Abstract
Keloid and hypertrophic scars are difficult to manage and remain a therapeutic challenge. Verapamil has shown a great potential in the management of keloid and hypertrophic scars. Comparing with conventional corticosteroid injections, verapamil could improve the appearance of keloid and hypertrophic scars, and is associated with a lower incidence of adverse effects. Is verapamil an effective alternative modality in the prevention and treatment of keloid and hypertrophic scars? The aim of this study was to assess the effectiveness of verapamil in preventing and treating keloid and hypertrophic scars. Searches were conducted in Medline, EMbase and Cochrane databases from 1974 to January 2015. The selection of articles was limited to human subjects. Five randomised controlled trials (RCTs) or cluster‐randomised trials or controlled clinical trials (CCTs) comparing the efficacy of verapamil with conventional treatments were identified. The results showed that verapamil could improve keloid and hypertrophic scars, and was not significantly different from conventional corticosteroid injections. Few adverse effects were observed. However, this result should be considered carefully, as most of the included studies have a high risk of bias because of issues with randomization, allocation concealment, blinding, incomplete outcomes and selective reporting. In conclusion, verapamil could act as an effective alternative modality in the prevention and treatment of keloid and hypertrophic scars. More high‐quality, multiple‐centre, large‐sample (RCTs) are required to define the role of verapamil in preventing and treating keloid and hypertrophic scars.
Keywords: Hypertrophic scar, Meta analysis, Therapy, Verapamil
Introduction
In clinical presentations, any abnormal wound healing will result in pathological scarring such as keloids and hypertrophic scars 1, 2. Keloids are elevated fibrous scars that extend beyond the borders of the original wound, do not regress and usually recur after excision. Patients at high risk of keloids are usually younger than 30 years and often are Africans, Caucasians and Orientals. Histologically, keloids are characterised by large, thick collagen fibres, with few or even no myofibroblasts 3, 4, 5.
Hypertrophic scars are similar, but remain within the boundaries of the original wound and usually regress over time. Histologically, hypertrophic scars are characterised by the presence of small blood vessels and collagen fibres randomly distributed in small groups of nodules. The presence of myofibroblasts is common 3, 4, 5.
They usually develop 3 months after the trauma. Symptoms such as erythema, itching, burning and pain may be present, and finally resulting in functional and cosmetic deformities, psychological stress and patient dissatisfaction 6, 7. High‐risk trauma includes burns, ear piercing and any factor that prolongs wound healing. Anterior chest, shoulders and upper arms, earlobes and cheeks are most susceptible to developing keloid and hypertrophic scars 8. They are difficult to treat, with a high recurrence rate.
First‐line management of keloid and hypertrophic scars include silicone sheeting, pressure dressings and corticosteroid injections. Surgical removal poses a high recurrence risk unless combined with one or several of these standard therapies 8, 9. Despite numerous proposed therapies reported in the literature, the management of keloid and hypertrophic scars is still challenging as there is no universally accepted treatment regimen 4, 10. Currently, there are many approaches for preventing and treating keloids and hypertrophic scars.
Verapamil, a calcium channel antagonist, has been recommended in the management of keloids and hypertrophic scars because of its ability to decrease extracellular matrix production in scars 11, 12, 13, 14, 15, 16, 17, 18, 19. Intralesional verapamil injection (2·5–10 mg/ml, at timed intervals) has already been successfully used in the past for keloid and hypertrophic scars 15, 16, 17, 18, 20, 21, 22. Moreover, topical verapamil at a concentration of 50 μM is also an excellent choice as a scar modulator 23.
Verapamil is a calcium channel blocker that acts specifically on the L‐type calcium channels present in the cell plasma membrane, blocking the influx of calcium from the extracellular matrix to the cytoplasm. By blocking the entrance of calcium into the cells, verapamil helps reduce the cytosolic concentration of this ion, inducing a series of morphological alterations and its functions 24, 25.
Lee et al. 17 were the first to report the response of burn scars to intralesional verapamil. Nowadays, numerous studies have shown the role of verapamil in the management of keloid and hypertrophic scars. Verapamil could inhibit the synthesis/secretion of extracellular matrix, induce fibroblast procollagenase synthesis and inhibit IL‐6, VEGF, TGF‐β1 and cellular proliferation of fibroblasts, resulting in depolymerization of actin filaments, alteration of cell shape, apoptosis and reduction of fibrous tissue production 11, 21, 22, 26, 27, 28.
Verapamil shows great potential for controlling the biosynthesis of its extracellular matrix, and has already been successfully used for treating keloid and hypertrophic scars. Recently, it is reported that verapamil can improve the appearance of keloid and hypertrophic scars, and have been associated with a lower incidence of adverse effects, compared with conventional corticosteroid injections 18, 20. Is verapamil an effective alternative modality in the prevention and treatment of keloid and hypertrophic scars? More evidences are needed for making this clinical decision.
Therefore, in this study, we attempt to assess the effects of verapamil in preventing keloid and hypertrophic scarring in people with newly healed wounds of any type, and also evaluate the effects of verapamil in treating established scarring in people with keloid and hypertrophic scars after any type of wound.
Materials and methods
Inclusive criteria of published studies
Types of studies
We considered any randomised controlled trials (RCTs), cluster‐randomised trials or controlled clinical trials (CCTs).
Types of participants
People of any age with healed full‐thickness wounds (damage to epidermis, dermis and subcutaneous tissue) where the skin is intact were included. Prevention studies that considered people with newly healed wounds and treatment studies that considered people with established scarring were considered.
Types of interventions
Comparisons of verapamil with all other conservative techniques (e.g. corticosteroids, silicon gel sheeting, 5‐fluorouracil or no intervention) were considered eligible. We included comparisons of verapamil at different doses. We excluded any compound preparation containing verapamil.
Types of outcome measures
Primary outcomes. Prevention studies, including (i) recurrence (the number of people who develop keloid and hypertrophic scarring) and (ii) adverse reactions.
Primary outcomes. Treatment studies, including (i) change in scar size, measured by length, volume, height or width – usually by ruler or Vancouver Scar Scale (VSS) (ii) scar colour, by VSS or colour charts (iii) skin elasticity, measured serially with the use of an elastometer or VSS (iv) complications (e.g. rashes, atrophy, skin breakdown, pain measured on a numbered scale) (v) cosmesis as defined by patient opinion and physician observations (using assessment scales).
Secondary outcomes. (i) Quality of life; (ii) economic burden; (iii) others.
All of the studies involved in this study have been approved by the ethics committee, and the patients have signed their informed consent.
Search methods for identification of studies
An electronic search was conducted through the Medline, EMbase and Cochrane databases from 1974 to January 2015. The references of published studies were also reviewed for relevant articles. The search was limited to human subjects.
Data collection and analysis
Two review authors independently assessed the titles and available abstracts of all the studies identified during initial search and excluded any clearly irrelevant studies. They independently assessed the full‐paper copies of reports of potentially eligible studies using the inclusion criteria. The authors resolved disagreements on inclusion by consensus and, when this failed, by arbitration by a third review author. Data were extracted by one review author and checked for accuracy by a second review author. We used a standard data form to capture the information. Data for prevention and treatment were dealt with separately. We requested additional unpublished data from primary authors and included when available.
For this review, two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias 29, which addresses random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other issues.
Statistical analysis
We allocated the results of the randomised controlled trial as dichotomous frequency data. Individual study relative risks (RRs) and 95% confidence intervals (CIs) were calculated from event numbers extracted from each trial before data pooling. Both fixed‐effects and random‐effects models were used to evaluate the pooled RR for verapamil treatment. All reported P values are two‐sided, and P values of less than 0·05 were regarded as statistically significant for all the included studies. Statistical analyses were performed using STATA software (StataCorp LP, College Station, Texas) (version 10.0).
Results
Searches conducted in the Medline, EMbase and Cochrane databases yielded a total of 99 articles. After examining the titles and abstracts, 87 studies were excluded because they were irrelevant. Eleven full‐text articles were assessed for further consideration. However, further seven articles were excluded (three were not RCTs or CCTs, one review, one case and two cytological experiments). Thus five trials were included finally, containing four English (two RCTs and two CCTs) and one Chinese (one CCT) (Figure 1) studies. Four trials compared verapamil injection with other treatments, and one trial compared verapamil gel with placebo. The included studies were conducted in four countries, and involved a total of 276 people aged 12 to 65 years. The controls included placebo, triamcinolone, interferon α‐2b and topical silicone without verapamil injections. Two prevention studies and three treatment studies were included. Characteristics of the included studies are shown in Table 1.
Figure 1.
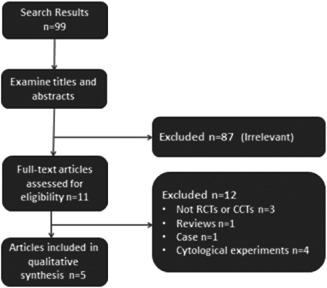
Flow diagram of the literature search and trials selection process.
Table 1.
Characteristics of the included studies
| Boggio 23 | Ahuja and Chatterjee 20 | Xu et al. 21 | Margaret Shanthi et al. 18 | D'Andrea et al. 15 | |
|---|---|---|---|---|---|
| Population characteristics | |||||
| Subjects (n) | 120 patients | 40 patients (48 scars) | 18 patients | 54 patients | 44 patients |
| Male:female | All females | Not specified | 1:1 | 1:1 | 28:16 |
| Mean age (years) | 43 | Not specified | Not specified | V:26, T:20 | Not specified |
| Age (years) | 31–65 | 15–60 | 12–48 | 10–50 | 22–45 |
| Site of scar | Abdomen, breast | Pre‐sternal, extremities, face, torso/back | Head, axilla, arm, pre‐sternal, shoulder, abdomen | Face/neck, earlobe, sternum, abdomen/chest, limb | Back, sternum and deltoid |
| Scar duration | 3 months | Under 2 years | 1–1·5 years | Not specified | 2–5 years |
| Cause of scar | Surgery | Post‐infective, surgery, burns, trauma | Not specified | Burns, trauma, surgery, insect bite and acne | Not specified |
| Treatment history | Untreated | Untreated | Untreated | Not specified | Not specified |
| Intervention | |||||
| Duration (ms) | 90 days | Until flattening or eight sessions | Not specified | Until flattened or 6 months | 2 months |
| Dosage | 50 μM | 2·5 mg/ml, maximum 1·5 ml | 2·5 mg/ml, 0·15 ml | 2·5 mg, 1 ml | 2·5 mg/ml, 0·5–2·0 ml |
| Frequency | Twice a day | Every 3 weeks | Once | Every 3 weeks | Four times |
| Treatments (n) | 60 | 26 scars | 6 | 27 | 22 |
| Comparator | Placebo | Triamcinolone | IFN a‐2b, triamcinolone, placebo | Triamcinolone | The same treatment except verapamil |
| Outcomes | |||||
| Primary outcomes | Stony Brook Scar Scale | Vancouver Scar Scale | PCNA, TGF‐β1, Apoptosis | Vancouver Scar Scale | Recurrence |
| Study duration | 3 months | 24 weeks | 7 days | 1 year | 18 months |
Verapamil compared with triamcinolone
Prevention studies
No prevention studies were identified.
Treatment studies
Two of the studies identified (involving 94 patients) used the VSS as the primary outcome 18, 20. Mean zero VSS scores were achieved with treatments in respect of scar height, vascularity and pliability at 24 weeks in both trials. Reported data were treated as continuous and used mean difference. When both trials were pooled (fixed effect, I 2 = 0%), there was no significant difference in the VSS in keloid and hypertrophic scars (mean difference = 0·14; 95% CI: −0·03 to 0·31) (Figure 2A). But Margaret Shanthi et al. found that the rate of reduction in vascularity, pliability, height and width of the scar with triamcinolone was faster than with verapamil 18.
Figure 2.
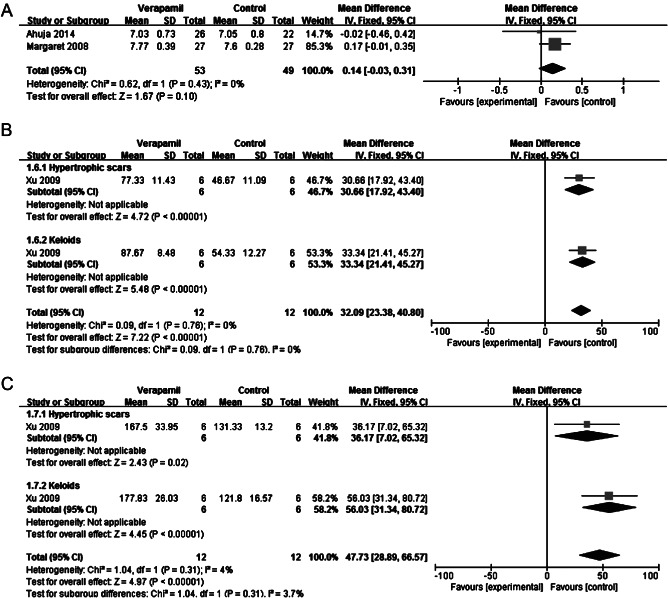
Forest plot of comparison: verapamil versus triamcinolone. (A) Mean difference in Vancouver Scar Scale. (B) Mean difference in TGF‐β1 expression. (C) Mean difference in apoptosis.
Xu et al. 21 compared the effects of intralesional triamcinolone with verapamil on TGF‐β1 expression and apoptosis in vivo. After intralesional triamcinolone acetonide injection, TGF‐β1 expression in keloid and hypertrophic scars could be significantly depressed (mean difference = 32·09; 95% CI: 23·38 to 40·80; fixed effect, I 2 = 0%) (Figure 2B). But intralesional verapamil injection could induce apoptosis in both keloid and hypertrophic scars (mean difference = 47·73; 95% CI: 28·89 to 66·57; fixed effect, I 2 = 3·7%) (Figure 2C) 21.
Verapamil compared with interferon α‐2b
Prevention studies
No prevention studies were identified.
Treatment studies
One study investigated the effects of intralesional interferon α‐2b and verapamil on apoptosis and TGF‐β1 expression in vivo. Verapamil could induce apoptosis in both keloid and hypertrophic scars (mean difference = 101·04; 95% CI: 83·20 to 118·89; fixed effect, I 2 = 0%) (Figure 3A), but interferon α‐2b could significantly prohibit TGF‐β1 expression (mean difference = 27·01; 95% CI: 18·14 to 35·87; fixed effect, I 2 = 15%) (Figure 3B) 21.
Figure 3.
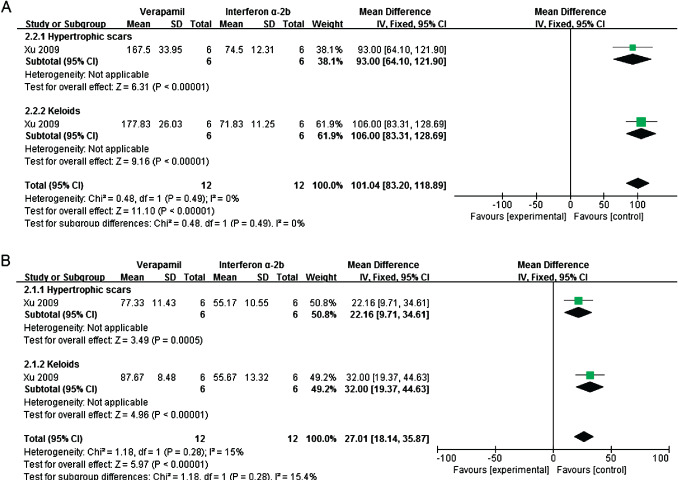
Forest plot of comparison: verapamil versus interferon α‐2b. (A) Mean difference in apoptosis. (B) Mean difference in TGF‐β1 expression.
Verapamil compared with placebo
Prevention studies
Boggio et al. 22 compared topical verapamil (a nonionic gel containing verapamil at a concentration of 50 μM) with no treatment. The scars were rated using the Stony Brook Scar Evaluation Scale by blinded observers, and differences in the quality of the scars in patients who used verapamil compared with scars of those who did not use any healing modulator were significant (P < 0·05). Patients treated with verapamil presented good quality scarring (80% of mammoplasty scars and 75·2% abdominoplasty scars), while patients in no‐treatment group showed 48% and 51·2% satisfaction for mammoplasty and abdominoplasty scars 23. The author was contacted for detailed data, but no response was received.
Treatment studies
One study reported the effects of intralesional verapamil injection and placebo on proliferation, apoptosis and TGF‐β1 expression in vivo. Compared with no‐treatment group, verapamil could inhibit fibroblast proliferation (mean difference = −52·17; 95% CI: −59·63 to −44·72; fixed effect, I 2 = 39%) (Figure 4A), reduce TGF‐β1 expression (mean difference = −20·49; 95% CI: –30·63 to −10·36; fixed effect, I 2 = 0%) (Figure 4B) and induce apoptosis (mean difference = 100·45; 95% CI: 82·04–118·86; fixed effect, I 2 = 0%) (Figure 4C) 21.
Figure 4.
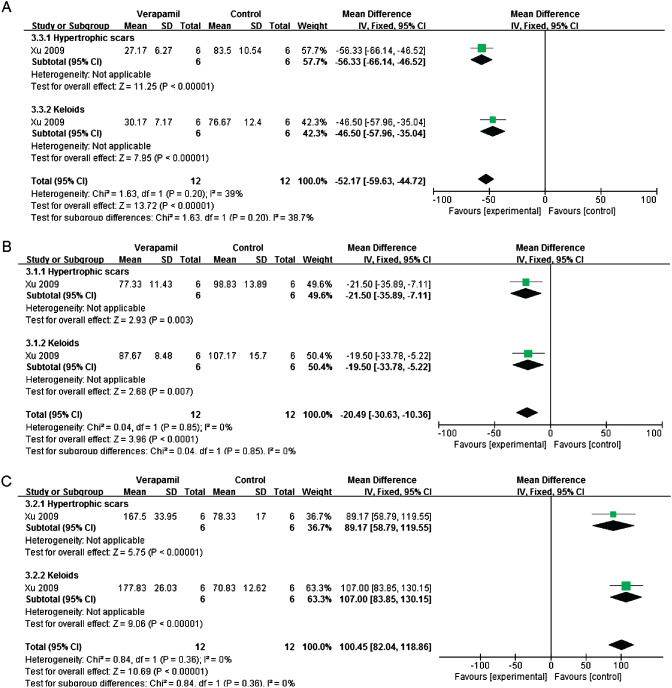
Forest plot of comparison: verapamil versus placebo. (A) Mean difference in fibroblast proliferation. (B) Mean difference in TGF‐β1 expression. (C) Mean difference in apoptosis.
Verapamil + silicone compared with silicone
Prevention studies
Recurrence was investigated by D'Andrea et al. 15 as a primary outcome. In the first group, treated by perilesional surgical excision of keloids and topical silicone, combined with intralesional verapamil hydrochloride injection, keloids were cured in 54% of the cases. But in the control group, receiving the same treatment except the verapamil hydrochloride, no complete regression of keloids occurred (RR 0·47, 95% CI: 0·30–0·73). Furthermore, an amelioration of the lesion could be demonstrated in 36% of the cases in the first group, compared with 18% in the control group.
Treatment studies
No treatment studies were identified.
Complications
In the trial by Boggio et al. 23, there were no adverse reactions such as erythema, pruritus, or bullous lesions found in any of the cases after the use of topical verapamil.
Two trials reported that adverse drug reactions were more with triamcinolone than with verapamil 18, 20. Only minor adverse effects (e.g. pain during injection of verapamil) were noted with verapamil 20.
Discussion
There were only two single‐blind, randomised, controlled trials in the five included studies without allocation concealment, resulting in high risk of selection bias and performance bias. Therefore, the quality of the trials showed high risk of selection bias, probably affecting the outcomes reported (Table 2).
Table 2.
Risk of bias
| Boggio 23 | Ahuja and Chatterjee 20 | Xu et al. 21 | Margaret Shanthi et al. 18 | D'Andrea et al. 15 | |
|---|---|---|---|---|---|
| Random sequence generation | High risk | Low risk | High risk | Low risk | High risk |
| Allocation concealment | Unclear | Unclear | Unclear | Unclear | Unclear |
| Blinding | Low risk | Low risk | Unclear | Low risk | Unclear |
| Incomplete outcome data | Low risk | Low risk | High risk | High risk | Unclear |
| Selective reporting | Unclear | Unclear | High risk | Unclear | Unclear |
| Other bias | Unclear | Unclear | Unclear | Unclear | Unclear |
There were plenty of studies about verapamil for treating keloid and hypertrophic scars; however, some problems existed that made meta‐analysis difficult. Firstly, the lack of a standardised protocol for keloid and hypertrophic scars, such as the duration of the scars, follow‐up time, a standardised treatment protocol and assessment scale, made the synthesis of results and analysis difficult. Secondly, pathological scars, including hypertrophic scars and keloids, were clubbed together without subgroup analysis. Even morphologically similar hypertrophic scars differed pathologically and clinically from keloids 30, 31. Therefore, the treatment details (e.g. dosage, frequency and duration) would be quite different. Thirdly, only figures were available in most articles and not original data, making it difficult to analyse data.
In summary, five RCTs or CCTs comparing the efficacy of verapamil with conventional treatments were identified 15, 18, 20, 21, 23, 32. Results of the trials showed that verapamil could improve keloid and hypertrophic scars, and was not significantly different with conventional corticosteroid injections. Fewer adverse effects were observed.
However, this result should be considered carefully, as most of the included studies have a high risk of bias because of issues with randomization, allocation concealment, blinding, incomplete outcomes and selective reporting.
In conclusion, verapamil could act as an effective alternative modality in the prevention and treatment of keloid and hypertrophic scars. More high‐quality, multiple‐centre, large‐sample randomised controlled trials are required to define the role of verapamil in preventing and treating keloid and hypertrophic scars.
Acknowledgements
The authors declared no competing interests. The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rekha A. Keloids, a frustrating hurdle in wound healing. Int Wound J 2004;1:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 3. Jackson IT, Bhageshpur R, DiNick V, Khan A, Bhaloo S. Investigation of recurrence rates among earlobe keloids utilizing various postoperative therapeutic modalities. Eur J Plastic Surg 2001;24:88–95. [Google Scholar]
- 4. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars. Arch Facial Plast Surg 2006;8:362–8. [DOI] [PubMed] [Google Scholar]
- 5. David TR, Daniel B. Abnormal wound healing: keloids. Clin Dermatol 2007;25:26–32. [DOI] [PubMed] [Google Scholar]
- 6. Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. Randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast Reconstr Surg 2005;116:1648–56. [DOI] [PubMed] [Google Scholar]
- 7. Baisch A, Riedel F. Hyperplastic scars and keloids. Part I: basics and prevention. HNO 2006;54:893–904. [DOI] [PubMed] [Google Scholar]
- 8. Juckett G, Hartman‐Adams H. Management of keloids and hypertrophic scars. Am Fam Physician 2009;80:253–60. [PubMed] [Google Scholar]
- 9. Bloemen MC, Van der Veer WM, Ulrich MM, Van Zuijlen PP, Niessen FB, Middelkoop E. Prevention and curative management of hypertrophic scar formation. Burns 2009;35:463–75. [DOI] [PubMed] [Google Scholar]
- 10. Mofikoya BO, Adeyemo WL, Abdus‐salam AA. Keloid and hypertrophic scars: a review of recent developments in pathogenesis and management. Nig Q J Hosp Med 2007;17:134–9. [DOI] [PubMed] [Google Scholar]
- 11. Doong H, Dissanayake S, Gowrishankar TR, LaBarbera MC, Lee RC. The 1996 Lindberg Award. Calcium antagonists alter cell shape and induce procollagenase synthesis in keloid and normal human dermal fibroblasts. J Brun Care Rehabil 1996;17:497–514. [PubMed] [Google Scholar]
- 12. Kang Y, Lee DA, Higginbotham EJ. In vitro evaluation of antiproliferative potential of calcium channel blockers in human Tenon's fibroblasts. Exp Eye Res 1997;64:913–25. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman S, Gopalakrishna R, Gundimeda U, Murata T, Spee C, Ryan SJ, Hinton DR. Verapamil inhibits proliferation, migration and protein kinase C activity in human retinal pigment epithelial cells. Exp Eye Res 1998;67:45–52. [DOI] [PubMed] [Google Scholar]
- 14. Porter GA Jr, Makuck RF, Rivkees SA. Reduction in intracellular calcium levels inhibits myoblast differentiation. J Biol Chem 2002;277:28942–7. [DOI] [PubMed] [Google Scholar]
- 15. D'Andrea F, Brongo S, Ferraro G, Baroni A. Prevention and treatment of keloids with intralesional verapamil. Dermatology 2002;204:60–2. [DOI] [PubMed] [Google Scholar]
- 16. Lawrence WT. Treatment of earlobe keloids with surgery plus adjuvant intralesional verapamil and pressure earrings. Ann Plast Surg 1996;37:167–9. [DOI] [PubMed] [Google Scholar]
- 17. Lee RC, Doong H, Jellema AF. The response of burn scars to intralesional verapamil. Report of five cases. Arch Surg 1994;129:107–11. [DOI] [PubMed] [Google Scholar]
- 18. Margaret Shanthi FX, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol 2008;74:343–8. [DOI] [PubMed] [Google Scholar]
- 19. Boggio RF, Freitas VM, Cassiola FM, Urabayashi M, Machado‐Santelli GM. Effect of a calcium‐channel blocker (verapamil) on the morphology, cytoskeleton and collagenase activity of human skin fibroblasts. Burns 2011;37:616–25. [DOI] [PubMed] [Google Scholar]
- 20. Ahuja RB, Chatterjee P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns 2014;40:583–8. [DOI] [PubMed] [Google Scholar]
- 21. Xu SJ, Teng JY, Xie J, Shen MQ, Chen DM. Comparison of the mechanisms of intralesional steroid, interferon or verapamil injection in the treatment of proliferative scars. Zhonghua Zheng Xing Wai Ke Za Zhi 2009;25:37–40. [PubMed] [Google Scholar]
- 22. Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg 2013;39:1745–57. [DOI] [PubMed] [Google Scholar]
- 23. Boggio RF, Boggio LF, Galvão BL, Machado‐Santelli GM. Topical verapamil as a scar modulator. Aesthetic Plast Surg 2014;38:968–75. [DOI] [PubMed] [Google Scholar]
- 24. Grossman E, Messerli FH. Calcium antagonists. Prog Cardiovasc Dis 2004;47:34–57. [DOI] [PubMed] [Google Scholar]
- 25. Burchiel SW, Edwards BS, Kuckuck FW, Lauer FT, Prossnitz ER, Ransom JT, Sklar LA. Analysis of free intracellular calcium by flow cytometry: multiparameter and pharmacologic applications. Methods 2000;21:221–30. [DOI] [PubMed] [Google Scholar]
- 26. Lee RC, Ping JA. Calcium antagonists retard extracellular matrix production in connective tissue equivalent. J Surg Res 1990;49:463–6. [DOI] [PubMed] [Google Scholar]
- 27. Giugliano G, Pasquali D, Notaro A, Brongo S, Nicoletti G, D'Andrea F, Bellastella A, Sinisi AA. Verapamil inhibits interleukin‐6 and vascular endothelial growth factor production in primary cultures of keloid fibroblasts. Br J Plast Surg 2003;56:804–9. [DOI] [PubMed] [Google Scholar]
- 28. Yang JY, Huang CY. The effect of combined steroid and calcium channel blocker injection on human hypertrophic scars in animal model: a new strategy for the treatment of hypertrophic scars. Dermatol Surg 2010;36:1942–9. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011; www.cochrane‐handbook.org. [Google Scholar]
- 30. Wang XQ, Liu YK, Qing C, Lu SL. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann Plast Surg 2009;63:688–92. [DOI] [PubMed] [Google Scholar]
- 31. Chen MA, Davidson TM. Scar management: prevention and treatment strategies. Curr Opin Otolaryngol Head Neck Surg 2005;13:242–7. [DOI] [PubMed] [Google Scholar]
- 32. Huang C, Akaishi S, Hyakusoku H, Ogawa R. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int Wound J 2014;11:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


