Abstract
Foot infections in diabetic patients are a common, complex and costly problem. They are potentially adverse with progression to deeper spaces and tissues and are associated with severe complications. The management of diabetic foot infection (DFI) requires a prompt and systematic approach to achieve more successful outcomes and to ultimately avoid amputations. This study reviews a multi‐step treatment for DFIs. Between September 2010 and September 2012, a total of about 37 patients were consulted for DFI. The treatment algorithm included four steps, that is, several types of debridement according to the type of wound, the application of negative pressure therapy (NPT), other advanced dressings, a targeted antibiotic therapy local or systemic as the case may, and, if necessary, reconstructive surgery. This treatment protocol showed excellent outcomes, allowing us to avoid amputation in most difficult cases. Only about 8% of patients require amputation. This treatment protocol and a multidisciplinary approach with a specialised team produced excellent results in the treatment of DFI and in the management of diabetic foot in general, allowing us to improve the quality of life of diabetic patients and also to ensure cost savings.
Keywords: DFI, DFUs, Diabetic foot infection, Diabetic foot treatment protocol, Diabetic foot ulcers
Introduction
The World Health Organization predicts that the prevalence of diabetes is expected to increase dramatically over the coming decades; about 24·5 million (8·9%) of the US population will have diabetes by the year 2025 1. The estimated number of diabetic people will increase to at least 228 million only in the developing countries and about 300 million in all nations by 2025 2. Foot ulcers in people with diabetes are an increasingly common problem and are associated with potentially serious complications. The cumulative incidence of developing a foot ulcer in patients with diabetes was 5·8% over 3 years and 15% of patients with diabetes will develop a foot ulcer during their lifetime 3, 4. Healing of neuropathic diabetic foot ulcers (DFUs) is a challenge for surgeons 5. In control groups of randomised control trials in which standard‐of‐care treatment alone is performed, healing rates remain low (approximately 30% at 20 weeks of care) 6. They are particularly prone to infection, with potentially adverse progression to deeper spaces and tissues, often resulting in limb amputation. The management of diabetic foot infection (DFI) requires a systematic approach to achieve more successful outcomes and ultimately avoid amputations. If not treated promptly and appropriately, DFI can become incurable or even lead to septic gangrene 7. At least 60% of non‐traumatic lower limb amputations occur among people with diabetes 8.
The base of DFU treatment includes restitution of skin perfusion if peripheral arterial disease is coexistent, offloading of the affected foot, infection control and good wound care. Good wound care includes disinfection, debridement, use of appropriate dressings and topical and/or systemic antimicrobials when needed 5.
This article reviews treatment and care for DFIs and offers a stepwise approach to an effective management.
Methods
Every year, more than 800 patients are treated at our facility of ulcers and chronic wounds. Skin wounds are especially of vascular, diabetic, traumatic and pressure ulcer origin.
A retrospective study was performed on about 37 patients with DFI, who received medical dressings for chronic wounds, from September 2010 to September 2012. Data were collected on gender, age, time of infection's onset, type of treatment and variations of wounds' aspect over the months.
The assessments were based on wound's aspect, microbiological buffers and photographs and comparative photography.
All patients followed a common algorithm of treatment (Figure 1). The inclusion criteria were as follows: infected wound, > 10 cm2 area, not responding for > 6 months to the advanced dressing treatment.
Figure 1.
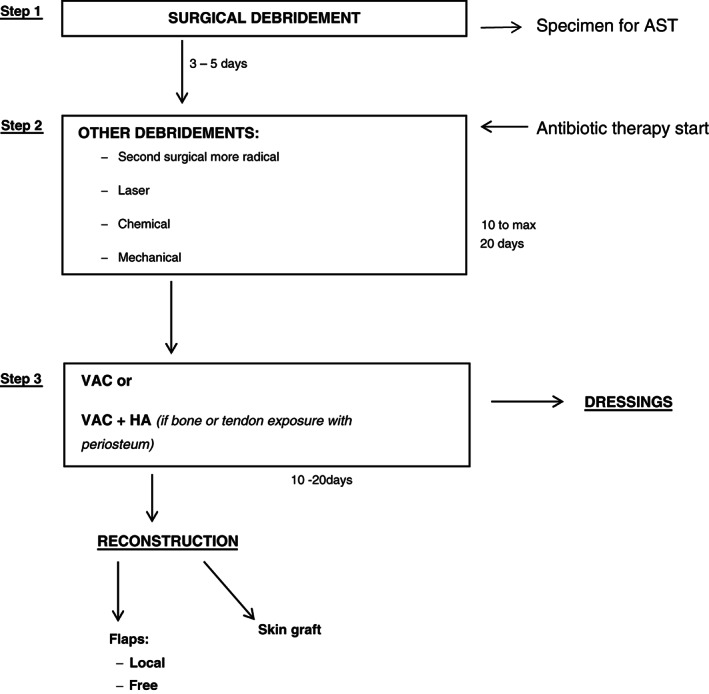
Therapeutic algorithm.
The possibility of infection occurring in any foot wound in a patient with diabetes has always to be considered. To detect infection in multiple specimens during wound debridement, to avoid contamination and to optimise identification of the right pathogens, the samples are sent for culture and antibiotic susceptibility testing (AST).
The therapeutic options are as follows:
mechanical, chemical, with erbium‐YAG‐laser or surgical debridement, depending on the wound response to the first debridement for a period of 2 weeks;
application of negative pressure therapy (NPT);
reconstructive surgery (with flaps or grafts).
For clinically infected wounds, antibiotic therapy is virtually always needed. This should be directed at the most commonly identified pathogens and should be started promptly 9. The regimen has to be typically targeted on culture and sensitivity results obtained from specimens' wound debridement. For severe infection, parenteral broad‐spectrum antibiotics are recommended.
Regarding patients with DFI, clinicians should attempt to provide a well‐coordinated treatment by a multidisciplinary team. Generally, antibiotic therapy was administered by an infectious disease specialist. Patients should be followed up by an endocrinologist, by monitoring the glucose and the glycated haemoglobin values.
In addition, the patient should request for the opinion of an orthopaedic specialist, who can advise them regarding a baropodometric examination in order to develop custom‐made insole and shoes. Lower limb unloading is a must in these patients.
Finally, the possibility of osteomyelitis onset should be considered, which is a potential complication of any infected, deep or large foot ulcer, especially the one that is chronic or overlies a bony prominence. It occurs in many diabetic patients with a foot wound and can be difficult to diagnose and treat. It should optimally be diagnosed by bone culture and histology, but in the clinical practice, we generally performed radiography and labelled leukocyte scintigraphy. The therapy was often surgical debridement and resection and/or prolonged systemic antibiotic therapy.
Results
A total of 37 patients, with a mean age of 69 ± 2·5 years (mean ± 2SD) (range: 50–90) at diagnosis and a male: female ratio of 1·2:1, with DFI being seen during the past 2 years.
At stage 2, seven patients, with cleaner wound bed after the first surgical debridement, without bone or tendon exposure, received only debridement with a chemical collagenase and healed only with advanced dressings (Figure 2A–C). Fifteen patients received skin grafts, of which three patients received the graft before a second surgical debridement, seven patients received a chemical debridement and six patients underwent laser therapy; in nine of these patients, it was appropriate to apply the vacuum‐assisted closure (VAC) therapy, while in six patients VAC therapy with hyaluronic acid (HA). Limb amputation was performed in 12 limbs, of which 4 were free limbs and 8 local limbs. Among these patients, after the first surgical debridement, four received a second surgical debridement, six patients a laser therapy and two patients a chemical debridement; nine of these patients underwent VAC therapy and three patients VAC therapy with HA (Figure 3A–C). Finally, only three patients underwent amputation (Figure 4A–C). These patients had not had good outcomes after the first surgical debridement, with vascular disease and/or advanced infection and necrosis of deep tissues, often with one or more foot fingers mummified or necrotic. These patients received the application of the VAC therapy after the first surgical debridement but the wound severity, deep and infected, however, required amputation.
Figure 2.
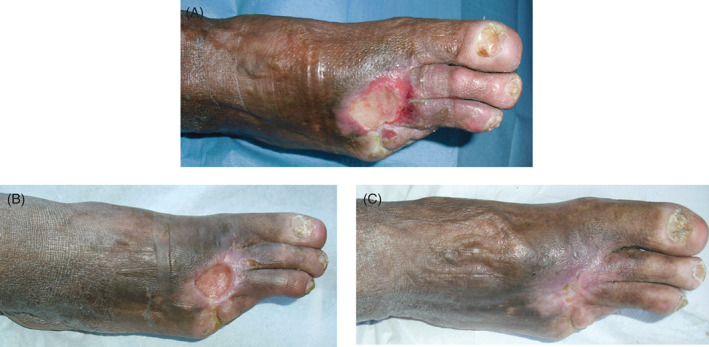
Chronic wound, in 75‐year‐old diabetic patient on right foot, treated by debridement and advanced dressings. (A) Pre, (B) intra, (C) after 2 months.
Figure 3.
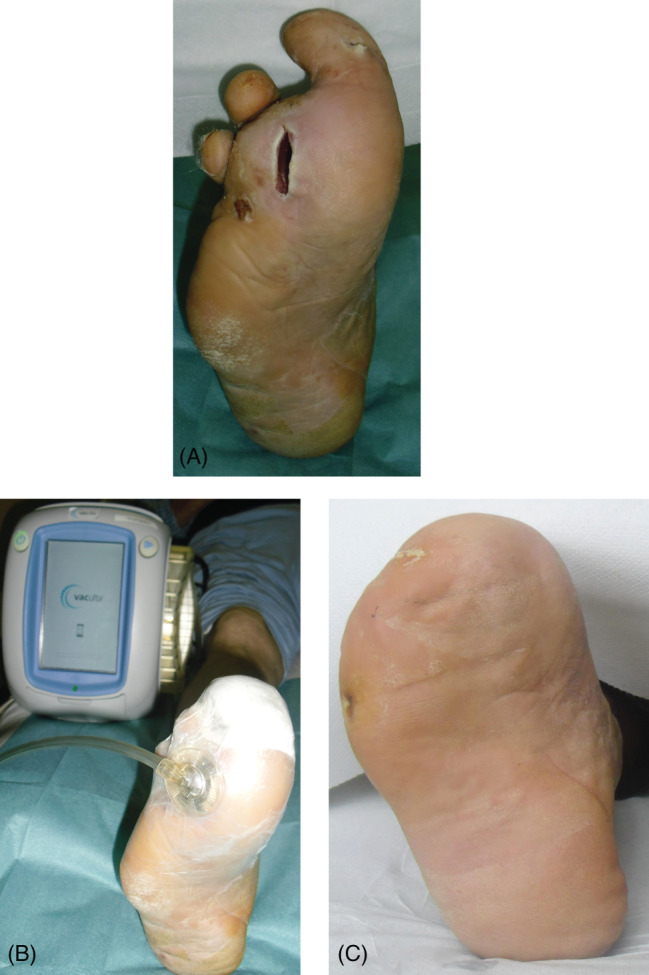
Chronic wound, in X‐year‐old diabetic patient on X foot, treated by debridement and limb X. Figure (A) Pre, (B) intra, (C) – after X month.
Figure 4.
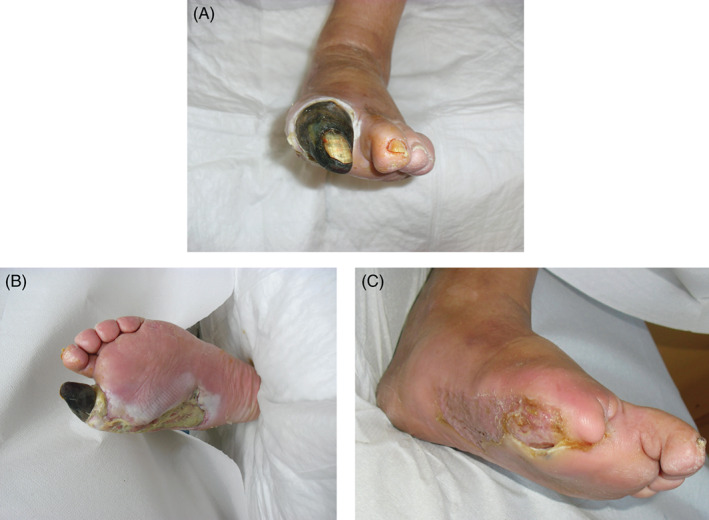
Chronic wound, in 69‐year‐old diabetic patient on right foot, treated by debridement and application of negative pressure therapy. (A), Pre, (B) intra, (C) after 1 month and half.
Discussion
Foot infections in diabetic patients are a common, complex and costly problem 3, 10. A DFI is defined as any inframalleolar infection in a person with diabetes mellitus 9. These include paronychia, cellulitis, myositis, abscesses, necrotizing fasciitis, septic arthritis, tendonitis and osteomyelitis 9.
Clinicians should consider the possibility of infection occurring in any foot wound in a patient with diabetes. Evidence of infection generally includes classic signs of inflammation or purulent secretions but may also include additional or secondary signs (e.g. non‐purulent secretions, friable or discoloured granulation tissue, undermining of wound edges and foul odour) 11. However, some infected patients may not manifest these findings, especially those who have peripheral neuropathy or limb ischaemia 11, 12, 13.
The goal is to determine the extent of infection, its microbial aetiology, the pathogenesis of the wound and the presence of any contributing biomechanical, vascular or neurological abnormalities 14, 15, 16. Our treatment protocol was applied on the infected diabetic wounds. The therapeutic protocol included a surgical debridement (step1), with the collection of specimens for AST. After the first debridement, the antibiotic therapy was started based on the AST result and further debridement should be considered depending on the result of the first treatment, including second radical surgery (in more serious cases), erbium‐YAG‐laser, chemical (with collagenase) and mechanical debridement. (This step lasts from 10 to 20 days maximum.)
For wounds yet infected, secreting and with the fibrin on the wound bed, after the first debridement, a second surgical debridement has been considered.
In patients with a reduction of the infection after the first surgical debridement of at least 1/2, laser therapy was considered.
In patients with a complete reduction of the infection, after the first surgical debridement, with only some infected areas, a chemical debridement, with collagenase containing vibrio alginolyticus and HA, was considered.
Step 3 includes the application of NPT alone, with VAC on clean and granulating wounds or VAC with HA (if bone or tendon exposure with periosteum).
Finally, to provide substance loss coverage, in smaller size wounds, dressings were continued until the complete healing, while, for great wounds, a reconstruction with local/free flaps or skin grafts was considered.
In patients, who had not had good outcomes after the first surgical debridement and the application of the VAC therapy, with vascular disease and/or advanced infection and necrosis of deep tissues, often with one or more foot fingers mummified or necrotic, the amputation was the only solution.
Our treatment algorithm applied to diabetic patients with foot ulcers showed good performances in the years. Careful observation of the patient's response to therapy is essential and should be performed daily.
Our algorithm was based on the assumption that any chronic wound has a subclinical or clinical infection. The first step allows to reduce the soft tissue infection through debridement immediately followed by antibiotic therapy. This combined approach allows the tissue to restart the healing process inhibited by the local infection. The second step of continuous debridements is useful because soft tissue infection is normally deeper than expected. The use of NPT (step 3) allows a booster of granulation tissue, a reduction of the oedema and of the inflammatory mediators 17. The use of NPT with a dermal matrix of an ester derivative of hyaluronic acid is indicated for cases of bone or tendon exposure in order to allow better granulation tissue before skin graft coverage at step 4 of treatment. Use of VAC therapy in large wounds obviated the need for a daily change of dressing, hence removing the trouble of a daily change of dressing, which was painful, difficult to perform and could lead to increased fluid loss 17, 18. An additional benefit observed was the ability of VAC therapy to alleviate bacterial infection in a wound.
The use of NPT is indicated for wound bed regeneration even in cases that will be reconstructed with local or free flaps. VAC therapy also provides a sterile, more controlled resting environment to large, exudating wound surfaces. Large DFUs were thus made more manageable 17, 18, 19. The flaps, particularly free flaps, usually bring new vascularisation to the recipient area allowing for an improved vascularisation of the limb.
In conclusion, the goals of treating a DFI are eradication of clinical evidence of infection and avoidance of soft tissue loss and amputations. Several studies have shown that high bacterial loads, ranging from 105 to 106 CFU/ml of wound fluid, are predictive of poor healing 20, 21, 22, 23.
In diabetic patients, high blood glucose and glycated haemoglobin levels delay the healing process of wounds. Therefore, it is important to include the achievement of an optimal glycemic control in the management of these patients. Properly identifying and counselling persons at risk of ulceration or infection can prevent the dire consequences of DFUs, such as lower extremity amputation. Cost savings may ensue, although this may be offset by an increased demand for preventive foot care, diagnostic testing and vascular interventions 24.
This treatment protocol and a multidisciplinary approach with a specialised team produced excellent results in the treatment of this disease, allowing us to improve the quality of life of diabetic patients and also to ensure cost savings.
Acknowledgement
We would like to thank Dr Franco Bartolomei for his help in preparing this manuscript.
References
- 1. King H, Aubert RD, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diabetes Care 1998;21:1414–31. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global burden of diabetes: WHO projects a 170% growth in the number of people with diabetes in developing countries by 2025. World Health Organization 1998. URL www.who.int/inf‐pr‐1998/en/pr98‐63.html [accessed on 26 January 2004]
- 3. Ramsey SD, Newton K, Blough D, McCollough DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 4. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetics. In: Diabetes in America, 2nd edn. Rockville: National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health, 1995:409–28. [Google Scholar]
- 5. Richmond NA, Vivas AC, Kirsner RS. Topical and biologic therapies for diabetic foot ulcers. Med Clin North Am 2013;97:883–98. [DOI] [PubMed] [Google Scholar]
- 6. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong DG, Lipsky BA. Diabetic foot infections: stepwise medical and surgical management. Int Wound J 2004;1:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention, US Department of Health and Human Services . National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2003. Atlanta: US Centers for Disease Control and Epidemiology, 2003. [Google Scholar]
- 9. El‐Tahawy AT. Bacteriology of diabetic foot infections. Saudi Med J 2000;21:344–7. [PubMed] [Google Scholar]
- 10. Tennvall GR, Apelqvist J, Eneroth M. Costs of deep foot infections in patients with diabetes mellitus. Pharmacoeconomics 2000;18:225–38. [DOI] [PubMed] [Google Scholar]
- 11. Armstrong DG, Lavery LA, Harkless LB, Van Houtum WH. Amputation and reamputation of the diabetic foot. J Am Podiatr Med Assoc 1997;87:255–9. [DOI] [PubMed] [Google Scholar]
- 12. McIntyre KE. Control of infections in the diabetic foot. the role of microbiology, immunopathy, antibiotics and guillotine amputation. J Vasc Surg 1987;5:787–90. [DOI] [PubMed] [Google Scholar]
- 13. Tan JS, Friedman NM, Hazelton‐Miller C, Flanagan JP, File TM. Can aggressive treatment of diabetic foot infections reduce the need for above‐ankle amputations? Clin Infect Dis 1996;23:286–91. [DOI] [PubMed] [Google Scholar]
- 14. Schaper NC, Apelqvist J, Bakker K. The international consensus and practical guidelines on the management and prevention of the diabetic foot. Curr Diab Rep 2003;3:475–9. [DOI] [PubMed] [Google Scholar]
- 15. Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, Tan JS, Infectious Diseases Society of America. Tan diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg 2004;117(7S). [DOI] [PubMed] [Google Scholar]
- 16. Tan T, Shaw EJ, Siddiqui F, Kandaswamy P, Barry PW, Baker M. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ 2011;342:d1280. [DOI] [PubMed] [Google Scholar]
- 17. Webb LX. New techniques in wound management: vacuum‐assisted wound closure. J Am Acad Orthop Surg 2002;10:303–11. [DOI] [PubMed] [Google Scholar]
- 18. Nather A, Chionh SB, Han AY, Chan PP, Nambiar A. Effectiveness of vacuum‐assisted closure (VAC) therapy in the healing of chronic diabetic foot ulcers. Ann Acad Med Singapore 2010;39:353–8. [PubMed] [Google Scholar]
- 19. Cigna E, Tarallo M, Bistoni G, Anniboletti T, Trignano E, Tortorelli G, Scuderi N. Evaluation of polyurethane dressing with ibuprofen in the management of split‐thickness skin graft donor sites. In Vivo 2009;23:983–6. [PubMed] [Google Scholar]
- 20. Elek SD. Experimental staphylococcal infections in the skin of man. Ann N Y Acad Sci 1956;65:85–90. [DOI] [PubMed] [Google Scholar]
- 21. Krizek TJ, Pobson MD, Kho E. Bacterial growth and skin graft survival. Surg Forum 1967;18:518. [Google Scholar]
- 22. Lookingbill DP, Miller SM, Knowles RC. Bacteriology of chronic leg ulcers. Arch Dermatol 1978;114:1765–8. [PubMed] [Google Scholar]
- 23. Bendy RH, Nuccio PA, Wolfe E, Collins B, Tamburro C, Glass W, Martin CM. Relationship of quantitative wound bacterial counts to healing of decubiti: effect of topical gentamicin. Antimicrob Agents Chemother 1964;10:147–55. [PubMed] [Google Scholar]
- 24. Ragnarson Tennvall G, Apelqvist J. Prevention of diabetes‐related foot ulcers and amputations: a cost‐utility analysis based on Markov model simulations. Diabetologia 2001;44:2077–87. [DOI] [PubMed] [Google Scholar]


