Abstract
Over the last decade, lymph node flap (LNF) transfer has turned out to be an effective method in the management of lymphoedema of extremities. Most of the time, the pockets created for LNF cannot be closed primarily and need to be resurfaced with split thickness skin grafts. Partial graft loss was frequently noted in these cases. The need to prevent graft loss on these iatrogenic wounds made us explore the possibility of attempting delayed skin grafting. We have herein reported our experience with delayed grafting with autologous banked split skin grafts in cases of LNF transfer for lymphoedema of the extremities. Ten patients with International Society of Lymphology stage II–III lymphoedema of upper or lower extremity were included in this study over an 8‐month period. All patients were thoroughly evaluated and subjected to lymph node flap transfer. The split skin graft was harvested and banked at the donor site, avoiding immediate resurfacing over the flap. The same was carried out in an aseptic manner as a bedside procedure after confirming flap viability and allowing flap swelling to subside. Patients were followed up to evaluate long‐term outcomes. Flap survival was 100%. Successful delayed skin grafting was done between the 4th and 6th post‐operative day as a bedside procedure under local anaesthesia. The split thickness skin grafts (STSG) takes more than 97%. One patient needed additional medications during the bedside procedure. All patients had minimal post‐operative pain and skin graft requirement. The patients were also reported to be satisfied with the final aesthetic results. There were no complications related to either the skin grafts or donor sites during the entire period of follow‐up. Delayed split skin grafting is a reliable method of resurfacing lymph node flaps and has been shown to reduce the possibility of flap complications as well as the operative time and costs.
Keywords: Banking split thickness skin graft, Delayed skin grafting, Lymph node flap transfer, Lymph node transfer, Lymphoedema
Introduction
Achievement of wound healing by application of split skin graft is the simplest and time‐tested treatment modality. The same can be performed with ease in the presence of a vascularised bed while ruling out any specific contraindications.
In a similar way, lymph node flap transfer (LNFT) is a relatively new approach for treating lymphoedema that has grown exponentially during the last few years because of its promising results 1, 2, 3, 4, 5. Several studies of different donor sites for LNFT have been described in the literature and have demonstrated that LNFT improves lymphatic drainage and function 1, 5, 6, 7, 8, 9, 10. At our unit, LNFT is one of the most common procedures performed for the treatment of lymphoedema. In some instances, primary closure may be achieved after insetting the flap. However, in most cases of LNFT, split thickness skin grafts (STSG) are required for closure owing to the inability of the flap to fit into the newly created skin pocket. The presence of surrounding fibrous tissue also precludes primary closure 6, 7, 8, 9, 10. Irrespective of the choice of flap, they all have a tendency towards swelling, especially in the first 48–72 hours. Thus, there is a possibility of pedicle compression between the swollen flap tissue and the overlying fixed skin graft in the first 48–72 hours. This may lead to pedicle displacement and kinking with subsequent vasospasm and ultimate complete or partial flap loss. To avoid this complication, we have started the practice of non‐coverage of the LNFT during the primary operation, allowing the initial flap swelling to settle. The skin graft is harvested and banked at the donor site. After confirming flap viability and allowing reduction of flap swelling, the skin grafting is performed 4–6 days after surgery as a bedside procedure.
Herein, we describe a surgical technique in the field of LNFT by banking the harvested skin graft at the donor site during the initial procedure followed by secondary grafting at the bedside. The purpose of this study was to critically evaluate the perioperative and postoperative benefits accrued in ten patients in whom the above technique was used.
Material and methods
Ten patients with stage II and III (International Society of Lymphology) lymphoedema of upper 6 or lower 4 extremity who underwent a vascularised lymph node transfer between June 2014 and January 2015 were included in this study. All patients had an exposed overlying skin defect after flap inset that necessitated coverage with a STSG. The skin graft was harvested as per the patient's preferred region of the thigh with an air dermatome during the primary procedure. Patients with diabetes mellitus and/ or coagulopathy and those having LNFT that could easily be closed primarily in a tension‐free manner were not included in this study. Patient data are summarised in Table 1.
Table 1.
Data of patients who underwent a delayed grafting for banked skin graft in lymph node flap transfer
| Patient | Age | Diagnosis | ISL lymphoedema stage | LNF used | Skin graft size (cm) | Day of grafting | Time of the procedure (min) | Length of hospital stay | Skin graft take at discharge (%) | Complications | Follow‐ up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Left upper limb | III | Gastroepiploic | 2 × 6 | 6 | 15 | 11 | 95 | No | 6 |
| 2 | 51 | Right upper limb | II | Gastroepiploic | 2 × 5 | 5 | 18 | 11 | 100 | No | 5 |
| 3 | 40 | Left upper limb | III | Groin | 1·5 × 4 | 5 | 20 | 10 | 98 | No | 6 |
| 4 | 57 | Right upper limb | II | Gastroepiploic | 2 × 5 | 4 | 18 | 9 | 100 | No | 6 |
| 5 | 61 | Left lower limb | III | Supraclavicular | 2·5 × 4 | 4 | 15 | 9 | 95 | No | 8 |
| 6 | 48 | Right lower limb | III | Gastroepiploic | 2 × 6 | 5 | 12 | 11 | 100 | No | 5 |
| 7 | 62 | Right lower limb | II | Supraclavicular | 2 × 5 | 5 | 15 | 10 | 95 | No | 6 |
| 8 | 48 | Left upper limb | II | Groin | 2·5 × 4·5 | 4 | 17 | 9 | 90 | No | 8 |
| 9 | 50 | Left lower limb | III | Gastroepiploic | 2 × 6 | 5 | 15 | 10 | 100 | No | 4 |
| 10 | 39 | Right upper limb | III | Supraclavicular | 2 × 5 | 6 | 17 | 11 | 100 | No | 8 |
| Mean (± SD) | 50·1 | 2·0 × 5·0 | 4·9 ± 0·7 | 16·2 ± 2·2 | 10·1 ± 0·87 | 97·3 ± 3·4 | 6·2 ± 1·4 |
ISL, International Society of Lymphology; LNF, lymph node flap.
A thin STSG (0·012 and 0·014 inch) skin graft was harvested (with one of the sides still attached) and was replaced on the donor site immediately. It was covered with a non‐adherent dressing material and an elastic bandage (Figure 1). The transfer of the STSG from the donor to the recipient site was carried out at bedside between the 4th and 6th postoperative days. This was performed with minimal intravenous sedation and analgesia while ensuring an asepsis and sterile technique. The skin was gently elevated from the donor site using the back end of a surgical blade depending on the defect size requirements. The skin graft was then washed in normal saline to remove any retained blood clot or debris. The recipient (flap site) wound was cleaned and any slough/devascularised tissue was carefully debrided. The skin graft was then laid in place and secured with a 5‐0 catgut sutures. The graft was dressed with a non‐adherent dressing material and moistened gauze, and the same was changed every 2 days. Donor sites were dressed with a semipermeable clear plastic dressing. All skin grafts were examined before discharge. Follow‐up was done once every month for the first 3 months and then every 3 months till the end of 1 year.
Figure 1.
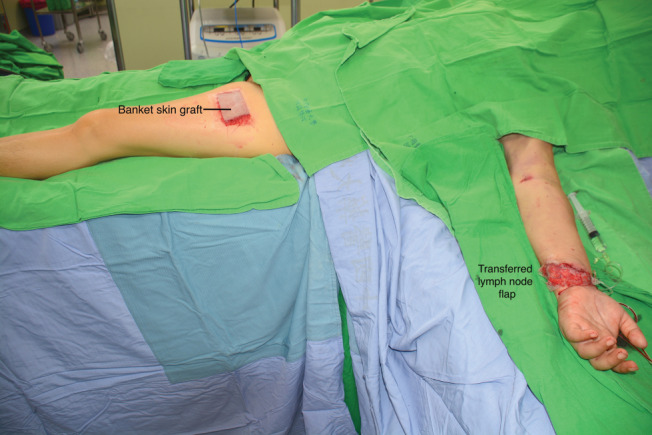
Immediate postoperative picture. Black arrow shows banked skin graft attached at donor site and flap has been inset at left wrist. Both sites covered with non‐adherent dressing material.
Results
The mean age of the patients was 50·1 years (range: 39–62 years). The average graft size was 2·05 × 5·05 cm2 (range: 1·5 × 4 cm2–2 × 6 cm2). The mean time for the secondary procedure was 16·2 ± 2·25 minutes (range: 12–20 minutes). The mean skin graft take at the time of discharge was 97·3% ± 3·43% (range: 90–100%). The delayed transfer of the STSG was carried out on the 4th 3, 5th 5 and 6th 2 postoperative day. In one patient, additional sedatives were required for relieving pain and anxiety. Except for this single case, delayed elevation of the STSG from the donor site was uneventful and well tolerated.
None of our patients reported any complications, and no donor‐site morbidity was encountered during the period of follow‐up. In the current report, the average length of hospital stay was 10·1 ± 0·87 days (range: 9–11 days). At a mean follow‐up of 6·2 ± 1·4 months (range: 4–8 moths), all grafts had healed. The results are summarised in detail in Table 1 and shown in Figures 2 and 3. All patients reported satisfaction with their cosmetic result.
Figure 2.
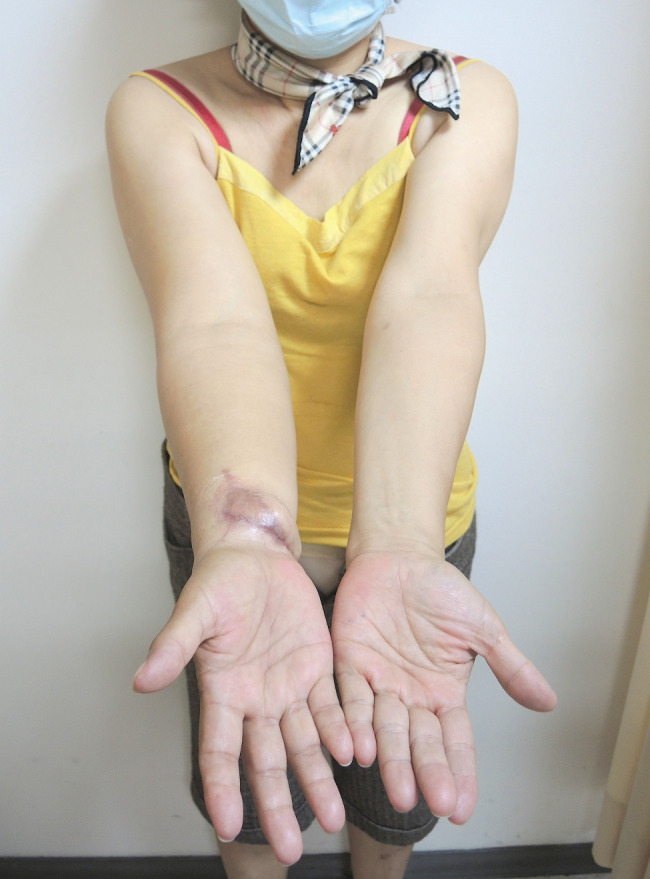
Nine‐month postoperative picture after delayed skin grafting for lymph node flap transfer (LNFT). Delayed split skin grafting allows reduced graft requirement, leading to better aesthetic outcome. Further improvement can be achieved by scar revision after 1 year of primary surgery.
Figure 3.
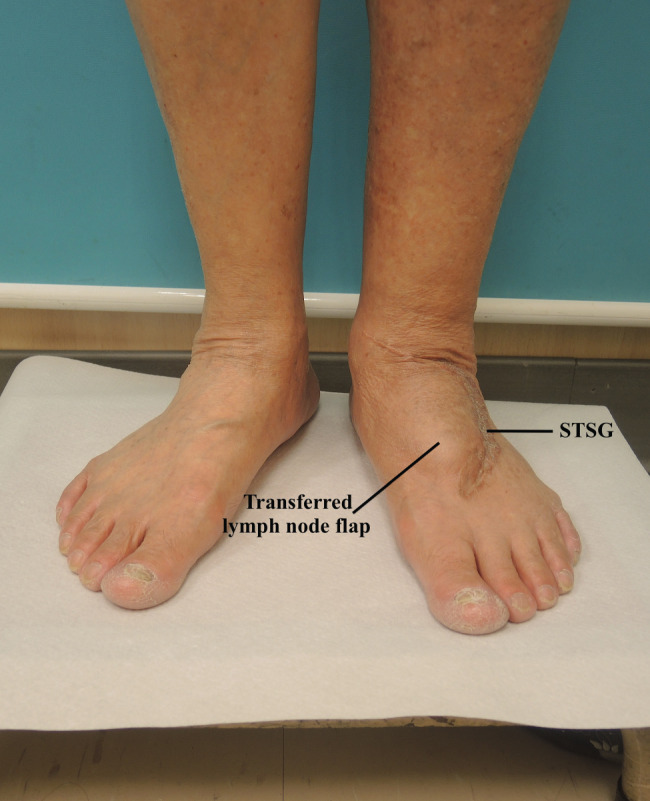
Eight month follow‐up picture shows a satisfactory result following lymph node flap transfer (LNFT) and delayed split thickness skin grafts (STSG) to the dorsum of the foot in case of lower extremity lymphoedema.
Discussion
In the literature, there are some series describing LNFT using STSG for coverage of the transferred flap when primary closure is not possible so as to avoid tension and also to facilitate monitoring 6, 7, 9. However, in our experience, even though we frequently use STSG to cover the exposed areas of the transferred flap, we found in some cases that the skin graft sutures had to be released after LNFT to relieve pressure on the flap because of pedicle compression. We find it difficult to predict the amount of swelling that such flaps develop in the initial period. This is especially true with intra‐abdominal flaps like the right gastroepiploic lymph node flap 9, where we have observed substantial swelling in the first 72 hours following surgery. The STSG also interfered with direct flap monitoring and defeated one of the purposes of using it. The frequent handling and exposure during the monitoring also resulted in partial graft loss in a few cases, requiring regrafting. Many patients reported less than satisfactory aesthetic outcomes as the flap swells up and increases the skin graft requirement. In our present series, all the above problems have been dealt with effectively. Thus, we have demonstrated a successful combination of a modern state of the art lymph node flap transfer with an old, time‐tested albeit overlooked method of resurfacing.
The classical method of preserving split‐thickness skin grafts has been in vogue for a long time. It consists of folding the graft with dermis‐to‐dermis contact followed by rolling in moistened gauze and finally storing it at 4°C 11, 12, 13, 14. Survival for up to 5 weeks has been reported with this method 15. Many methods have been described later with some variations in storage technique 12, 13, 14. There have also been other accepted practices like storage on the patient's body. Shepard described this technique and successfully used it for delayed grafting for up to 3 weeks post‐harvest in cases of traumatic injuries, burns and chronic ulcers 16. We found this method to be highly effective and have demonstrated its successful application in our study. We performed the same between the 4th and 6th post operative day. This was performed as a bedside procedure, ensuring adequate asepsis and patient safety. However, we have used the techniques in elective cases of LNFT on the upper or lower extremity in patients with moderate to advanced lymphoedema. The delayed grafting has not only ensured complete survival of the flaps but has also reduced the skin graft requirement. This leads to significant reduction in the scarring and improves satisfaction levels in many patients who increasingly demand aesthetically acceptable outcomes. Secondly, if the graft is not required or a lesser amount is required, it is simply left back on the donor site itself and has been shown to heal in an uneventful manner 16. In our study, the indication for delayed grafting was to safeguard the flap viability by allowing direct clinical monitoring and avoiding pressure on the pedicle because of the sutured skin graft. The delayed grafting allowed the flap swelling to settle, and the final skin graft requirement was substantially reduced. It thus helped us to provide improved aesthetic results without compromising on the functional aspect. It also minimised donor site morbidity. In order to provide better aesthesis, the transferred flap may be debulked along with partial or complete removal of the STSG after 1 year of the primary surgery using the microscopic debulking technique 17, taking due care to avoid damage to the lymphatic architecture.
Finally, in cases where the flap shows signs of failing or ultimately fails in the first few days itself, needless sacrifice of the skin graft is avoided. Fortunately, in our study, we did not encounter such a scenario. Another interesting benefit is the avoidance of any issues arising out of ex vivo storage, which may not comply with the strict norms laid down for skin and tissue preservation by various regulatory authorities. It eliminates potential complications like clerical errors, contamination and wrong patient graft application, which may occur in scenarios where the graft is stored ex vivo in the hospital's skin banks. An additional advantage derived from this method is the cost effectiveness. The primary procedure is shortened by reducing the time required in graft inset and the delicate graft dressing. Moreover, the delayed procedure can be easily managed at the bedside in simple analgesia cover, thus avoiding the operation theatre and anaesthesia expenses. The analgesia requirement for the skin graft donor site is reduced as the graft itself acts as a biological dressing. Some patients may, however, be uncomfortable to have an additional procedure, especially when performed without complete anaesthesia. Yet, we firmly believe that this combined technique is largely comfortable and safe for most of the patients and allows flexibility in the coverage of the donor site while providing additional security against tension and compression of the flap pedicle in the critical early postoperative period.
Conclusion
We found the use of delayed autologous split skin graft banking to be a novel and effective approach in lymph node flap surgery. It is a minimally invasive, successful procedure that avoids potential pedicle compression and minimises the use of a skin graft at the recipient site while reducing donor site morbidity.
Acknowledgements
All authors hereby declare not to have any potential conflict of interests and not to have received funding for this work from any of the following organizations: National Institutes of Health (NIH); Welcome Trust; Howard Hughes Medical Institute (HHMI); and other(s). Each author participated sufficiently in the work to take public responsibility for the content.
References
- 1. Raju A, Chang DW. Vascularized lymph node transfer for treatment of lymphedema: a comprehensive literature review. Ann Surg 2015;261:1013–23. [DOI] [PubMed] [Google Scholar]
- 2. Cheng MH, Huang JJ, Wu CW, Yang CY, Lin CY, Henry SL, Kolios L. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg 2014;133:192. [DOI] [PubMed] [Google Scholar]
- 3. Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema long‐term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granzow JW, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol 2014;21:1195–201. [DOI] [PubMed] [Google Scholar]
- 5. Lin CH, Ali R, Chen SC, Wallace C, Chang YC, Chen HC, Cheng MH. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265–75. [DOI] [PubMed] [Google Scholar]
- 6. Cheng MH, Huang JJ, Nguyen DH, Saint‐Cyr M, Zenn MR, Tan BK, Lee CL. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol 2012;126:93–8. [DOI] [PubMed] [Google Scholar]
- 7. Sapountzis S, Singhal D, Rashid A, Ciudad P, Meo D, Chen HC. Lymph node flap based on the right transverse cervical artery as a donor site for lymph node transfer. Ann Plast Surg 2014;73:398–401. [DOI] [PubMed] [Google Scholar]
- 8. Barreiro GC, Baptista RR, Kasai KE, Dos Anjos DM, Busnardo FD, Modolin M, Ferreira MC. Lymph fasciocutaneous lateral thoracic artery flap: anatomical study and clinical use. J Reconstr Microsurg 2014;30:389–96. [DOI] [PubMed] [Google Scholar]
- 9. Ciudad P, Maruccia M, Socas J, Lee MH, Chung KP, Constantinescu T, Kiranantawat K, Nicoli F, Sapountzis S, Yeo MS, Chen HC. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: technique and outcomes. Microsurgery 2015; doi: 10.1002/micr.22450. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10. Sapountzis S, Ciudad P, Lim SY, Chilgar RM, Kiranantawat K, Nicoli F, Constantinides J, Wei MY, Sönmez TT, Singhal D, Chen HC. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery 2014;34:439–47. [DOI] [PubMed] [Google Scholar]
- 11. Cram AE, Domayer MA. Short‐term preservation of human autografts. J Trauma 1983;23:872–3. [DOI] [PubMed] [Google Scholar]
- 12. DeBono R, Rao GS, Berry RB. The survival of human skin stored by refrigeration at 4°C in MCC medium: does oxygenation of the medium improve storage time. Plast Reconstr Surg 1998;102:78–83. [DOI] [PubMed] [Google Scholar]
- 13. Sheridan R, Mahe J, Walters P. Autologous skin banking. Burns 1998;24:46–8. [DOI] [PubMed] [Google Scholar]
- 14. Sterne GD, Titley OG, Christie JL. A qualitative histological assessment of various storage conditions on short term preservation of human split skin grafts. Br J Plast Surg 2000;53:331–6. [DOI] [PubMed] [Google Scholar]
- 15. Titley OG, Cooper M, Thomas A, et al. Stored skin—stored trouble? Br J Plast Surg 1994;47:24–9. [DOI] [PubMed] [Google Scholar]
- 16. Shepard GH. The storage of split‐skin grafts on their donor sites. Plast Reconstr Surg 1972;49:115–22. [DOI] [PubMed] [Google Scholar]
- 17. Ciudad P, Yeo MS, Sapountzis S, Lim SY, Nicoli F, Maruccia M, Kiranantawat K, Chen HC. Microsurgical debulking procedure after free lymph node flap transfer. Microsurgery 2014;34:670–1. [DOI] [PubMed] [Google Scholar]


