing was evaluated on patients with challenging wounds from over 60 health care facilities (hospitals, clinics, nursing homes) and community settings across the United Kingdom and Ireland between February and September 2014. The evaluating clinicians were all experienced in tissue viability or podiatry and had previous experience with sodium carboxymethylcellulose (Na‐CMC) wound dressings. While there were no strict inclusion or exclusion criteria, the clinicians were asked to use their discretion in the selection of patients with particularly challenging wounds that were failing to demonstrate progression towards healing (i.e. stagnant or deteriorating) and that were considered to be impeded by biofilm and/or infection. As the NGAD had gained regulatory clearance for clinical use in Europe, ethical committee approval was not required 16. As this was not a clinical research study, written informed consent was not essential; however, verbal consent was obtained between clinician and patient before commencement.
Dressing usage
Clinicians were requested to continue managing their patients with their own standard protocols of wound care (i.e. no biofilm‐based protocols were followed) but to replace their previously used primary dressing with the NGAD for up to 4 weeks, or as long as deemed clinically appropriate. Although it is acknowledged that variations in protocols of care would be expected by clinical setting, it was expected that clinical best practice was followed, for example, compression for venous leg ulcers and antibiotics as per institutional protocols. Each clinician was primarily asked to evaluate each wound before and after the NGAD evaluation using standardised evaluation forms as used previously 12.
Baseline assessment
The evaluation form was used by each clinician to record relevant patient demographic information, medical history and the following baseline wound information:
Type of wound;
Duration of wound (months);
Wound status (stagnant; deteriorating);
Previously used wound management (including antimicrobial agents and dressings);
Wound infection status (yes; no), as judged by clinician, based on multiple signs of infection 11, 17;
Signs and symptoms of infection (pain; erythema; oedema; heat/warmth; foul odour; purulent exudate; discolouration of granulation tissue; friable granulation tissue) 15 and suspected biofilm 13, 14;
Estimate of approximate percentage of tissue types present on the wound bed [necrotic; sloughy; suspected biofilm 13, 14; granulation];
Exudate level (low; moderate; high); and
Condition of surrounding skin (healthy; macerated; dry/eczematous).
Final assessment
At final assessment, the following information was recorded on the evaluation form to assess wound progress:
Evaluation duration (weeks);
Overall wound status (healed; improved; same; deteriorated);
Approximate percentage of tissue types present on the wound bed [necrotic; sloughy; suspected biofilm 13, 14; granulation; epithelialisation];
Exudate level (low; moderate; high);
Change in surrounding skin condition (improved; same; deteriorated);
Overall performance of NGAD when compared to previous dressing (less effective; same; more effective);
Frequency of dressing changes compared to previous dressing used (less; same; more);
Whether the clinician would continue to use NGAD (yes; no);
Whether they would recommend NGAD to a colleague (yes; no);
Any adverse events or reasons why dressing use was stopped; and
Additional comments/feedback.
Results
Sample
A total of 111 patients were evaluated. One patient had two wounds suitable for inclusion, so the final sample size was n = 112.
Patients
The final sample (n = 112) consisted of 63 males and 47 females (one patient's gender was unrecorded) with a median age of 72 years (mean: 69 years; range: 18–96 years; Table 1). Venous leg ulcers (VLUs) accounted for 30% of the sample population (n = 31; Table 1). Wounds were further categorised by duration, with the median duration before evaluation being 12 months (mean: 32·2 months; range: 1 week to 30 years; Table 1; Figure 1). Of particular note were the median durations of the 31 VLUs (12 months), 13 arterial ulcers (24 months) and 11 pressure ulcers (24 months).
Table 1.
Evaluation sample patients by wound type, age and duration
| Wound type | No. of wounds (%) | Mean patient age (range) [years] | Wound duration [months] | |
|---|---|---|---|---|
| Median | Mean (range) | |||
| Venous leg ulcer | 31 (28) | 73 (41–92) | 12·0 | 58·4 (3·0–360) |
| Mixed aetiology | 16 (14) | 74 (43–89) | 11·0 | 32·8 (0·5–240) |
| Arterial ulcer | 13 (12) | 75 (57–96) | 24·0 | 27·3 (1·8–84) |
| Pressure ulcer | 11 (10) | 64 (26–84) | 24·0 | 16·7 (3·0–30) |
| Diabetic foot ulcer | 10 (9) | 58 (18–90) | 3·0 | 21·9 (0·3–96) |
| Leg ulcer | 4 (4) | 70 (57–83) | 6·0 | 11·0 (3·0–24) |
| Traumatic | 2 (2) | 90 | 12·0 | – |
| Cyst | 1 (1) | 45 | 10·0 | – |
| Other | 16 (14) | 70 (31–93) | 5·5 | 14·7 (0·3–48) |
| Not given | 8 (7) | 57 (30–85) | 12·0 | 20·4 (6·0–54) |
| ALL | 112 | 69 (18–96) | 12·0 | 32·2 (0·3–360) |
Figure 1.
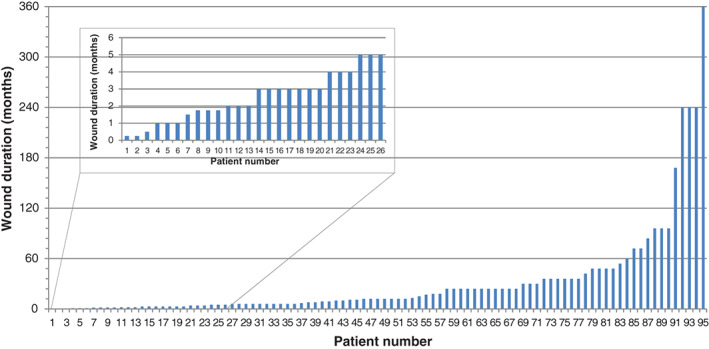
Wound durations. Note: information was not given for 17 wounds.
The antimicrobial products used prior to the NGAD evaluations are shown in Figure 2. Silver dressings were the most frequently used product, with AQUACEL® Ag dressings the most used. Systemic antibiotics followed by iodine dressings, honey products and PHMB products were the next most frequently used products on these stagnant or deteriorating wounds.
Figure 2.
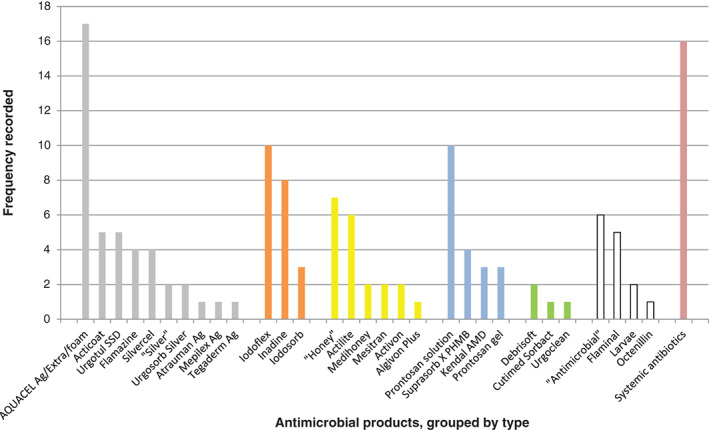
Antimicrobial products used prior to the NGAD evaluations. Products are grouped by type ( , silver;
, silver;  , iodine;
, iodine;  , honey;
, honey;  , PHMB;
, PHMB;  , debridement;
, debridement;  , other;
, other;  , systemic antibiotics). ‘Silver’, ‘Honey’ and ‘Antimicrobial’ were recorded as such. In some cases, multiple antimicrobial products were used either concurrently or sequentially; in 19 cases, no antimicrobial product was used; and in 9 cases, no product information was recorded.
, systemic antibiotics). ‘Silver’, ‘Honey’ and ‘Antimicrobial’ were recorded as such. In some cases, multiple antimicrobial products were used either concurrently or sequentially; in 19 cases, no antimicrobial product was used; and in 9 cases, no product information was recorded.
Baseline measurements
At baseline, 73 wounds (65%) were judged to be stagnant, while 30 (27%) were judged to be deteriorating (9 were not classified). Thirty‐five wounds (31%) were judged to be infected 15, 54 (48%) were judged not to be infected, while 23 wounds (21%) were not classified on infection status. The number of wounds with clinical signs of infection and suspected biofilm 13, 14 is shown in Figure 3. There was some correlation between the clinician's judgment on infection status and the average number of clinical signs reported in that wounds judged to be infected showed an average of 3·7 (median: 4) signs of infection, whereas wounds judged not to be infected showed only 1·9 (median: 2) signs. Biofilm was suspected more than the observation of any other clinical sign of infection (in 54% of wounds), suggesting that biofilm presence was a likely contributory factor in the non‐healing status of many of these wounds. Figure 4 shows the relationship between suspected biofilm and increased numbers of other clinical signs of infection. While there was a possible slight increase in biofilm suspicion with increasing numbers of clinical signs, biofilm presence was also apparent in wounds that lacked multiple signs of infection (i.e. biofilm‐impeded wounds without infection).
Figure 3.
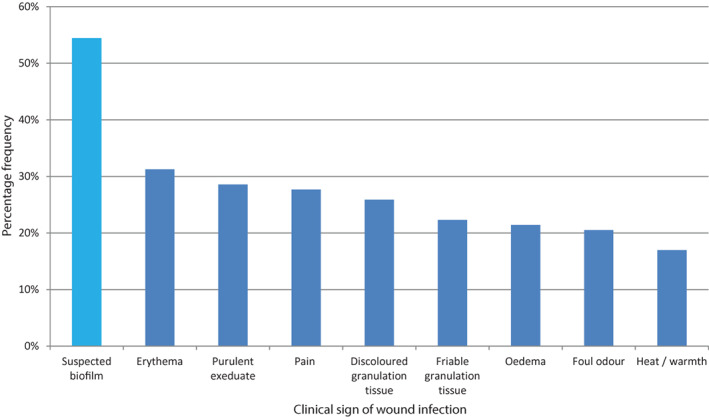
Percentage of patients with clinical signs of wound infection 10 [plus suspected biofilm 8, 9;  ].
].
Figure 4.
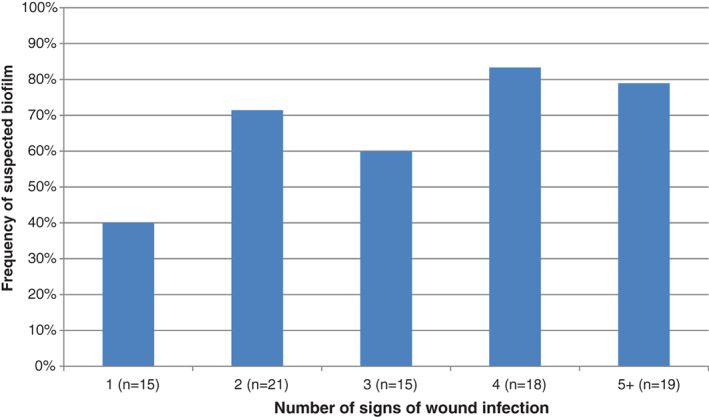
Relationship between the number of signs of wound infection observed 10 and frequency of suspected biofilm 8, 9.
Baseline versus final measurements
Exudate
Exudate levels were shifted from predominantly high (n = 43; 38%) or moderate (n = 54; 48%) at baseline (Figure 5A) to predominantly low (n = 48; 43%) or moderate (n = 44; 39%) after evaluation (Figure 5B). The number of wounds with high levels of exuate reduced from 43 (38%) to 10 (9%).
Figure 5.
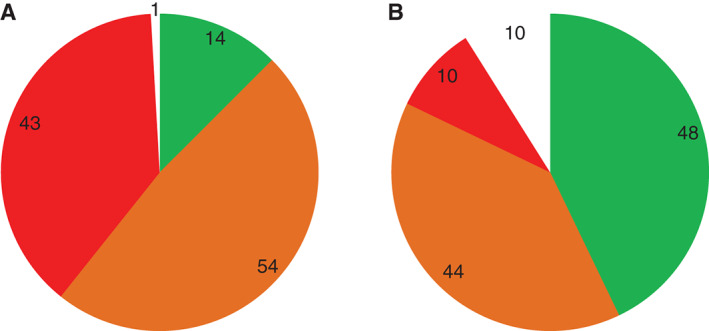
Frequencies of exudate levels recorded at (A) baseline and (B) after evaluation ( , low/healed;
, low/healed;  , medium;
, medium;  , high;
, high;  , no information given).
, no information given).
Suspected biofilm
In the absence of an available point‐of‐care biofilm detection method, biofilm suspicion and approximate coverage was judged by clinicians according to the suggested visible and indirect signs of biofilm previously published 13, 14. At baseline, over half of all wounds (n = 61; 54%) were judged to contain biofilm, which was reduced to 27% of wounds (n = 30) after evaluation (Table 2). Suspected biofilm coverage of all wound beds at baseline was judged to be 40% on average, falling to 16% after the evaluations. Of those wounds that were identified as containing suspected biofilm at baseline and after evaluation, this biofilm coverage reduced from an average of 82% (of 61) to 59% (of 30).
Table 2.
| Baseline | Final assessment | |
|---|---|---|
| Suspected biofilm in wound | 54% (n = 61) | 27% (n = 30) |
| Mean approx. biofilm coverage of wound bed – all wounds (n = 112) | 40 ± 45% | 16 ± 33% |
| Mean approx. biofilm coverage of wound bed – biofilm‐suspected wounds only (n = 61/30) | 82 ± 28% | 59 ± 40% |
Wound bed
Clinicians were asked to judge the approximate coverage of wound beds by different tissue types. Figure 6 shows how suspected biofilm, as an average percentage of all wound beds, shifted from 40% at baseline to 16% after evaluation. Similarly, sloughy tissue was judged to reduce from an average of 31–17% coverage. These reductions in undesirable tissue types were accompanied by a shift in granulation tissue, from an average of 27% coverage at baseline to 46% after evaluation.
Figure 6.
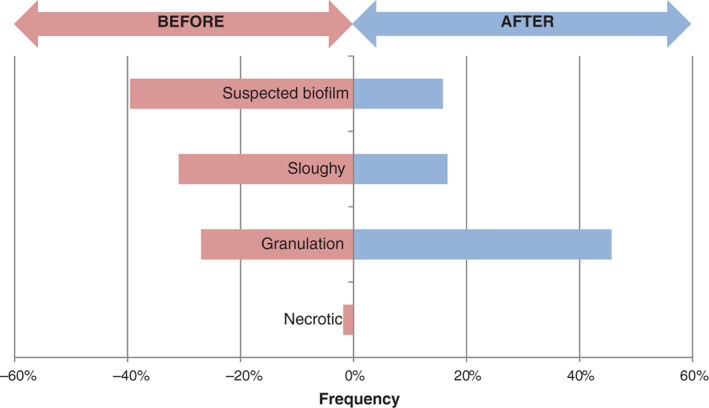
Average estimated wound bed coverage as a percentage of different tissue types at baseline ( ) and after evaluation (
) and after evaluation ( ).
).
Wound status
There was a marked shift from stagnant wounds (n = 73; 65%) or deteriorating wounds (n = 30; 27%) at baseline to improved (n = 73; 65%) or healed (n = 14; 13%) after the NGAD evaluations, while only 6 (5%) were still classed as deteriorating (Table 3; Figure 7). Overall, 87 wounds (78%) demonstrated wound progression either towards or obtaining healing.
Table 3.
Wound status and skin health at baseline and after evaluation
|
Wound status* Deteriorating |
Stagnant/same | Improved | Healed |
Skin health† Healthy |
Macerated | Dry/eczematous | |
|---|---|---|---|---|---|---|---|
| Baseline | 30 | 73 | 20 | 41 | 40 | ||
| Deteriorating | Same | Improved | |||||
| Final assessment | 6 | 15 | 73 | 14 | 8 | 27 | 70 |
Information not given for nine wounds at baseline and five wounds at final assessment.
Information not given for 11 wounds at baseline and seven wounds at final assessment.
Figure 7.
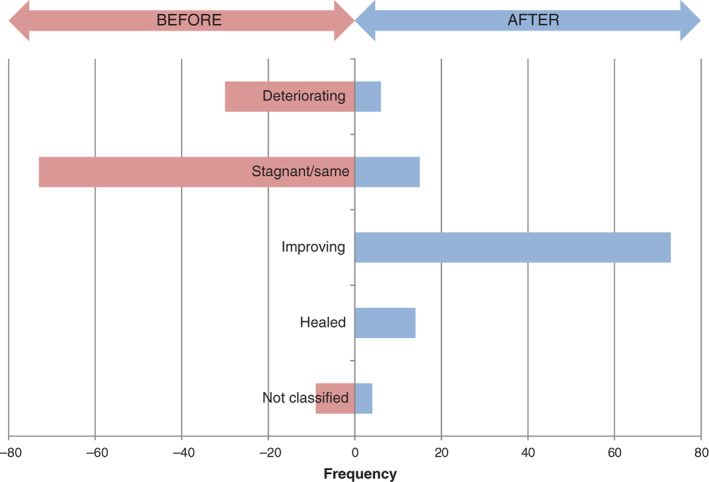
Wound status at baseline ( ) and after evaluation (
) and after evaluation ( ).
).
Skin health
Skin health was generally poor at baseline with 81 reports of macerated or dry/eczematous skin (72%). However, skin health was judged to have improved in 70 wounds (63%) after evaluation (Table 3).
Dressing usage
For all evaluations, the evaluation period ranged from 1–16 weeks, and the average evaluation time was 3·9 weeks. Clinicians judged the NGAD to be more effective than their previously used primary dressing in at least 72% (n = 81) of cases (Table 4). Only three clinicians judged the dressing to be less effective, and this related to three of the wounds that failed to progress. Frequency of use of the NGAD was rated as mainly the same (43%) or less (42%) when compared to the previously used dressing. Ninety clinicians (80% of total; 91% of respondents) said they would continue to use the NGAD, while 95 clinicians (85% of total; 99% of respondents) said they would recommend the dressing to a colleague (Table 4).
Table 4.
NGAD dressing usage
| Less | Same | More | |
|---|---|---|---|
| Overall efficacy of NGAD compared to previous dressing* | 3 | 22 | 81 |
| Frequency of dressing changes compared to previous dressings* | 47 | 48 | 1 |
| Yes | No | ||
| Will you continue to use NGAD?* | 90 | 9 | |
| Would you recommend NGAD to a colleague?* | 95 | 1 | |
Information not given by 6, 16, 13 and 17 clinicians, respectively.
Dressing‐related adverse events
There were three reported adverse events related to the NGAD dressing (Table 5). One was described as a reaction to the dressing, with no further details provided, and so cannot be classified. In two cases, a stinging sensation was described by the patients on initial dressing application. In the first of these cases, the stinging was intermittent for 3 days, but the patient then reported a reduction in this sensation, accompanied by phantom pains at the next two dressing changes. In the final case, the stinging resolved itself after the third application. Adverse events relating to tolerability of dressings are common and to be expected within such a patient population. Overall, the NGAD appeared to have been well tolerated.
Table 5.
Adverse events related to the NGAD *
| Wound type | Wound duration (months) | Age of patient (years) | Other details | Final wound assessment | Details of possible dressing‐related adverse event |
|---|---|---|---|---|---|
| Traumatic (leg) | † | 90 | Five signs of infection; moderate exudate | Same | Evaluation stopped after 1 week as patient had a reaction to the dressing |
| Other (leg) | 36 | 76 | Judged infected; suspected biofilm of 100% coverage; moderate exudate | Same | Patient complained of stinging which started after initial application, continuing intermittently for 3 days. This was a theme throughout. Patient reported a reduction in stinging, but also phantom pain for the first two applications, with a reduction near the end of the evaluation |
| Other (foot) | 36 | 80 | Judged infected; four signs + suspected biofilm of 100% coverage; high exudate | Deteriorated | Patient complained of stinging on first few applications; resolved on third application |
Language is taken from evaluation forms verbatim.
Information not given.
Discussion
This clinical user evaluation involved a real‐life assessment of an NGAD that has been designed to address biofilm‐associated delayed wound healing and infection through its synergistic combination of anti‐biofilm and antimicrobial components (Ag + Technology). Related antimicrobial wound dressings (the AQUACEL® Ag family of products) have a long history of safe use in wound care, with approximately 246 million silver‐containing Na‐CMC (Hydrofiber®; ConvaTec Ltd., Deeside, UK) dressings being used on patients worldwide since 2002 (unpublished data, ConvaTec Ltd., Deeside, UK). The NGAD (AQUACEL® Ag + Extra™) is the latest addition to this family of silver‐containing Na‐CMC dressings and contains additional anti‐biofilm agents to maximise the effectiveness of antimicrobial ionic silver. While product safety has been demonstrated pre‐clinically 18 and clinically 11, continued post‐market surveillance on the clinical safety and effectiveness of the NGAD was undertaken.
Evidence of wound healing progression (improved exudate management, reduction in suspected biofilm, improved or healed wound) was recorded in 93 of the 112 wounds (83%) in this evaluation. These improvements can be attributed largely to the design of the NGAD because switching to this dressing was the only aspect that changed in otherwise standard protocols of care. Firstly, the shift in wound exudate levels to predominantly low (Figure 5) is attributable to the absorptive nature of the Na‐CMC dressing base, which not only gels rapidly when in contact with fluid but also contours intimately to the wound bed to facilitate exudate management 19 and immobilisation of bacteria within the gelled dressing, as demonstrated in vivo 20. Secondly, the addition of anti‐biofilm components to the Na‐CMC dressing, along with the well‐characterised antimicrobial ionic silver 21, 22, 23, resulted in a general reduction in the frequency and extent of suspected biofilm (Figure 6). This reduction of biofilm would also consequentially reduce exudate production associated with biofilm‐induced inflammation. The anti‐biofilm excipients were selected based on their biofilm‐disruptive and surface‐acting properties, which have been demonstrated to expose microorganisms within biofilm to the antimicrobial action of ionic silver 9, 10 and prevent biofilm re‐formation 9. These reductions in exudate levels and biofilm coverage were accompanied by improvements in skin health (Table 3), shifts in wound bed appearance towards granulation tissue (Figure 6) and shifts from deteriorating/stagnant wounds to mainly improving or healed wounds (Figure 7). A growing body of evidence exists that supports this finding, independent of the NGAD, where numerous clinical 1, 24, 25, 26, 27 and in vivo 24 studies have demonstrated how reducing wound biofilm correlates with progression towards healing.
A wide variety of antimicrobial wound care products had been used on the wounds in these evaluations before switching to the NGAD (Figure 2). Notably, alongside systemic antibiotics, AQUACEL® Ag dressings (which do not contain the anti‐biofilm components of the NGAD) and iodine dressings were the most frequent antimicrobial products used previously. This suggests that standard antimicrobial agents, such as ionic silver, iodine or systemic antibiotics, may not be optimally effective in stagnant or deteriorating wounds that are likely to be compromised by biofilm. This was backed by the NGAD being rated by most clinicians as being more effective than the previous dressings used. These high levels of dressing acceptance (Table 4) and recorded wound healing improvements (Figures 5, 6, 7, Tables 2, 3) were accompanied by 42 positive comments in the evaluation forms. These comments were separated and, where necessary, split into themes of healing, responding, infection, exudate, skin, dressing changes, patient comments and clinician comments (Table 6). The comments on healed (n = 10) and responding wounds (n = 18) and improved management of infection, exudate and skin health (12 in total) correlate well with the observations already discussed, and of additional interest are the comments on dressing usage. Almost half of respondents reported that the NGAD dressing change frequency was less than the previously used dressing (Table 4), which was supported by several comments that dressing changes were notably reduced (Table 6). The direct implications of this finding include improved patient comfort, fewer clinician visits, less clinician time spent on dressing changes and possible reductions in dressing spend. Additionally, considering the time and cost benefits of healing wounds more quickly, reducing infection and improving skin health, the potential cost benefits associated with an effective antimicrobial wound dressing are apparent, as discussed previously 12.
Table 6.
Positive comments from evaluation forms by theme*
| Healed wounds | Responding wounds | Infection | Exudate | Skin | Dressing changes | Patient comments | Clinician comments | |
|---|---|---|---|---|---|---|---|---|
| Performance of the dressing was excellent; the main reason the wound accelerated to healing point | Marked improvement seen in long standing, stagnant ulcer; vast reduction in slough and increase in granulation tissue | Initial improvement was slow due to oedematous leg and inability to elevate; marked, continuing improvement now noted | Patient reported reduction in pain over 2 weeks use; pleased with odour reduction | Exudate much better; patient noted far less leakage, which had been a concern previously | No sign of macerated peri‐wound skin; looks healthy now | Dressing was removed in one piece which made dressing changes much easier | Patient so pleased that wound has stopped deteriorating and started to improve | Reason for using this dressing: only get one shot at applying a dressing; it addresses infection, absorbs exudate, stays in place and is easy/quick to apply |
| Patient had compression therapy with various dressings with little effect; in two weeks wound is healed | Wound has improved with application of the NGAD for 7 weeks, reduction in size, wound bed clean | Wound remains same in size but much less slough and improvement in healthy granulating tissue | Since starting offensive exudate has reduced to almost nil | Any exudate removed from wound and held in dressing | Reduced maceration around wound edges | Dressing change reduced from every 3–5 days | Patient wishes to continue using the NGAD and pleased with progression | Wound improvement excelled my expectations |
| Patient now healed after using the dressing for 3 weeks; now in compression hosiery | Since using the dressing we have seen the most improvement in wound direction | Marked improvement in wound appearance and size in last 4 weeks | Odour has improved (was prone to colonisation) | Much more absorbent than previously tried dressings | Dressing change frequency reduced to twice weekly from 3 × weekly | She reports that the product was comfortable to wear | This gentleman's ulcer is looking the best it has ever been | |
| Wound of over 3 years completely healed 17 weeks after initiating | Care will remain the same as wound continues to respond | Showed colleagues who agreed a visible improvement | Pain decreased when we began using the dressing | By week 4 there was a reduction in exudate | Reduction of dressing changes to twice weekly | Patient comfort on wound change has improved | This gentleman's ulcer is looking the best it has ever been | |
| The quickest I have ever healed a wound of this size | Wound had improved greatly by week 4 | Seen a marked improvement in wounds using the dressing | No odour | Managed exudate levels well | Able to reduce dressing changes to 2 weekly | Patient delighted with result | Great product, very impressed and will continue to use | |
| Wound healed almost within 4 weeks | Improvement to both leg wounds is pleasing | Wound is now smaller in size, less sloughy | Dressing changes have reduced | Patient happy with improvement | Dressing very effective | |||
| This wound healed after one treatment | Wound began to heal and slough reduced greatly | Definite improvement noted | Patient says comfortable | Will definitely use again | ||||
| It healed sufficiently | Significant improvement noted with wound | Ulcer had decreased in size | ||||||
| Wound healed | Wound was improving | Vast improvement | ||||||
| Healed | ||||||||
Language is taken from evaluation forms verbatim.
This clinical user evaluation was primarily concerned with the safety of the NGAD, so unfortunately clinical signs of infection after the evaluations were not recorded, although there were a small number of comments about reductions in signs of infection (purulent exudate, pain and odour). However, the detailed collection of these signs of infection, along with suspected biofilm at baseline, is still instructive. The links between the classic clinical signs of infection 15 and biofilm have been recently explored 13, 14. In the present evaluations, suspected biofilm was reported more frequently than any classic sign of infection (Figure 2), which was also the case prior to a previous 113‐patient evaluation of the NGAD 12. Of particular note was the finding that there was no obvious link between the number of classic clinical signs of infection reported and the frequency of suspected biofilm reported (Figure 3). For example, biofilm accompanied by two clinical signs of infection (71%) was almost as common as biofilm accompanied by five clinical signs (79%). This suggests that the presence of wound biofilm may interfere with wound healing despite the absence of clear signs of clinical infection. On the other hand, a wound with multiple signs of infection, and which may be classed as overtly infected, may not exhibit any visible signs of biofilm. Perhaps this paradox could be addressed by the emergence of infection diagnostic and biofilm detection point‐of‐care devices in the future.
The safety of an antimicrobial wound dressing is of utmost importance and is the key contributor to its clinical effectiveness. In addition to the three dressing‐related adverse events (Table 5), six of the 112 wounds (5%) included in these evaluations were classed as deteriorating at final assessment. Examination of the evaluation forms for these cases revealed that none reported any apparent safety‐related events alongside these observed deteriorations (Table 7). Reasons stated by the clinicians for wound deterioration in these 5% of cases briefly included established local or systemic infection (in five of the six cases), high levels of suspected biofilm (three cases) and clinical comorbidities (one case). Each of these issues requires consideration of additional wound management practices to antimicrobial dressings, for example, antibiotics, cleansing/debridement and addressing comorbidities. Further examination of all evaluation forms revealed nine further case comments (8%) that may be classified as negative. Table 8 lists these cases, of which six were additional to those deteriorating wounds listed in Table 7. Three of these wounds briefly contained suspected biofilm at baseline, one was sloughy, and one was a sacral pressure ulcer with a non‐visible wound bed (3 cm deep). Only one wound was reported to have no reported extenuating circumstances; this was a 20‐year‐old leg ulcer in an 84‐year‐old patient. Further investigation of this particular case was not possible because of the independent nature of information gathering via the evaluation forms.
Table 7.
Wounds judged to have deteriorated*
| Wound type | Wound duration (months) | Age of patient (years) | Other details | Clinician comment |
|---|---|---|---|---|
| DFU | 9 | 82 | Judged infected; moderate exudate | Recently infected, resistant to many antibiotics. On verge of being admitted for IV antibiotics |
| Other (leg) | 6 | 82 | Judged infected; moderate exudate | No improvement in wound (same patient as above) |
| Arterial (leg) | 84 | † | Not infected; high exudate | Deterioration could be due to clinical comorbidities, rather than the dressing. Dressing discontinued. |
| Arterial (leg) | 3 | 57 | Judged infected; moderate exudate; suspected biofilm of 50% coverage | None |
| Cyst | 10 | 45 | Three signs of infection + suspected biofilm of 100% coverage | Initially improvement noted. Wound then deteriorated to original size. |
| Other (foot) | 36 | 80 | Judged infected; four signs + suspected biofilm of 100% coverage; high exudate | Stinging which resolved on third application. (see Table 5, row 3) |
Language is taken from evaluation forms verbatim.
Information not given.
Table 8.
Examination of negative comments for dressing‐related adverse events*
| Wound type | Wound duration (months) | Age of patient (years) | Other details | Final wound assessment | Clinician comment |
|---|---|---|---|---|---|
| Other (leg) | 6 | 82 | Judged infected; moderate exudate | Deteriorated | No improvement in wound (see Table 7, row 2) |
| Arterial (leg) | 24 | 70 | Suspected biofilm; moderate exudate | Same | No noticeable difference following the evaluation |
| VLU (stage II) | 12 | † | Judged infected; four signs + suspected biofilm of 100% coverage; high exudate | Same | Stopped using after 5 weeks as no change. Wound remained static, size and wound bed did not change |
| Mixed (leg) | 240 | 86 | Not infected; moderate exudate | Same | No improvement to wound bed after 2–3 weeks |
| Arterial (leg) | 84 | † | Not infected; high exudate | Deteriorated | Deterioration could be due to clinical comorbidities, rather than the dressing. Dressing discontinued (see Table 7, row 3) |
| Mixed (leg) | 2 | † | Painful; sloughy wound bed | Improved | Although this ulcer is smaller with less slough, it is still painful and not healed |
| PU (sacrum) | 30 | 60 | Purulent exudate; suspected biofilm | Improved (skin deteriorated) | Wound was static until NPWT dressing applied |
| PU (sacrum) | † | 75 | Moderate exudate; 1·5 × 1 × 3·0 cm deep | Same | Wound bed cannot be seen, so improvements cannot be seen. Dressing not suitable for this wound |
| Cyst | 10 | 45 | Three signs of infection + suspected biofilm of 100% coverage | Deteriorated | Initially improvement noted. Wound then deteriorated to original size (see Table 7, row 4) |
Language is taken from evaluation forms verbatim.
Information not given.
Limitations
There was no standardised protocol used for these real‐life evaluations, as previously discussed 12. However, because the only change in clinician's protocols was to replace the antimicrobial primary dressing with the NGAD, any changes in wound health could be reasonably attributed to the dressing, and its safety was closely observed. There were several instances of missing information in various sections of the evaluation forms. However, these clinical user evaluations were independent activities with no assistance or reminders provided to clinicians in data capture. It was therefore deemed appropriate and ethical to include all collected evaluation forms in order to allow as true a representation of the safety and effectiveness of the NGAD as possible. As discussed above, a limitation of the post‐evaluation data collected was that clinical signs of infection were only captured at baseline. These signs of infection, and those of suspected biofilm and approximations of wound bed tissue type, were also subjective. Future studies in this area could utilise more sophisticated methods of wound bed assessment, such as planimetry or wound image analysis software as well as microbiological assessment by culture or molecular analysis of wound samples. Finally, these clinical user evaluations were not designed to compare the safety and effectiveness of the NGAD to previously used or other antimicrobial dressings. Therefore, more controlled, exhaustive and comparative clinical studies are required to support the emerging safety and effectiveness of this new dressing.
Conclusion
This clinical user evaluation aimed to further assess the safety and effectiveness of the NGAD by assessing its performance in the evaluation of challenging wounds in a variety of clinical settings. The results further indicate that the incorporation of anti‐biofilm agents in the NGAD does not compromise patient safety but does encourage resolution of biofilm‐associated wound recalcitrance and improved wound and skin health.
Participating clinicians
Linda Astell, St Paul's Nursing Home, East Sussex.
Heidi Ball, South Warwickshire NHS Foundation Trust, Warwickshire.
Patricia J. Barnes‐Moss, Hailsham.
Jessica Bates, Shropshire Community Health, Shropshire.
Alison Bendall, Abbotsbury Road Surgery, Weymouth.
Tanya Brandon, St John's Hospital, West Lothian.
Helen Buck, Haxby Health Centre, York.
Rachel Bucknell, Heathville Medical Practice, Gloucester.
Tracey Burwell, South Warwickshire NHS Foundation Trust, Warwickshire.
Mari G. Clarke, District Nurse, Port Talbot Resource Centre, Port Talbot.
Romie Clarke, Station Plaza Health Centre, East Sussex.
Joanne Claxton, Heathfield Health Centre, Doncaster.
Fiona Concannon, Community Care, Dublin.
Lynn Crump, Winchester Wound Clinic, Winchester.
Fiona Cunningham, St John's Hospital, West Lothian.
Debra Darke, Sunderland.
Leanne Davies, St Stephens Centre, Birmingham.
Jane Denney, Winchester Wound Clinic, Winchester.
Jenny Dyson, Grange Dene Medical Centre, West Yorkshire.
Laura Fotheringham, Bo'ness Health Centre, West Lothian.
Wendy Fraser, St John's Hospital, West Lothian.
Patricia Gay, St John's Hospital, West Lothian.
Alison Hale, Govanhill Health Centre, Glasgow.
Richard Hall, Inverleith Medical Practice, Edinburgh.
Susan Hammett, Winchester Wound Clinic, Winchester.
C. Hancock, Oak Park Community Clinic, Hampshire.
Jim Hickton, Southam Clinic, Southam.
Sue Hillier, The Lydney Practice, Gloucestershire.
Jane James, Hywel Dda Health Board, Carmarthenshire.
Sarah Kane, Easingwold Health Centre, York.
Delia Keen, Powys Health Board, South Powys.
H. Lidden, Acomb Health Centre, York.
Donna MacAskill, Bishopton Health Centre, Renfrewshire.
Ashley Mackie, St John's Hospital, West Lothian.
Susan Mason, Dedridge Health Centre, Livingston.
Sarah McClanigan, St John's Hospital , West Lothian.
Bernie McGlynn, St Stephens Centre, Birmingham.
Alison McGrath, South Tees NHS Trust, Middlesbrough.
Kaye McIntyre, Monklands Diabetes Centre, Airdrie.
Georgina McKay, Fieldhouse Medical, Grimsby.
Ann Marie McNee, Airdrie Health Centre, Lanarkshire.
Sarah McLadrigan, St John's Hospital, West Lothian.
Kate Murray, Craiglockhart Medical Group, Edinburgh.
Nicole Narguizian, Royal South Hants, Southampton.
E. Pollock, Zetland Medical Practice, Teeside.
Tracey Ponkin, Unity House District Nursing Service, Middlesbrough.
Roz Puzey, Royal Derby Hospitals, Derby.
Susie Rathie, Queen Margaret University, Edinburgh.
Vanessa Reynolds, Tranent Health Centre, East Lothian.
Natasha Ross, Muirhouse Medical Group, Edinburgh.
V. Sempeswa, Erskine Glasgow Home, Glasgow.
Nicola Vaughan, Yarm Medical Practice, Teeside.
R. Walker, 3 The Green Care Management Group, Surrey.
Eleanor Williamson, Strathbrock Heath Centre, Broxburn.
Gillian Williamson, St John's Hospital, West Lothian.
R. Young, Sandown Health Centre, Isle of Wight.
Rachel Wong, St Stephens Centre, Birmingham.
12 on file.
Acknowledgements
The authors gratefully acknowledge all participating clinicians and Paula Evans, Clinical Trials Associate, Clinical Affairs, ConvaTec, for the collation of the evaluation form data. The authors are all employed by ConvaTec Ltd. Dressings were provided to the clinicians free of charge.
References
- 1. Wolcott RD, Rhoads DD. A study of biofilm‐based wound management in subjects with critical limb ischaemia. J Wound Care 2008;17:145–55. [DOI] [PubMed] [Google Scholar]
- 2. Dowd SE, Wolcott RD, Kennedy J, Jones C, Cox SB. Molecular diagnostics and personalised medicine in wound care: assessment of outcomes. J Wound Care 2011;20:232–9. [DOI] [PubMed] [Google Scholar]
- 3. Serralta VW, Harrison‐Balestra C, Cazzaniga AL, Davis SC, Mertz M. Lifestyles of bacteria in wounds: presence of biofilms? Wounds 2001;13:29–34. [Google Scholar]
- 4. Percival SL, Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004;16:234–40. [Google Scholar]
- 5. James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 6. Kirketerp‐Møller K, Jensen PØ, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker‐Nielsen T, Høiby N, Givskov M, Bjarnsholt T. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008;46:2712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall‐Stoodley L, Stoodley P, Kathju S, Høiby N, Moser C, Costerton JW, Moter A, Bjarnsholt T. Towards diagnostic guidelines for biofilm‐associated infections. FEMS Immunol Med Microbiol 2012;65:127–45. [DOI] [PubMed] [Google Scholar]
- 8. Parsons D. WO/2012/136968. Composition comprising antimicrobial metal ions and a quaternary cationic surfactant. ConvaTec Technologies Inc.
- 9. Parsons D. Designing a dressing to address local barriers to wound healing. In: Next‐generation antimicrobial dressings: AQUACEL™ Ag+ Extra™ and Ribbon. London: Wounds International, 2014. (Suppl). URL www.woundsinternational.com
- 10. Said J, Walker M, Parsons D, Stapleton P, Beezer AE, Gaisford S. An in vitro test of the efficacy of an anti‐biofilm wound dressing. Int J Pharm 2014;474:177–81. [DOI] [PubMed] [Google Scholar]
- 11. Harding KG, Szczepkowski M, Mikosiński J, Twardowska‐Saucha K, Blair S, Ivins NM, Saucha W, Cains J, Peters K, Parsons D, Bowler P. Safety and performance evaluation of a next‐generation antimicrobial dressing in patients with chronic venous leg ulcers. Int Wound J 2015. DOI: 10.1111/iwj.12450 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walker M, Metcalf D, Parsons D, Bowler P. A real‐life clinical evaluation of a next‐generation antimicrobial dressing on acute and chronic wounds. J Wound Care 2015;24:11–22. [DOI] [PubMed] [Google Scholar]
- 13. Metcalf DG, Bowler PG, Hurlow J. A clinical algorithm for wound biofilm identification. J Wound Care 2014;23:137–42. [DOI] [PubMed] [Google Scholar]
- 14. Metcalf DG, Bowler PG. Clinician perceptions of wound biofilm. Int Wound J 2014. DOI: 10.1111/iwj.12358 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care 1994;3:198–201. [DOI] [PubMed] [Google Scholar]
- 16. National Research Ethics Service . Guidance for Researchers & Reviewers, 2011. URL http://tinyurl.com/q86vty4 [accessed on 30 October 2015].
- 17. Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Guyadec TL, Zagnoli A, Perrot JL, Sauvadet A, Bohbot S. The role of a silver releasing lipido‐colloid contact layer in venous leg ulcers presenting inflammatory signs suggesting heavy bacterial colonization: results of a randomized controlled study. Wounds 2008;20:158–66. [PubMed] [Google Scholar]
- 18. Seth AK, Zhong A, Nguyen KT, Hong SJ, Leung KP, Galiano RD, Mustoe TA. Impact of a novel, antimicrobial dressing on in vivo, Pseudomonas aeruginosa wound biofilm: quantitative comparative analysis using a rabbit ear model. Wound Repair Regen 2014;22:712–9. [DOI] [PubMed] [Google Scholar]
- 19. Williams C. An investigation of the benefits of Aquacel Hydrofibre wound dressing. Br J Nurs 1999;8:676–80. [DOI] [PubMed] [Google Scholar]
- 20. Tachi M, Hirabayashi S, Yonehara Y, Suzuki Y, Bowler P. Comparison of bacteria‐retaining ability of absorbent wound dressings. Int Wound J 2004;1:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver‐containing hydrofiber dressing. Wound Repair Regen 2004;12:288–94. [DOI] [PubMed] [Google Scholar]
- 22. Bowler PG, Jones SA, Walker M, Parsons D. Microbicidal properties of a silver‐containing hydrofiber dressing against a variety of burn wound pathogens. J Burn Care Rehabil 2004;25:192–6. [DOI] [PubMed] [Google Scholar]
- 23. Bowler PG, Welsby S, Towers V, Booth R, Hogarth A, Rowlands V, Joseph A, Jones SA. Multidrug‐resistant organisms, wounds and topical antimicrobial protection. Int Wound J 2012;9:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Metcalf DG, Bowler PG. Biofilm delays wound healing: a review of the evidence. Burns Trauma 2013;1:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P. Clinical biofilms: a challenging Frontier in wound care. Adv Wound Care 2015;4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurlow J. Biofilm as the cause of non‐healing wounds. Medsurg Nurs 2015;24:12–3. [PubMed] [Google Scholar]
- 27. Wolcott RD. Disrupting the biofilm matrix improves wound healing outcomes. J Wound Care 2015;24:366–71. [DOI] [PubMed] [Google Scholar]


