Abstract
This study aimed to evaluate the effect of strategies of a lifestyle orientation programme on patients with venous ulcer in elastic compression therapy. This was a single‐blind, 2‐arm, randomised clinical controlled trial. The primary outcome included the reduction of the wound surface area. The secondary outcomes included the perception of pain, questionnaire of ulcer status, and quality of life. Seventy‐one patients with ulcers of venous aetiology were randomised into 2 arms: control group (CG) and intervention group (IG), with a 12‐week follow up. The CG was provided with the routine guidelines of the health services. Meanwhile, the IG was provided with lifestyle guidelines regarding the physiopathology of a venous ulcer, importance of compression therapy, physical exercises and rest in 4 face‐to‐face and 2 telephone interviews. The IG had significant improvement on the wound healing on 30, 60, and 90 days of follow up when compared with the CG (P = .0197; P = .0472; P = .0116). There were no statistical differences between groups; both had improvement in the quality of life and pain perception. Our results demonstrated that elastic compression therapy along with guidelines on lifestyle is effective adjunctive treatment to promote wound healing in patients with leg ulcers.
Keywords: compression therapy, exercise, healing, venous leg ulcers
1. INTRODUCTION
Among chronic ulcers, an ulcer of venous aetiology is responsible for 70% of all wounds in lower extremities1 and is associated with vascular complications and other morbidities, with high indexes of recurrences and slowness in wound healing.2, 3
Venous leg ulcers (VLUs) affect between 1% and 3% of the population worldwide and are considered a health problem of high treatment costs throughout history. According to a study, the first VLU appears around 30–40 years of age,4 taking long periods for complete healing in addition to recurrence indexes that reach 70% until the second year after the cure. Therefore, the treatment incurs a high cost for health care services, patients, and family members.5
VLU management is complex: the international literature, through guidelines, shows that, among the care intervention, proper understanding accompanied by recommendations that involve changes in lifestyle, such as rest, elevation of lower extremities, nutrition, hydration, reduction of smoking/drug abuse, physical activity, and cleansing and moisturising of skin, are fundamental care factors for wound healing and prevention.1, 6, 7, 8, 9, 10, 11, 12
However, to change lifestyles, aiming to promote the change of attitudes is a difficult task that requires educational health guidelines as a priority, emphasising the guidelines for risk factors evident in this population that may be implemented in the health care environment.13, 14 Furthermore, to follow the orientations listed by the professional and achieve better health outcomes, individuals need information to clarify their doubts and concerns about their health condition.
Studies have shown that the guidelines on lifestyle are fundamental to promote self‐care and, consequently, the recovery of the patient.10, 14 Thus, the objective of this study was to evaluate the effect of the strategies of an orientation programme on the lifestyle of VLU patients and the wound‐healing process.
2. MATERIALS AND METHODS
This study followed the Consolidated Statement of Reporting Trials (CONSORT), as shown in Figure 1.
Figure 1.
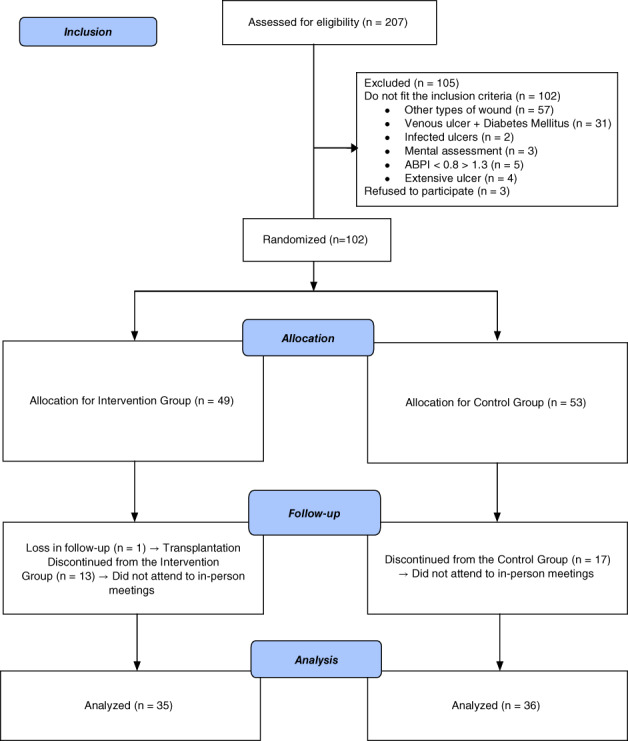
Flow of participants through study
2.1. Design
This was a single‐blind, 2‐arm, randomised controlled trial (RCT) with an intervention group (IG) and control group (CG). The study was registered with the International Clinical Trial Registration Platform (ICTPR) via the Brazilian Clinical Trial Registry (REBEC—RBR‐52fq9q). Written consent was obtained after the patients agreed to participate in the research. The process followed the precepts of the Declaration of Helsinki.
2.2. Participants
Patients with VLUs in compression therapy were included according to the following inclusion criteria: ankle brachial pressure index (ABPI) > 0.8 and < 1.313, 15, minimum wound duration of 6 weeks, and size ≥0.2 cm2. 16
Individuals with a medical diagnosis of osteomyelitis, diabetes mellitus, and infected wounds, evaluated by the presence of 2 or more clinical signs/symptoms of infection (pain, erythema, oedema, heat, purulent exudate, serous exudate with simultaneous inflammation, slow wound healing, pale granulation tissue, friable granulation tissue, and odour), were excluded from the research.17
The sample size was estimated using the methodology of a repeated measures ANOVA model and assuming a 5% significance level, a test of 80% power, and an effect size of 0.25, which can be considered a medium effect size.18 The calculation resulted in a sample of 82 individuals (41 individuals per group). However, considering a 20% rate of possible losses, the sample size included 98 subjects (49 individuals per group).
2.3. Intervention
This study had 2 arms, IG and CG, for 12 weeks (90 days) through 4 steps of data collection. The period of 12 weeks (90 days) was based on the research by Harrison,19 who identified that a period of 9 to 11 weeks is the appropriate time required for ulcer healing in patients undergoing compression therapy.
The IG was provided with nursing guidelines regarding the lifestyle. They focused on specific physical exercises for lower extremities, especially daily repetitive movements of the calves and feet for 3 to 4 times, intermittent rest throughout the day, and the importance of compression therapy in the wound‐healing process. The orientations were based on the guidelines available for management of VLUs related to the lifestyle of these patients.7, 11
The orientations lasted an average of 40 minutes and were conducted in person by the lead researcher through informative brochures in a private room. Four meetings were held every 4 weeks (30 days). The meetings were meant to reinforce the initial instructions and answer possible doubts. Contact was maintained by phone between the in‐person meetings of the IG. According to specialists, the orientations must have been remembered every 2 to 6 weeks.6, 10
In the CG, the initial meeting was held to provide information on the study, including the usual routine guidelines of the unit, that is, cleansing of the wound with saline solution, application of the therapeutic agent prescribed by the nurse of the unit, and compression therapy. The CG returned every 15 days according to the protocol of the health unit.
2.4. Outcomes
As the primary outcome, the evolution of the wound was analysed through the reduction of the wound area in centimetres2, determined by the measure of the height and width of the wound. In addition, ulcer characteristics, such as the type of wound bed tissue and the amount of exudate, were evaluated through by the PUSH tool (Pressure Ulcer Scale for Healing).15, 20 Each wound was measured every 30 days until the conclusion of the follow up or until complete wound healing.
The secondary outcome was the improvement of the pain perception by the numeric rating scale for pain (NRS Pain), determined by the numerical scale and quality of life (QoL), using the Freiburg Life Quality Assessment for Wounds (FLQAw) instrument, both measured before randomization and conclusion of the study or until the complete wound healing.
2.5. Instruments
The instrument used in data collection to characterise the sample has socio‐demographic and health data, developed specifically for this study. Socio‐demographic data include the following variables: age, gender, marital status, income, and education level. Regarding health data, the variables are: venous ulcer duration (months), ulcer area, current treatment, ABPI, and compression therapy duration.
To assess the evolution of the wound, the PUSH tool was used, after being adapted to Portuguese in 2005 and made suitable for use in patients with chronic wounds in lower extremities.15
The PUSH tool covers 3 parameters or subscales: wound area, amount of exudate, and appearance of wound bed tissue. After obtaining the score of each subscale, the values were summed to generate a total score, which can range from 0 to 17. The higher the score, the worse the wound conditions; the lower the score, the better the tissue repair.15
The evolution of the wound was assessed at the beginning of the collection, and every 30 days thereafter, by photographing the ulcer. To analyse the evolution of the wound, the wound area was measured by photographing the ulcer with an 8‐megapixel digital camera, ƒ/2.4 aperture, LED flash, backlight sensor, and resolution of 3264 × 2448 pixels. A computerised planimetry tool (Texas Health Science Center in San Antonio Image Tool, version 3.0), downloaded from (www.ddsdx.uthsca.edu/dig/itdesc.html), was used for calculation.
The NRS Pain was chosen for pain assessment in this study. The scale has 11 scores, rated from 0 to 10, in which the final scores are the extremes, being 0 (no pain) and 10 (worst pain imaginable). Patients select the number of the scale that best represents their pain.20 Patients with more than one wound will classify their wound with the worst pain.
The abbreviated version of the FLQAw instrument was adapted for use in Brazil in 2013 to assess the QoL of patients with chronic wounds of any aetiology.21 The instrument has 24 questions, grouped into 6 scales: physical ailments, everyday life, social life, psychological well‐being, therapy, and satisfaction. The score ranges from 1 to 5, and the higher the score, the worse the QoL.
2.6. Recruitment, randomisation, and allocation of participants
The participants of this study were recruited in units specialised in wound treatment. The original invitation was given in the unit on a day of routine care. Subsequently, the invitation to participate in the study was given, and a day and time to demonstrate the objectives of this study were scheduled, as well as the achievement of patients consenting to participate in the research.
On the day scheduled, the lead researcher clarified the objective of the study and every stage of the research. At the end of this meeting and after the patients agreed to participate in the study and signed the written consent form, they were randomised into the 2 groups using the randomised block design through randomly selected block sizes. A member of the research team not in contact with the participants prepared the list in a randomised sequence generated by the website randomization.com, with 120 participants in numerical sequence concealed from the investigators. Subsequently, each of these numbers was sealed individually in opaque envelopes. Thus, only after the conclusion of the first interview (baseline) did the investigator open an envelope and allocate the participant into 1 of the groups. A number exceeding the sample calculation was prepared because of possible losses during the data collection.
The assessment at the end of the follow up was conducted by a collaborator previously instructed on the research procedures and who was not in contact with participants.
2.7. Data analysis
Descriptive analysis was used for socio‐demographic and clinical variables; relative and absolute frequencies were used for continuous and categorical variables.
For the comparison between the groups in relation to measures of QoL and wound area over the periods considered, linear mixed‐effect models were proposed. The linear mixed‐effect models (random and fixed effects) are used in data analysis, in which answers of a single individual are grouped, and the supposition of independence between observations of a single group is not suitable.
For cases in which presuppositions of the model were not achieved, the Mann–Whitney U and Wilcoxon (for paired samples) non‐parametric tests were applied, considering the Bonferroni correction at the significance level.
3. RESULTS
A total of 207 individuals met the eligibility criteria; of these, 102 individuals did not fit the inclusion criteria, and 3 refused to participate in the study. After providing written consent, the remaining 102 participants were randomised into the IG and CG, with 49 and 53 participants in each group, respectively. Seventeen individuals of the CG and 13 of the IG were discontinued from the arm because they did not attend the in‐person meetings of orientation and assessment of variables. One individual of the IG was excluded for having transplantation during the follow up (Figure 1). No participants reported complications or any damages related to the intervention during the study programme.
Table 1 shows the socio‐demographic and clinical characteristics of the baseline sample. The mean age among participants was 66.50 (12.8) years; most (57.65%) were females with no partners (53.52%). Participants’ income was around US$500/month and they had an average of 3 years of education.
Table 1.
Socio‐demographic and health characteristics at baseline of the intervention and control groups
| Variable | Group | n | % | Mean (standard deviation) | P‐value |
|---|---|---|---|---|---|
| Age (y) | Control | 36 | 68.17 (12.63) | .2735* | |
| Intervention | 35 | 64.83 (12.86) | |||
| Total | 71 | 66.50 (12.8) | |||
| Wound duration (mo) | Control | 36 | 55.89 (67.62) | .7859** | |
| Intervention | 35 | 58.57 (81.47) | |||
| Total | 71 | 57.21 (74.24) | |||
| Compression duration (wk) | Control | 36 | 62.86 (81.71) | .4675** | |
| Intervention | 35 | 83.74 (107.91) | |||
| Total | 71 | 90.53 (95.39) | |||
| Education level (y) | Control | 36 | 3.89 (2.96) | .8334** | |
| Intervention | 35 | 3.71 (2.72) | |||
| Total | 71 | 3.80 (2.83) | |||
| Income (US$500/mo) | Control | 36 | 1.63 (1.14) | .5445** | |
| Intervention | 35 | 1.49 (1.07) | |||
| Total | 71 | 1.56 (1.09) | |||
| ABPI (mm Hg) | Control | 36 | 1.06 (0.10) | .4996** | |
| Intervention | 35 | 1.05 (0.10) | |||
| Total | 71 | 1.06 (0.10) | |||
| Gender (female) | Control | 20 | 55.56 | .7047*** | |
| Intervention | 21 | 60.00 | |||
| Total | 41 | 57.65 | |||
| Marital status (no partner) | Control | 20 | 55.56 | .7274*** | |
| Intervention | 18 | 51.43 | |||
| Total | 38 | 53.52 | |||
| Treatment+ | Control | 20 | 80.00 | .4265**** | |
| Intervention | 20 | 80.00 | |||
| Total | 40 | 56.34 | |||
| Compression (elastic) | Control | 31 | 86.11 | 1.0000**** | |
| Intervention | 31 | 88.57 | |||
| Total | 62 | 87.32 |
Treatment+ Calcium Alginate, Hydrogel, and Polyhexamethylene Biguanide.
P‐value obtained through the unpaired Student's t test;
P‐value obtained through the Mann–Whitney U test;
P‐value obtained through the χ 2 test;
P‐value obtained through the Fisher's exact test.
Regarding health data, the venous ulcer duration was 57.21 months, and ABPI was an average of 1.06 mm Hg, most using elastic compression (87.32%), for 62.86 months. The most common treatment used was polyhexamethylene biguanide, calcium alginate, and hydrogel (56.34%). There were no differences between socio‐demographic and clinical characteristics between individuals allocated into the CG and IG.
Considering the QoL assessment within and between groups, we observed significant difference in the satisfaction domain of QoL (P = .0300) for IG by the end of the follow up. In the wound area assessment, the IG had significant area reduction on days 30, 60, and 90 of follow up when compared with the CG (P = .0197; P = .0472; P = .0116, respectively). The comparison of the follow‐up in both groups showed significant improvement regarding time (P < .0001); however, the IG had greater difference between means (Table 2).
Table 2.
Comparisons within and between groups according to the quality of life and the wound area variables over time: Baseline and 90 days after the follow up
| Dependent variable | Comparison | Mean difference | Confidence interval (95%) | P‐value | |
|---|---|---|---|---|---|
| Inferior limit | Superior limit | ||||
| Physical ailments | Intervention—Control (D0) | 0.48 | 0.03 | 0.94 | .0385 |
| Intervention—Control (D90) | 0.16 | −0.21 | 0.54 | .3899 | |
| Control (D90–D0) | −0.48 | −0.75 | −0.21 | .0005 | |
| Intervention (D90–D0) | −0.80 | −1.14 | −0.46 | <.0001 | |
| Everyday life | Intervention—Control (D0) | 0.22 | −0.35 | 0.78 | .4520 |
| Intervention—Control (D90) | −0.04 | −0.53 | 0.45 | .8750 | |
| Control (D90–D0) | −0.67 | −0.93 | −0.40 | <.0001 | |
| Intervention (D90–D0) | −0.92 | −1.31 | −0.53 | <.0001 | |
| Social life | Intervention—Control (D0) | 0.18 | −0.34 | 0.70 | .4976 |
| Intervention—Control (D90) | 0.07 | −0.38 | 0.51 | .7729 | |
| Control (D90–D0) | −0.44 | −0.75 | −0.14 | .0039 | |
| Intervention (D90–D0) | −0.56 | −0.95 | −0.17 | .0053 | |
| Psychological well‐being | Intervention—Control (D0) | −0.03 | −0.53 | 0.47 | .9070 |
| Intervention—Control (D90) | −0.10 | −0.45 | 0.24 | .5521 | |
| Control (D90–D0) | −0.52 | −0.77 | −0.28 | <.0001 | |
| Intervention (D90–D0) | −0.60 | −0.91 | −0.28 | .0002 | |
| Therapy | Intervention—Control (D0) | 0.05 | −0.37 | 0.47 | .8178 |
| Intervention—Control (D90) | −0.24 | −0.62 | 0.15 | .2245 | |
| Control (D90–D0) | −0.52 | −0.79 | −0.25 | .0001 | |
| Intervention (D90–D0) | −0.81 | −1.13 | −0.49 | <.0001 | |
| Satisfaction | Intervention—Control (D0) | −0.21 | −0.57 | 0.15 | .2596 |
| Intervention—Control (D90) | −0.39 | −0.75 | −0.04 | .0300 | |
| Control (D90–D0) | −0.26 | −0.53 | 0.01 | .0592 | |
| Intervention (D90–D0) | −0.44 | −0.72 | −0.17 | .0016 | |
| FLQAw | Intervention—Control (D0) | 0.15 | −0.20 | 0.51 | .4056 |
| Intervention—Control (D90) | −0.07 | −0.38 | 0.24 | .6446 | |
| Control (D90–D0) | −0.50 | −0.68 | −0.32 | <.0001 | |
| Intervention (D90–D0) | −0.72 | −0.92 | −0.51 | <.0001 | |
| Wound area | Intervention—Control (D0) | 10.57 | 3.18 | 17.97 | .0051 |
| Intervention—Control (D30) | 7.64 | 1.22 | 14.07 | .0197 | |
| Intervention—Control (D60) | 6.90 | 0.09 | 13.70 | .0472 | |
| Intervention—Control (D90) | 8.31 | 1.86 | 14.75 | .0116 | |
| Control (D30—D0) | 0.76 | −0.58 | 2.09 | .2666 | |
| Control (D60–D0) | 0.75 | −0.67 | 2.17 | .3016 | |
| Control (D90–D0) | −1.56 | −3.22 | 0.10 | .0654 | |
| Intervention (D30–D0) | −2.17 | −5.18 | 0.84 | .1571 | |
| Intervention (D60–D0) | −2.93 | −5.91 | 0.05 | .0542 | |
| Intervention (T90–T0) | −3.83 | −6.54 | −1.12 | .0056 | |
FLQAw, Freiburg Life Quality Assessment for Wounds.
There were no statistical differences between groups considering the self‐referred variables (pain, health, wound, treatment, and QoL) and the total PUSH score (Table 3). However, the health (P = .0019) and wound (P < .0001) variables, assessed by the total PUSH score, had a significant difference in time. Considering the PUSH, the IG had significant results in 60 and 90 days when compared with the initial time.
Table 3.
Comparisons within and between groups according to pain, health, wound, quality of life, and total PUSH score variables over time: Baseline and 90 days after follow up
| Variable | Comparison | P‐value |
|---|---|---|
| Pain | Intervention—Control (D0) | .0159* |
| Intervention—Control (D90) | .4437* | |
| Control (D90–D0) | .1084** | |
| Intervention (D90–D0) | .5868** | |
| Health | Intervention—Control (D0) | .7213* |
| Intervention—Control (D90) | .1023* | |
| Control (D90–D0) | .5124** | |
| Intervention (D90–D0) | .0019** | |
| Wound | Intervention—Control (D0) | .9481* |
| Intervention—Control (D90) | .6434* | |
| Control (D90–D0) | .0423** | |
| Intervention (D90–D0) | .0278** | |
| Quality of life | Intervention—Control (D0) | .7933* |
| Intervention—Control (D90) | .6685* | |
| Control (D90–D0) | .0483** | |
| Intervention (D90–D0) | .5380** | |
| Score—total (PUSH) | Intervention—Control (D0) | .0167* |
| Intervention—Control (D90) | .1696* | |
| Control (D90–D0) | .4289* | |
| Intervention (D90–D0) | .2629* | |
| Control (duration) | .1058*** | |
| Intervention + (duration) | <.0001*** |
P‐value obtained through the Mann–Whitney U test;
P‐value obtained through the unpaired Wilcoxon test;
P‐value obtained through the Friedman test.
On the other hand, the CG had significant differences in the QoL domains (physical ailments, everyday life, social life, psychological well‐being, therapy) and total score of QoL. However, the differences of the averages are greater in the IG, proving that the evolution of variables was better in IG (Figure 2). Regarding the pain assessment, we observed that the CG had worsening of pain during the follow up when compared with the IG.
Figure 2.
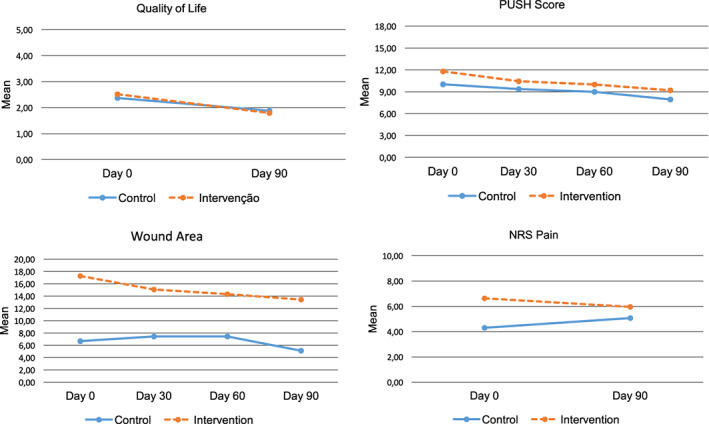
Mean of variables: Quality of life, PUSH score, wound area, and NRS Pain
4. DISCUSSION
The VLU is a chronic disease that requires recurring treatment. Therefore, guidelines that provide self‐care allow individuals to be involved in their own care and promote greater adherence to the therapeutic regimen, reducing complications.22 We are aware that VLU treatment consists of the use of adequate compression therapy, associated with patient education.23 Therefore, our study approached the guidelines on practice of exercises, rest, and importance of the understanding and had obtained positive results in the tissue restoration of the wound, pain, and QoL of patients with VLUs.
Considering the characterisation of the participants, the predominant population included women and older people with low income and education. A study discovered that the low educational level influences the adoption of practices of self‐care guidelines24 because it can interfere with the assimilation of the content transmitted by the health care professional. When also associated with low income, it can make the acquisition of materials required for treatment difficult.25 Therefore, the guidelines were provided in a simple and objective manner, in person, following the informative brochure and focusing on the importance of lifelong treatment.8, 9
The IG achieved significant results in relation to the reduction of the wound area, pain, and improvement of QoL when compared with CG at the end of the follow up. On the other hand, we observed that the CG improved throughout the follow up with regard to QoL, without significant improvement in wound reduction and pain. In addition, we observed, on baseline, that there were statistic differences between IG and CG groups on the physical ailments item regarding QoL appraisal. On the other hand, 90 days after follow up, there were significant differences between D90 and D0 for both IG and CG, but not when both groups are compared with one another. A recent systematic review26 that investigates the impact of chronic VLUs demonstrated that it negatively impacts all areas of daily living, and specifically in the physical area, the studies revealed a negative effect regarding pain, mobility, sleep, and daily activities over quality life. However, when the participants adhered to the programme as an adjunctive treatment, they demonstrated better outcomes over treatment with standard care.27 Although our results showed improvement over physical ailments in both groups, the IG participated in an adjunctive exercise programme, which may have contributed to the better score when compared with CG.
According to the literature, the constant pressure application from 20 to 40 mm Hg in lower extremities is the best outcome for VLU treatment; however, the period of time needed to achieve success using this treatment is long and may make the adherence to the compression therapy difficult.28, 29 A study showed that, with proper compression treatment, 30% to 60% of VLUs heal in 24 weeks and 70% to 85% in 1 year,30 corroborating our results for the CG, in which the use of compression alone demands a longer time to act positively on tissue repair. Therefore, there is evidence that the use of compression associated with the guidelines for change in lifestyle promotes wound healing in less time and pain reduction.
Research has demonstrated that the compression associated with guidelines on exercises for the legs and feet are effective practices for the proper functioning of the calf muscle pump, promoting wound healing.31, 32
Thus, there is no doubt that compression therapy is the gold standard in care for the patient with VLUs.33 On the other hand, the non‐adherence to compression devices is influenced by several factors; among them are the lack of correct, objective, and easy‐to‐understand information for the population that receives them.34 Because of this, the promotion of adherence to continuous use of compression therapy through education/orientation is a fundamental component in the evolution of the wound.35 Taken together, these dates suggest that the guidelines and follow up by the health care professional during the therapeutic regimen contribute to the wound‐healing process.33
Another point to be highlighted is that confidence in the professional is a factor that increases the rates of adherence to VLU treatment.36 Thus, the in‐person orientations and phone reinforcements may have assisted in the adherence to the guidelines, favouring the orderly recovery of the wound.
There is evidence in the literature that wound area reduction, as well as pain relief, improves the QoL of the patients with an ulcer.35 A double‐blind RCT evaluated 2 types of therapeutic agents in patients with wounds and showed that the wounds that healed in a shorter time led to significant reduction of the pain perception, discomfort, and anxiety of the patient, resulting in improvement of QoL and social and emotional disorders.37 Our results corroborate these pieces of evidence because the intervention had a positive impact on the improvement of QoL, reduction of the wound area, and pain.
The presence of a wound is a factor that affects the QoL because VLUs have a slow healing process with high recurrence rates and are usually painful, the pain being one of the main signs/symptoms that interfere negatively with the QoL.38 These alterations interfere with the daily life of the patient and have an impact on physical and social aspects, functional capacity, and physical health,38 reinforcing the fact that the effective planning of assistance promotes tissue repair and improves the pain perception, promoting the well‐being and QoL of patients with VLUs.39
On the other hand, our results demonstrated that the effect changes in the lifestyle had did not interfere with QoL because both groups demonstrated improvement in QoL, regardless of lifestyle guidelines. This fact may be related to the period of 12 weeks, which can be insufficient to affect well‐being.
Thus, our results demonstrate that the effectiveness of the orientation programme for changes in lifestyle during the daily routine of the patient with regard to compression therapy assists in VLU treatment.
4.1. Study limitation
Study limitations are: the sample size, because 30 patients discontinued from the study, and the unsupervised follow up on lifestyle, because patients only self‐reported that they performed the activities proposed.
5. CONCLUSION
Elastic compression therapy is crucial for the treatment of a venous ulcer; however, it needs to be associated with a change in lifestyle, so patients can achieve better results regarding reduction of wound area, QoL, and pain.
ACKNOWLEDGEMENTS
This work was supported by grants from Fundacão de Amparo a Pesquisa do Estado de Sao Paulo‐FAPESP (2015/15134‐8).
Domingues EAR, Kaizer UAO, Lima MHM. Effectiveness of the strategies of an orientation programme for the lifestyle and wound‐healing process in patients with venous ulcer: A randomised controlled trial. Int Wound J. 2018;15:798–806. 10.1111/iwj.12930
Funding information São Paulo Research Foundation ‐ Fapesp, Grant/Award Number: 2015/15134‐8
REFERENCES
- 1. Gillespie DL. Venous ulcer diagnosis, treatment, and prevention of recurrences. J Vasc Surg. 2010;52(5 suppl):8S‐14S. [DOI] [PubMed] [Google Scholar]
- 2. Markova A, Mostow EN. US skin disease assessment: ulcer and wound care. Dermatol Clin. January 2012;30(1):107‐111. [DOI] [PubMed] [Google Scholar]
- 3. Abbade LPF, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44:449‐456. [DOI] [PubMed] [Google Scholar]
- 4. Alavi A, Sibbald R, Phillips T, et al. What's new: management of venous leg ulcers. Approach to venous leg ulcers. J Am Acad Dermatol. 2016;74(4):627‐640. [DOI] [PubMed] [Google Scholar]
- 5. da Silva FA, Freitas CH, Jorge MS, Moreira TM, de Alcântara MC. Nursing in stomatherapy: clinical care for the patient with varicose ulcer. Rev Bras Enferm. 2009;62(6):889‐893. [DOI] [PubMed] [Google Scholar]
- 6. Hecke AV, Grypdonck M, Beele H, Bacquer DD, Defloor T. How evidence‐based is venous leg ulcer care? A survey in community settings. J Adv Nurs. February 2009;65(2):337‐347. [DOI] [PubMed] [Google Scholar]
- 7. Edwards H, Courtney M, Finlayson K, et al. Chronic venous leg ulcers: effect of a community nursing intervention on pain and healing. Nurs Stand. September 7–13, 2005;19(52):47‐54. [DOI] [PubMed] [Google Scholar]
- 8.Scottish Intercollegiate Guidelines Network. Sign Guideline 120: management of chronic venous leg ulcers. http://www.sign.ac.uk/guidelines/fulltext/120/recommendations.html. Published 2010. Accessed January 4, 2018.
- 9.Australian Wound Management Association Inc. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. http://www.awma.com.au/publications/2011_awma_vlug. Published 2011. Accessed January 4, 2018.
- 10. Heinen M, Borm G, Van der Vleuten C, Evers A, Oostendorp R, Van Achterberg T. The lively legs self‐management programme increased physical activity and reduced wound days in leg ulcer patients: results from a randomized controlled trial. Int J Nurs Stud. 2012;49(2):151‐161. [DOI] [PubMed] [Google Scholar]
- 11. Miller C, Kapp S, Donohue L. Sustaining behavior changes following a venous leg ulcer client education program. Healthcare. September 2014;2(3):324‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding K, Dowsett C, Fias L, et al. Simplifying venous leg ulcer management: consensus recommendations. http://www.woundsinternational.com/consensus-documents/view/simplifying-venous-leg-ulcer-management. Published 2015. Accessed January 4, 2018.
- 13. Van Hecke A, Goeman C, Beeckman D, Heinen M, Defloor T. Development and psychometric evaluation of an instrument to assess venous leg ulcer lifestyle knowledge among nurses. J Adv Nurs. 2011;67(12):2574‐2585. [DOI] [PubMed] [Google Scholar]
- 14. Heinen MM, Van Achterberg T, Scholte Op Reimer W, Van De Kerkhof PCM, De Laat E. Venous leg ulcer patients: a review of the literature on lifestyle and pain‐related interventions. J Clin Nurs. 2004;13(3):355‐366. [DOI] [PubMed] [Google Scholar]
- 15. Santos VLCDG, Azevedo MAJ, Silva TSD, Carvalho VMJ, Carvalho VFD. Adaptação Transcultural do Pressure Ulcer Scale for Healing (PUSH) para a Língua Portuguesa. Rev Latinoam Enferm. 2005;13(3):305‐313. [DOI] [PubMed] [Google Scholar]
- 16. Malaquias SG, Bachion MM, Martins MA, Nunes CAB, Torres GV, Pereira LV. Impaired tissue integrity, related factors and defining characteristics in persons with vascular ulcers. Texto e Contexto—enferm. 2014;23(2):434‐442. [Google Scholar]
- 17. Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs. 2009;11(2):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988:75‐108. [Google Scholar]
- 19. Harrison MB, Van Den Kerkhof EG, Hopman WM, Graham ID, Carley ME, Nelson A. The Canadian bandaging trial: evidence‐informedleg ulcer care and the effectiveness of two compression technologies. BMC Nurs. 2011;10(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073‐1093. [DOI] [PubMed] [Google Scholar]
- 21. Rocha EA, Alexandre NMC, Silva JV. Cultural adaptation and validation of the Freiburg life quality assessment—wound module to Brazilian Portuguese. Rev Latinoam Enferm. 2016;24:e2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. dos RLS GMT, da SV JJM. Self‐care in nursing: self‐management, self‐monitoring, and the management of symptoms as related concepts. Rev Min Enferm. January/March 2013;17(1):225‐230. [Google Scholar]
- 23. Ratliff CR, Yates S, McNichol L, Gray M. Compression for primary prevention, treatment, and prevention of recurrence of venous leg ulcers: an evidence‐and consensus‐based algorithm for care across the continuum. J Wound Ostomy Continence Nurs. 2016;43(4):347‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodrigues FFL, dos SMA, de Teixeira CR S, Gonela JT, Zanetti ML. Relação entre conhecimento, atitude, escolaridade e tempo de doença em indivíduos com diabetes mellitus. Acta Paul Enferm. 2012;25(2):284‐290. [Google Scholar]
- 25. de LCG M, Guedes MVC, Oliveira RM, de Oliveira SKP, de Meneses LST, de Castro ME. Self‐care practice of ostomy patients: contributions of the orem's theory. Rev Rene. 2013;14(2):301‐310. [Google Scholar]
- 26. Green J, McKinley R, Pooler A. The impact of chronic venous leg ulcers: a systematic review. J Wound Care. 2014;23(12):401‐409. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien J, Finlayson K, Kerr G, Shortridge‐Baggett L, Edwards H. Using a theoretical approach to identify factors influencing adherence to an exercise programme for adults with venous leg ulcers. J Health Psychol. 2018;23(5):691‐700. [DOI] [PubMed] [Google Scholar]
- 28. Herschthal J, Kirsner RS. Current management of venous ulcers: an evidence based review. Surg Technol Int. 2008;17:77‐83. [PubMed] [Google Scholar]
- 29. Raju S, Neglén P. Clinical practice. Chronic venous insufficiency and varicose veins. N Engl J Med. May 28, 2009;360(22):2319‐2327. [DOI] [PubMed] [Google Scholar]
- 30. Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg. 2007;21:652‐662. [DOI] [PubMed] [Google Scholar]
- 31. O'Brien J, Finlayson K, Kerr G, Edwards H. Evaluating the effectiveness of a self‐management exercise intervention on wound healing, functional ABPIlity and health‐related quality of life outcomes in adults with venous leg ulcers: a randomised controlled trial. Int Wound J. 2017;14:130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Araujo DN, Ribeiro CTD, Maciel ACC, Bruno SS, Fregonezi GAF, Dias FAL. Physical exercise for the treatment of non‐ulcerated chronic venous insufficiency. Cochrane Database Syst Rev. December 2016;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folguera‐Alvarez C, Garrido‐Elustondo S, Verdú‐Soriano J, et al. ECAMulticapa: effectiveness of doublelayered compression therapy for healing venous ulcers in primary care: a study protocol. BMC Nurs. 2016;15(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liberato SMD, Araújo R de O, de Souza AJG, Marconato AMP, Costa IKF, Torres GV. Adesão ao tratamento de pessoas com úlceras venosas atendidas na atenção primária à saúde. Aquichán. 2017;17(2):128‐139. [Google Scholar]
- 35. Latz CA, Brown KR, Rl B. Compression therapies for chronic venous leg ulcers: interventions and adherence. Chron Wound Care Manage Res. 2015;12:11‐21. [Google Scholar]
- 36. Folguera Álvarez MC, Soriano JV. Adherence to compression therapy in patients with venous ulcers. Gerokomos. 2015;26(3):104‐108. [Google Scholar]
- 37.Meaume S, Dompmartin A, Lok C, et al. Quality of life in patients with leg ulcers: results from CHALLENGE, a double‐blind randomised controlled trial. J Wound Care. July 2, 2017;26(7):268‐379. [DOI] [PubMed] [Google Scholar]
- 38. Dias TYAF, Costa IKF, Melo MDM, da Torres SM SGSO, Maia EMC, Torres G de V. Quality of life assessment of patients with and without venous ulcer. Rev Latinoam Enferm. August 2014;22(4):576‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs. 2009;18:1541‐1549. [DOI] [PubMed] [Google Scholar]


