Abstract
Infections in burn patients are still the principal cause of complications in burn injuries. The aim of this study is to assess a new strategy for burn wound management in view of infection prevention and treatment in the experience of the Burn Treatment Center in Siemianowice Śląskie. The applied methodology involved the analysis of patient records describing the hospital's epidemiological situation between 2014 and 2016. The analysis also included the use and cost of antibiotics, silver‐containing dressings, and other antiseptics relative to the number of sepsis cases, including those caused by Pseudomonas aeruginosa, as well as the mortality ratio. The total costs of prevention and treatment of infections were reduced, while the use of silver‐containing dressings and antiseptics increased. The number of patients with sepsis decreased, including cases caused by P. aeruginosa, and the mortality ratio was reduced. Introducing a strategy for burn wound‐oriented infection prevention and treatment in burn patients provides a number of benefits. It is also cost‐effective. Using locally applied active dressings and antiseptics can be a welcome choice for often‐unnecessary antibiotic therapy of a suspected or existing burn wound infection.
Keywords: antibiotics, burns, infections, silver‐containing dressings
1. INTRODUCTION
A burn and its origin determines its treatment pathway. Regardless of the depth and extent of a thermal injury, the principal focus is the prevention of burn wound infection and, potentially, systemic infection as both can significantly complicate the treatment. Despite the fact that great progress has recently been made in the local and systemic treatment of burn patients, infections are still the principal cause of mortality. This is primarily due to numerous risk factors found in burn patients, which include: burn shock, prolonged treatment of shock, immune disorders, the use of vascular catheters, performing mechanical ventilation, parenteral nutrition, prevention of upper gastrointestinal bleeding, presence of necrotic tissue in the wound, and associated comorbidities. In addition to the factors directly associated with the burn injury, an increasing resistance of the microorganisms responsible for infections in burn patients has been reported. In addition, the flora of a burn wound changes and becomes more pathogenic over time, which therefore bolsters the reasons for early intervention. Thus, the prevention of infections in burn patients should be introduced at the beginning of the treatment, even if the burn wound immediately after the burning event is bacteriologically clean.1, 2
1.1. Antibiotic therapy of burn wound infections
In stage I superficial burns, antibacterial therapy should not be introduced. In deep II b‐degree burns and in full‐thickness burns, antibacterial treatment should be considered but only administered when appropriate, depending on the patient's overall clinical condition, in order to prevent infection development. Due to disturbed skin function and poor blood supply, the penetration of antibiotics into the burn wound is impaired. Administration of antibiotics through intravenous infusion protects the patient from potentially developing bacteraemia or helps to treat the condition if it occurs; it does not eradicate colonisation or wound infection.3 In the group of patients with a injury, contamination with multi‐resistant microorganisms is often observed and, with all the associated adverse consequences, significantly increases therapeutic challenges. Therefore, preventative antibiotic therapy should be considered in appropriate burned patients based on both their clinical and microbiological status.4 In this group of patients, therapy should be limited to the cases when complications, for example, sepsis, may occur. Situations justifying the administration of preventative antibiotics include burns of the respiratory tract accompanying skin burns; protection in case of extensive necrectomy, especially in late necrectomy; gastrointestinal haemorrhage; or presence of other comorbidities (diabetes, systemic disease). Preventative antibiotic therapy should be applied only when a severe clinical condition is because of an infection posing a direct risk to the patient's life. Implementation of empirical therapy should always be determined by the patient's clinical condition. The selection of antibiotics for preventative therapy should be based on numerous factors, related to both the patient (general condition, including renal function, albumin levels—carrier for antibiotics) and to the established sensitivity of microorganisms specific for a given unit offering treatment for burn patients. The decision to introduce targeted antibiotic therapy is based on a positive microbiological test result revealing the cause of the infection and its sensitivity to drugs. It should be noted that the microbiological test is only complimentary to the existing clinical symptoms suggesting the development of the presence of an infection. The application of targeted antibiotic therapy should be based on factors highlighted in Table 1.
Table 1.
Selection criteria for targeted antibiotics for the treatment of burn infections
| 1. | The patient's general condition (phase of the burn injury, surgical interventions, comorbidities, and existing infection risk factors) |
| 2. | Symptoms suggesting the presence of infection |
| 3. | Results of biochemical tests and blood counts |
| 4. | Previous microbiological test results (microbiological analysis of all the tests, regardless of the type of specimen) |
| 5. | Pharmacokinetic and pharmacodynamic properties of the antibiotic (burns cause major hemodynamic modifications) |
| 6. | In vitro minimal inhibitory concentration values of antibiotics effective for individual microorganisms should be verified with the EUCAST guidelines, considering bioavailability, pharmacokinetics and pharmacodynamics |
| 7. | Current epidemiological situation in a given center (the most frequently isolated microorganisms, current drug sensitivity profile) |
The more criteria considered while choosing targeted antibiotic therapy, the higher the chances for a successful therapeutic effect. From the clinical and epidemiological points of view, the worst‐case scenario involves the presence of endemic, multidrug‐resistant Pseudomonas aeruginosa or Acinetobacter baumannii strains in the local hospital microflora, resistant to all antibiotics used.5, 6, 7, 8, 9, 10 In such cases, the only active chemotherapeutic agent available is colistin, which is of limited use in the treatment of sepsis in burn patients due to its strongly nephrotoxic effect.11, 12 Another challenge in the treatment of infections caused by non‐fermenting Gram‐negative rods is their ability to create biofilm on the surface of tissue or dead structures.13 Bacteria living in the biofilm demonstrate a high resistance to antimicrobials (after the development of biofilm, the minimal bactericidal concentration, Minimal Bactericidal Concentration (MBC), increases by up to 150‐fold), which impairs penetration of antibiotics and antibodies.14 The biofilm may become fragmented and may be released, together with the microorganisms, into the circulation, causing bacteraemia, septic congestion, or secondary infection foci, and during disintegration of bacterial cells, endotoxins responsible for septic shock may be released.15, 16
1.2. Prevention and treatment of burn wound infections
Infection prevention in burn patients starts immediately after the injury, and it should be multi‐directional, including quick (acute) resection of necrotic tissue, which is a starting point for the development of infection, and covering the deficits with autologous or allogeneic skin grafts or skin substitutes.17 Furthermore in the early strategy of burn treatment the counteracting of immunosuppression and introduction of en'teral nutrition should be considered when deciding between infection prevention options. Parenteral nutrition should always be treated as complimentary to enteral nutrition. Local prevention of burn wound infection, apart from aseptic measures (following the highest standards of cleanliness during performance of all the diagnostic and therapeutic procedures), also involves the use of antiseptics, lavaseptics, and advanced wound dressings. Lavaseptics assist wound cleansing by a physical removal (irrigation) of harmful substances, such as endo‐ and exogenous toxic substances, tissue residues, toxins of various origin, and microorganisms, including mechanical removal of the bacterial biofilm. It is currently believed that the impairment of burn wound healing after the removal of necrotic tissue may be caused by the presence of bacterial biofilm in the wound, which is difficult to remove.18 Direct application of a lavaseptic on the wound using high pressure (using a hydrosurgical device) should suffice to remove the biofilm from the wound. There are several antiseptics available for treating burn and general wounds on the Polish market, with different active substances. When choosing a particular antiseptic in the prevention or treatment of burn wound infections, the following features of the product should be considered: low cytotoxicity, wide spectrum of antimicrobial activity (including antimycotic effect),rapid speed of kill, prevention of biofilm formation, no pain on application and during wear, no impact on the wound healing process, prevents development of resistance, compatibility with the material and substances used in dressings, and lack of neutralisation due to protein burden and wound pH.
Antimicrobial dressings and preparations are most frequently used in the prevention and/or treatment of burn wound infections. Many silver‐containing wound covers are considered advanced active dressings and used in local treatment of wounds of various aetiology, including burn wounds. The high antimicrobial activity of nanocrystalline silver, combined with an advanced delivery system that is in direct contact with the injured surface, makes them the product of choice in our unit, especially for treating complicated and infected wounds. Most silver‐containing dressings combine a carrier dressing with various chemical forms of silver. Individual products differ in structure, but most of them create an environment associated with moist wound healing. This creates a wound microenvironment that promotes autolysis and the healing processes. Adding the antimicrobial component of silver to these active dressings significantly extends their effectiveness in the treatment of wounds with various aetiologies.19, 20 In 2015, none of the isolated microbial strains demonstrated drug sensitivity of over 50% for any antibiotic. In that year, a sudden increase of up to several dozen percentages in resistance to certain antibiotics was observed in microorganisms, which was probably an adverse consequence of the extensive use of antibiotics in 2014, as confirmed by the cost analysis. This alarming situation necessitated implementation of an alternative method for prevention and treatment of infections in burn patients. Therefore, the use of local antimicrobial products for prevention and treatment of infections in the Burn Treatment Centre was increased.
2. METHODS
This study is a retrospective analysis of epidemiological assessment reports of the Burn Treatment Center in Siemianowice Śląskie in 2014 to 2016, containing data about microbiological profiles, types of aetiological factors isolated from all the specimens, and their drug sensitivity profiles. The microbiological data analysis was conducted by a microbiological laboratory with the use of the Kamsoft statistical programme. The analysis also involved a comparison of costs incurred to purchase antibiotics, relative to the costs of antiseptics and active silver‐containing dressings. The demographic data of patients, including the number of burn patients, gender, age, body surface area affected by the burn, comorbidity of respiratory tract burns as well as data on the use of antibiotics, and silver‐containing dressings, were obtained from the Solmed hospital programme (Table 2). The summarised data was analysed relative to the number of sepsis cases caused by P. aeruginosa and the mortality ratio.
Table 2.
Demographic data of burn patients hospitalised between 2014 and 2016
| Data | Overall | Female | Male |
|---|---|---|---|
| Hospitalised patients | 2000 | 605 (30.25%) | 1395 (69.75%) |
| Mean age (years) | 45.7 | 49.7 | 44.04 |
| Mean body surface area affected by the burn | 18.4% | 15.34% | 19.69% |
| Burns of the respiratory tract | 656 | 157 (23.9%) | 499 (76.1%) |
3. RESULTS
The number of burn patients hospitalised in the years analysed is similar, although a slight increasing trend has been observed. The overall number of burn patients treated within the analysed time frame was 2000.
Further analysis consisted of the comparison of costs incurred for antimicrobial prevention and treatment in the years 2014 to 2016. Analysed data were based on actual drug dispensation into hospital departments according to pharmacy department reports. It was assumed that antimicrobial treatment involved antibiotics and antimycotic chemotherapeutics in one group and antiseptics and silver‐containing dressings in the other group.
The analysis of costs incurred due to antimicrobial prevention and treatment revealed that the greatest amount was spent in 2014, whereas the lowest amount was paid in 2016. In 2016, a reduction of just over 20% in the expenses for antibiotics, antimycotics, antiseptics, and silver‐containing dressings was observed, compared with 2014. The authors estimated the percentage share of both groups of agents in the global costs of antimicrobial prevention and treatment (Table 3). The comparison of the costs of antibiotics and antimycotics with those of antiseptics and active silver‐containing dressings revealed that the spend profiles changed. The share of antibiotics and antimycotics in infection prevention and treatment was reduced by nearly 20%. At the same time, the share of local preventative and therapeutic agents (antiseptics + active silver‐containing dressings) increased (Table 4).
Table 3.
Total costs of the antimicrobial prevention and treatment in 2014 to 2016
| Year | Amount in USD |
|---|---|
| 2014 | 308 407 |
| 2015 | 301 088 |
| 2016 | 236 906 |
Conversion according to the exchange rate from April 12, 2017.
Table 4.
Share of antibiotics and antimycotics, as well as antiseptics and silver‐containing dressings in the total costs of infection prevention and treatment
| Year | Total costs | Costs of antibiotics and antimycotics | Costs of antiseptics and Ag‐containing dressings | Share in the total costs of antibiotics and antimycotics (%) | Share in the total costs of antiseptics and Ag‐containing dressings (%) |
|---|---|---|---|---|---|
| 2014 | USD 308 407 | USD 256 357 | USD 52 049 | 83.2 | 16.8 |
| 2015 | USD 301 088 | USD 234 804 | USD 66 284 | 78 | 22 |
| 2016 | USD 236 906 | USD 150 302 | USD 86 603 | 63.4 | 36.6 |
The assessment demonstrated that sepsis was confirmed clinically and microbiologically in 60 patients hospitalised in the Burn Treatment Centre in 2014 (Figure 1). The patients constituted 8.4% of all the patients hospitalised in the analysed year. Considering the fact that P. aeruginosa were the most common isolates from burn wounds, the rate of sepsis cases induced by this microorganism was determined. The evaluation revealed that P. aeruginosa was the causative factor in 20% of sepsis cases in burn patients.
Figure 1.

Comparison of the number of sepsis cases in 2014, including those induced by Pseudomonas aeruginosa, and the mortality rate
In 2015, sepsis was confirmed again in 60 patients, that is, in 8.2% of all the patients hospitalised due to burn injuries (Figure 2). However, the presence of P. aeruginosa decreased radically from 20% in 2014 to 5% in 2015. This is a very positive situation considering that this microorganism is responsible for sepsis and mortalities due to sepsis originating directly from the infected burn wound in patients with burns of 30% TBSA. Interestingly, in 2015, P. aeruginosa resistance to all the recommended antibiotics was the highest in the analysed range. This may suggest that despite limited therapeutic options for this type of infection and implementation of restrictions to the antibiotic therapy policy in wound infections, the mortality due to P. aeruginosa‐induced sepsis did not increase but was significantly reduced. It should be noted that the financial contributions to local antimicrobial treatment were increased in 2015, which helped to control a possible development of systemic infections through burn wound management.
Figure 2.
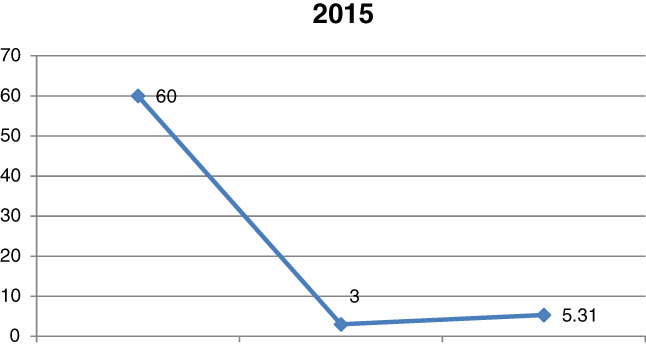
Comparison of the number of sepsis cases in 2015, including those induced by Pseudomonas aeruginosa, and the mortality rate
The analysis of the year 2016 demonstrated a reduction in the total number of sepsis cases from 60 to 46, that is, 6.1% of all the patients hospitalised at that time, as well as a further decrease in the number of P. aeruginosa‐induced sepsis to one case, that is, 2.1% of all the systemic infections (Figure 3). Continuation of the 2015 strategy for infection management from the burn wound level by an increased use of antiseptics and silver‐containing dressings brought visible results in the following year. One of the effects is the reduction in the total number of sepsis cases from 60 to 46, that is, 6.1% of all the patients hospitalised at that time, as well as a further decrease in the number of P. aeruginosa‐induced sepsis to one case, that is, 2.1% of all the systemic infections. The introduced antimicrobial policy also significantly restored drug sensitivity to most antibiotics used in P. aeruginosa infections.
Figure 3.
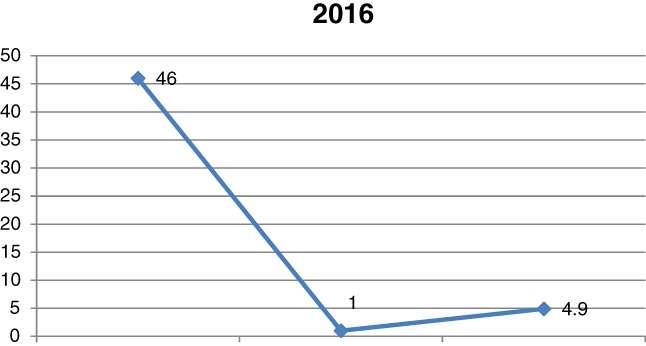
Comparison of the number of sepsis cases in 2016, including those induced by Pseudomonas aeruginosa, and the mortality rate
4. DISCUSSION
Due to an absence of guidelines for microbiological diagnostics and preventative treatment of burn wound infections in Poland and other countries, a change in the strategy regarding infections in burn injuries is necessary. The results of these retrospective analyses conducted by the authors of this study demonstrate that introducing restrictions in antibiotic therapy use will not contribute to higher rates of sepsis originating directly in the burn wound and related mortalities assuming early intervention with additional preventative strategies. It is acknowledged that the preliminary data summarised were obtained from our microbiological laboratory, finance, and pharmacy departments and should be seen as a pilot exercise. However, the trends that have been observed are encouraging prospect for further studies that will provide statistically compelling results. An increasing lack of therapeutic options using antibiotics had to be compensated for with other approaches, that is, nanocrystalline silver dressings and antiseptic wound cleansers. In numerous studies, other researchers have demonstrated that an effective antimicrobial local agent significantly reduces the quantity of microorganisms on the wound surface and decreases the risk of generalised infection.21, 22 The selection of the local antimicrobial therapy should be based on the product's ability to rapidly inhibit the microorganisms and also on its low toxicity for the newly forming tissue.23
Recently, dressings that release silver in a controlled manner to provide an environment that promotes wound healing by reducing the bioburden on the wound have been increasingly popular.24, 25, 26 Randomised studies using nanocrystalline silver (Acticoat) and silver nitrate solution demonstrated that the frequency of dressing changes and number of septic complications in the group receiving Acticoat were lower.27 The use of nanocrystalline silver dressings in the Burn Treatment Center in Siemianowice Śląskie is multi‐directional. It means that the dressings are used from the beginning of the treatment, immediately after the injury, both for prevention and treatment of infections. The dressings are applied first after deep excision of necrotic tissue to prevent penetration of the microorganisms colonising the wound into the deeper tissue layers. The next stage is wound cleansing, where antiseptics and hydrosurgery, or nanocrystalline silver dressings combined with Negative Pressure Wound Therapy (NPWT) are used to improve the antimicrobial outcomes. In the case of confirmed colonisation with microorganisms, using antiseptics and nanocrystalline silver dressings is invaluable. Preliminary reports regarding the use of nanocrystalline silver‐containing dressings in the prevention of local burn wound infection in a group of 45 patients, published by the authors in 2007, confirmed the product's exceptional effectiveness in the eradication of the Gram‐negative flora colonising the wounds.28 In 2004, Dunn presented reports from the 2003 European Burn Association meeting regarding benefits of nanocrystalline silver dressings in burn patients observed by a few doctors from various European countries. The dressings were used in children with partial‐ to full‐thickness burns. Apart from their antimicrobial efficacy, the condition of wounds following the use of nanocrystalline silver was generally improved, and natural or surgically supported healing was observed. Reduction of pain symptoms, frequency of dressing changes, the amount of exudation from the wound, and the number of surgical procedures were reported.29 The study conducted in 2005 by Tonkin and Wood clearly confirms the observations made by the authors of this study during data analysis. The study examined the use of nanocrystalline silver in reducing the need for antibiotic therapy in the treatment of burn wounds, and it involved 72 burn patients treated in an Australian burn treatment centre. The control group comprised patients who received silver sulfadiazine. There was a statistically significant decrease of the need for antibiotic usage with Acticoat dressings. This dressing also considerably reduced the mean hospitalisation time and the number of burn wound infections. Another important issue is mentioned in the publication, namely, wound swabs prior to antibiotic therapy as a standard practice in the treatment of burn wounds. The authors confirm that the basis for introduction of both preventive and targeted antibiotic therapy should include not only a positive microbiological test result but also other symptoms suggestive of symptomatic infection of the burn wound.30 The analysis of the costs of prevention and treatment in burn wound infections conducted by the authors of this study demonstrated a 20% reduction in the total costs incurred; the expenditure on dressings and antiseptics increased, whereas costs associated with antibiotics were reduced. Similar observations were made by the authors of a review of studies on nanocrystalline dressings in wound treatment. Authors who firmly state the financial benefits of using nanocrystalline silver dressings are cited.31
5. CONCLUSIONS
Antimicrobial stewardship has been elevated to the highest priority by many institutions worldwide to combat the ever‐present challenges associated with the emergence of antibiotic resistance. Much of the focus is on ensuring appropriate antibiotic use at appropriate concentrations for appropriate time periods. These programmes are overseen by trained professionals in this discipline, including medical microbiologists, pharmacists, and intensivists, and the implementation of global surveillance programmes has helped to evaluate success or failure. Within the field of wound care, topical antiseptics should also be included with other antimicrobial agents under the banner of stewardship, and additional choices can be made available to be considered for appropriate wounds. Introducing a strategy for burn wound‐oriented infection prevention and treatment in burn patients provides various benefits, not only for patients but also for the local and global epidemic situation. It is also cost‐effective. Using effective local active dressings containing nanocrystalline silver and antiseptics can be an alternative for an often unnecessary antibiotic therapy of a suspected or existing burn wound infection. This study highlights if such an approach can create new opportunities for the optimisation of the treatment of infections; decrease the use of antibiotics, thus reducing the development of drug resistance; and, primarily, if it can improve treatment outcomes patients’ safety.
Conflict of interest
No conflict of interest to declare.
Glik J, Łabuś W, Kitala D, et al. A 2000 patient retrospective assessment of a new strategy for burn wound management in view of infection prevention and treatment. Int Wound J. 2018;15:344–349. 10.1111/iwj.12871
REFERENCES
- 1. Schwacha MG, Holland LT, Chaudry IH, Messina JL. Genetic variability in the immune‐inflammatory response after major burn injury. Shock. 2005;23(2):123‐128. [DOI] [PubMed] [Google Scholar]
- 2. Lawrence JC, Soothill J. Burn wound infection. Burns. 1989;15(5):342. [DOI] [PubMed] [Google Scholar]
- 3. Riaz I, Babar AH. Burn wound infections and antibiotic susceptibility patterns at Pakistan Institute of Medical Sciences, Islamabad, Pakistan. World J Plast Surg. 2015;4:9‐15. [PMC free article] [PubMed] [Google Scholar]
- 4. Bagdonas B, Tamelis A, Rimdeika R, et al. Analysis of burn patients and the isolated pathogens. Lithuania Surg. 2004;2:190‐193. [Google Scholar]
- 5. Bahmani N, Ramazanzadeh R. Detection of SHV type extended‐spectrum B‐lactamase and risk factors in Pseudomonas aeruginosa clinical isolates. Pak J Med Sci. 2013;29:788‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayan M, Durmaz R, Aktas E, Durmaz B. Bacteriological, clinical and epidemiological characteristics of hospital‐acquired Acinetobacter baumannii infection in a teaching hospital. J Hosp Infect. 2003;54:39‐45. [DOI] [PubMed] [Google Scholar]
- 7. Livermore DM. The impact of carbapenemases on antimicrobial development and therapy. Curr Opin Invest Drugs. 2002;3(2):218‐224. [PubMed] [Google Scholar]
- 8. Heggers JP, Hawkins H, Edgar P, et al. Treatment of infections in burns. In: Herndon DN, ed. Total Burn Care. London, England: WB Saunders; 2002:120‐169. [Google Scholar]
- 9. Elliott TS, Lambert PA. Antibacterial resistance in the intensive care unit: mechanisms and management. Br Med Bull. 1999;55:259‐276. [DOI] [PubMed] [Google Scholar]
- 10. Khadijah Y. Microbial profile of burn wound infections in burn patients, Taif, Saudi Arabia. Arch Clin Microbiol. 2016;7(2):1‐9. [Google Scholar]
- 11. Glik J, Kawecki M, Gażdzik T, et al. The impact of types of microorganisms isolated from blond and wounds on the results of treatment in burn patients with sepsis. Pol J Surg. 2012;1:10‐27. [DOI] [PubMed] [Google Scholar]
- 12. Levin AS. Treatment of Acinetobacter spp infections. Expert Opin Pharmacother. 2003;4(8):1289‐1129. [DOI] [PubMed] [Google Scholar]
- 13. Percival SL, McCarty SM, Lipsky B. Biofilms in wounds. An overview of the evidence. Adv Wound Care. 2015;4(7):375‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reid AS. Biofilm in infections disease and on medical devices. Int J Antimicrob Agents. 2003;22:223‐226. [DOI] [PubMed] [Google Scholar]
- 15. Erol SU, Altoparlak U, Akcay MN, Celebi F, Parlak M. Changes of microbial flora and wound colonization in burned patients. Burns. 2004;30:357‐361. [DOI] [PubMed] [Google Scholar]
- 16. deMacedo JL, Santos JB. Bacterial and fungal colonization of burn wounds. Mem Inst Oswaldo Cruz. 2005;100:535‐539. [DOI] [PubMed] [Google Scholar]
- 17. Atiyeh BS, Gunn SW, Hayek SN. State of the art in burn treatment. World J Surg. 2005;29:131‐148. [DOI] [PubMed] [Google Scholar]
- 18. Roberts CD, Leaper DJ, Assadian O. The role of topical antiseptic agents within antimicrobial stewardship strategies for prevention and treatment of surgical site and chronic open wound infection. Adv Wound Care. 2017;6(2):63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demling RH, De Santi L. Effect of silver on wound management. Wounds. 2001;13:4‐9. [Google Scholar]
- 20. Thoma S, McCubbin P. A comparison of the antimicrobial effects of four silver‐containing dressings on three organisms. J Wound Care. 2003;3(2):101‐107. [DOI] [PubMed] [Google Scholar]
- 21. Namias N, Samiian L, Detal N. Incidence and susceptibility of pathogenic bacteria vary between intensive care units within a single hospital: implications for empiric antibiotic strategies. J Trauma. 2000;49:638‐645. [DOI] [PubMed] [Google Scholar]
- 22. Japoni A, Farshad S, Alborzi A. Pseudomonas aeruginosa: burn infection, treatment and antibacterial resistance. Iran Red Crescent Med J. 2009;11(3):244‐253. [Google Scholar]
- 23. Templeton S. Management of chronic wounds: the role of silver‐containing dressings. Prim Inten. 2005;13:170‐179. [Google Scholar]
- 24. White R, Cooper R, Kingsley A. A topical issue: the use of antibacterials in wound pathogen control. In: White R, ed. Trends in Wound Care. London UK: Bath Press; 2002:6‐17. [Google Scholar]
- 25. Wright B, Lam K, Buret A, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141‐151. [DOI] [PubMed] [Google Scholar]
- 26. Wright B, Lam K, Olsen M, et al. Is antimicrobial efficacy sufficient? A question concerning the benefits of new dressings. Wounds. 2003;15:133‐114. [Google Scholar]
- 27. Tredget E, Shankovsky R, Groenveld A, Burrell R. A matched‐pair, randomized study evaluating the efficacy and safety of Acticoat silver‐coated dressing for the treatment of burn wounds. J Burn Care Rehabil. 1998;19:531‐537. [DOI] [PubMed] [Google Scholar]
- 28. Glik J, Kawecki M, Nowak M. Ocena zastosowania opatrunków z nanokrystalicznym srebrem w zapobieganiu miejscowej infekcji rany oparzeniowej. Zakażenia. 2007;7(3):97‐103. [Google Scholar]
- 29. Dunn K, Edwards‐Jones V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns. 2004;30(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 30. Tonkin C, Wood F. Nanocrystalline silver reduces the need for antibiotic therapy in burn wounds. Prim Inten. 2005;13(4):163‐168. [Google Scholar]
- 31. Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine. 2006;1(4):441‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]


