Abstract
Various types of wound dressings have been designed for different purposes and functions. Controlling bacterial burden in a wound during the early phase is important for successful wound repair. Once bacterial burden is under control, the active promotion of wound healing is another important factor for efficient wound healing. This study investigated the potential of three silver‐containing dressings, namely KoCarbonAg®, Aquacel® Ag and Acticoat 7, in reducing bacterial survival and promoting wound healing. The ability of these dressings to block the entry of bacteria from external environment and retain intrinsic bacteria was studied in vitro. In addition, the study used a rat model to compare the healing efficiencies of the three dressings and investigate the quantity of collagen synthesis in vivo. In vitro results indicated that the silver‐containing dressings prevented bacterial growth in wounds by blocking the entry of external bacteria and by retaining the bacteria in the dressing. In vivo study indicated that reduction in bacterial burden accelerated wound healing. Wounds treated by the silver‐containing dressings showed better healing than those treated with gauze. Moreover, KoCarbonAg® further accelerated wound healing by promoting collagen synthesis and arrangement.
Keywords: Bacterial burden, Collagen synthesis, KoCarbonAg®, Silver‐containing dressing
Introduction
The skin is the biggest organ in the body that performs protective, sensory and thermoregulatory functions. Wound healing involves three phases: inflammation, proliferation and remodelling 1. The inflammatory phase begins immediately after a skin injury. It is characterised by the release of platelet‐derived growth factor by the platelets in the thrombus that attracts neutrophils and macrophages towards the wound bed to remove necrotic tissue, debris and bacteria 2, 3. Macrophages then become the dominant cells in the inflammatory phase and further release various growth factors such as fibroblast growth factor and transforming growth factor beta (TGF‐β) to induce fibroblast proliferation and collagen synthesis, respectively, thus, entering into the proliferation phase 2, 4. Fibroblast proliferation and collagen synthesis are the two prominent events in the proliferation phase. Meanwhile, this phase also involves other events, such as angiogenesis and epithelialisation, which promote the repair of the wound cavity and the formation of reepithelialised skin. In the remodelling phase, the irregularly arranged collagen would be degraded and replaced by regularly aligned collagen fibres to strengthen the newly formed skin 2, 5.
Open wounds contain a certain degree of bacteria on their surface. States of contamination, colonisation, critical colonisation and invasive infection can be categorised depending on the amount and effects of bacteria in the wound 6, 7. Contamination is defined as the presence of non‐replicating bacteria in the wound. Colonisation is defined as the presence of replicating bacteria in the wound without any tissue damage. These two states do not delay the healing processes. However, critical colonisation, in which bacteria do not invade the skin tissue, would delay wound healing. Invasive infection is characterised by bacterial density of 105 microorganisms per gram of the skin tissue 7, 8, 9. Delayed wound healing occurs because of the increased levels of endotoxins produced by bacteria 7. Endotoxins are known to elevate the levels of proinflammatory cytokines, which in turn decrease the production of growth factors and the deposition of collagen in wounds, leading to critical colonisation and invasive infection 7, 10, 11. Therefore, it is important to keep the bacterial burden in the wound bed below the level of critical colonisation.
All the currently available wound dressings promote wound healing and prevent further harm to the wounded region. An ideal wound dressing maintains moisture in the wound, removes excess exudates, is non‐adherent, prevents the entry of microorganisms, controls infection, allows gaseous exchange and leaves no foreign particles in the wound 12, 13. Although different dressings have different properties, all the dressings function as a barrier (at various degrees) to prevent the entry of external microorganisms. A recent study by Fujiwara et al. put forth a new concept of bacterial retention in a wound dressing. They proposed that the ability of a dressing to retain bacteria could be important for reducing bacterial burden in the wound bed. In addition to controlling bacterial burden, a wound dressing actively promotes wound healing, thus accelerating the healing processes 14. In this study, we examined three silver‐containing dressings made of different raw materials and compared their efficiency with that of gauze (control). These three dressings are as follows: (i) KoCarbonAg® (Biomedical Carbon Technology Co., Ltd., Taichung, Taiwan) containing activated carbon fibre (ACF) supported by silver particles, (ii) Acticoat 7 (Smith & Nephew, London, UK) containing alternate layers of rayon and silver‐coated polyethylene film and (iii) Aquacel® Ag (ConvaTec Inc., Skillman, NJ) containing hydrofibres and ionic silver. We investigated the ability of these three dressings to block and retain bacteria in vitro and their wound healing efficacy in vivo in a rat model.
Methods
Bacteria preparation
The bacteria Pseudomonas aeruginosa (ATCC 27853) were grown on tryptic soy broth (TSB) agar plates under microaerophilic conditions for 24 hours at 37°C. A single colony was selected and cultured in TSB medium to an optical density of 1·0 at 590 nm (OD590) measured by a visible spectrophotometer (Biochrom, England, UK). The suspension was diluted in TSB medium to achieve a final concentration of 1 × 105 colony‐forming unit/ml (CFU/ml) for further use.
Analysis of bacterial blocking activity
For analysing the bacterial blocking activity, 6 × 6 cm2 pieces of the dressings were placed on agar plates with their skin‐contacting surface facing downwards. Then, 3 ml suspension of P. aeruginosa (1 × 105 CFU/ml) was inoculated in the centre of the dressing on the exposed upper surface. The plates were incubated at 37°C for 24 hours. The test pieces of the dressings were then removed aseptically, and the plates were further incubated for 24 hours at 37°C. The area of bacterial growth in each plate was measured using Image Tool 3.0 (UTHSCSA, San Antonio, TX).
Analysis of bacterial retention activity
In a laminar flow cabinet, 3 ml suspension of P. aeruginosa (1 × 105 CFU/ml) was placed in the centre of the 6 × 6 cm2 piece of each test dressing on the skin‐contacting surface. The pieces were incubated for 5 minutes to allow the complete absorption of the bacterial suspension. The pieces were then placed on plates, with the skin‐contacting surface facing downward, and the plates were incubated at 37°C for 24 hours. The dressing pieces were then removed aseptically, and the agar plates were further incubated for another 24 hours at 37°C. The area of bacterial growth in each plate was measured using Image Tool 3.0.
Quantification of bacterial quantity
The areas on the agar plates covered by the dressing pieces were cut and transferred to a new culture plate. Then, 10 ml of TSB was added, and the plates were incubated at 37°C. Next, 100 μL cultures incubated for 1 hour and 6 hours were used for water‐soluble tetrazolium salt (WST‐1) assay (Roche Diagnostics, Mannheim, Germany).
Wound healing and histologic analysis of wound tissue
Animal care and use complied with the 1996 revision of the ‘guide for the care and use of laboratory animals’ prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press. A total of 20 Sprague–Dawley (SD) rats weighing 250–300 g were individually housed in polycarbonate cages maintained at constant temperature (22 ± 2°C) and humidity (55%). The rats were subjected to a 12:12 hour light–dark cycle and were provided access to food and water. Their average weight and behaviour did not change significantly during the experiment. Rats were anaesthetised with an intraperitoneal injection of ketamine (90 mg/kg) with xylazine (10 mg/kg) 15. The dorsal skin was shaved, application fields were outlined with a marker pen before skin excision and the surgical area was disinfected with 70% ethanol. On the back of each rat, a full‐thickness wound with dimensions of 1 × 1 cm2 was created on each side of the spine by dermo‐epidermic excision. Each of the wounded rats received 100 µl of P. aeruginosa (1·5 OD590) on the wound surface. Wound dressings with dimensions of 2 × 2 cm2 were applied onto the wounded rats after 24‐ hour infection. Each dressing was covered with a sterile compress secured with a hypoallergenic elastic adhesive bandage. Animals were caged individually following identification. The dressings were changed every 3 days; the wounds were photographed and examined to determine wound size reduction. Wound size measurements taken during surgery and at biopsy were used to calculate percentage size reductions of wounds 16.
where A 0 and A t denote initial wound area and wound area after time interval ‘t’, respectively. Wound area was measured from photographs using the Image‐Pro Plus (Media Cybernetics, Silver Spring, MD) following calibration.
Histologic analysis on day 3 was carried out using light microscopy. Briefly, biopsies were fixed in buffered paraformaldehyde and embedded in paraffin wax 17, 18. Sections of 5 µm were stained with Masson's trichrome. The stained sections of each test sample were then examined under a light microscope for the analysis of collagen synthesis.
Western blotting analysis of wound tissue
Wound tissue extracts were fractionated by performing sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE). The separated proteins were transferred onto a polyvinylidene difluoride membrane by using a transfer apparatus (Bio‐Rad Laboratories Inc., Hercules, CA), according to the manufacturer's protocols. After incubation with 5% non‐fat milk in TBST (10 mM Tris pH 8·0, 150 mM NaCl, and 0·05% Tween‐20) for 60 minutes, the membrane was washed once with TBST and was incubated with primary antibodies against rabbit anti‐collagen‐1 antibody (1:500 dilution) or mouse anti‐glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; 1:5000 dilution) at 4°C for 16 hours. The membrane was then washed three times and was incubated with horseradish peroxidase–conjugated anti‐rabbit or anti‐mouse antibodies (1:5000 dilution) for 1 hour. The blots were washed three times with TBST and the proteins were visualised by enhanced chemiluminescence western blotting detection reagents (GE Healthcare, Little Chalfont, UK) and detected by X‐ray films (Kodak, New York, NY). Concentration of collagen‐1 was quantified using ImageJ software (National Institute of Health, Bethesda, MD) after normalisation with GAPDH protein.
Results
Ability of the dressings to block the entry of bacteria from external environment
An important function of a wound dressing is to protect the wound area from any contamination from the external environment. In this study, the ability of the three dressings to block the entry of bacteria from the external environment was tested. Percentage bacterial growth on the agar plates after inoculating P. aeruginosa on the exposed surface of the dressings was investigated. Data obtained from image analysis showed that growth area of P. aeruginosa on plates covered by gauze was 80·3% ± 4·9% of the covered area, indicating that gauze barely protected the wound area from the external environment (Figure 1A and B). The gauze blocked only 20% of the inoculated bacteria, and the remaining bacteria penetrated through the gauze and colonised the wound area. The growth area of P. aeruginosa on plates covered by KoCarbonAg® was 0·41% ± 0·3% of the covered area, indicating that KoCarbonAg® blocked the entry of >99% bacteria and prevented them from colonising on the wound area. The growth areas of P. aeruginosa on plates covered by Acticoat 7 and Aquacel® Ag were 18·6% ± 9·6% and 30·5% ± 2·4%, respectively, of the covered areas. However, this could be because of the interference by brown colour developed on the plate covered by Acticoat 7 or by fibres left on the plate covered by Aquacel® Ag (Figure 1A).
Figure 1.
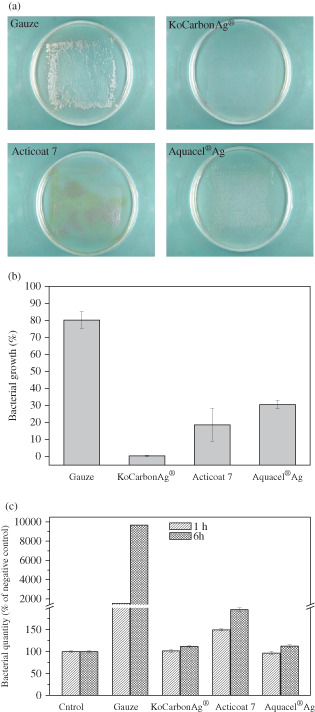
Blockage assay. (A) Representative photographs of growth of Pseudomonas aeruginosa beneath indicated dressings. (B) Percentage area of P. aeruginosa growth beneath indicated dressings. (C) The quantity of P. aeruginosa growth beneath indicated dressings by WST‐1 assay.
The amount of bacteria on each plate was quantified by performing the WST‐1 assay. Bacteria were harvested from the plates and were cultured in TBS for up to 6 hours. Relative bacterial counts at 1 hour and 6 hours are represented in Figure 1C. The plate covered with gauze had large amount of bacteria (15‐ and 96‐fold higher than that in the control plate at 1 and 6 hours, respectively), which was consistent with the results of image analysis. Plates covered by KoCarbonAg® and Aquacel® Ag had similar amounts of bacteria at both 1 and 6 hours, whereas the plate covered with Acticoat 7 had slightly increased amount of bacteria (1·5‐ and 2‐fold higher amount of bacteria than that in the control plate at 1 and 6 hours, respectively). Results of the WST‐1 assay indicated that both Aquacel® Ag and KoCarbonAg® had a similar capacity to protect wounds from environmental contamination, indicating that Aquacel® Ag could also block 99% of external bacteria (Figure 1C). The bacterial amount obtained from the plate covered with Acticoat 7 at 1 hour was approximately 1/10th of that obtained from the plate covered by gauze. Bacterial growth area in the plate covered by gauze was 80·3%, while it was estimated to be 8% in the plate covered by Acticoat 7. This indicated that Acticoat 7 blocked approximately 92% of external bacteria.
Ability for retaining bacteria in the dressing
Wound dressing is usually applied to the wounded area from a couple of hours to a few days. Besides the surrounding environment, the dressing itself is another source of bacterial contamination. If the dressing cannot retain the absorbed bacteria, then the bacteria would leak into the wound area to induce inflammation or to colonise, thus delaying wound healing. To examine the ability of the wound dressings to retain the absorbed bacteria, the percentage growth of P. aeruginosa inoculated on the skin‐contacting surface of the dressings was investigated. The image analysis data showed that the growth area of P. aeruginosa on the plate covered by gauze was 93·2% ± 9·6% of the covered area, indicating that the gauze could not retain the absorbed bacteria (Figure 2A and B). Only approximately 7% of bacteria were retained in the gauze, and the remaining leaked out and colonised the wound area. The growth area of P. aeruginosa on the plate covered by KoCarbonAg® was only 1·1% ± 0·3% of the covered area, whereas those on the plates covered by Acticoat 7 and Aquacel® Ag were 50·8% ± 4·9% and 32·4% ± 2·4%, respectively, of the covered areas. We also observed brown colour on Acticoat 7‐covered plate and fibres on Aquacel® Ag‐covered plate (Figure 2A). Bacterial quantity was analysed by performing the WST‐1 assay to confirm the amount of bacteria in each plate (Figure 2C). The results indicated that large amount of bacteria grew on the plate covered with gauze (14‐ and 101‐fold higher than that on the control plate at 1 and 6 hours, respectively). The plate covered by KoCarbonAg® and Aquacel® Ag had similar amounts of bacteria compared with that in the control plate at both 1 and 6 hours. However, the plate covered with Acticoat 7 showed slightly increased amount of bacteria compared with that in the control plate (2·2‐fold higher than that in the control plate at both 1 and 6 hours). The results of the WST‐1 assay indicated that Aquacel® Ag and KoCarbonAg® had a similar capacity to retain bacteria, indicating that Aquacel® Ag could also prevent 99% of bacteria from leaking out. The bacterial amount in the Acticoat 7‐covered plate at 1 hour was approximately 2·2/14 of that in the gauze‐covered plate (Figure 2C). Bacterial growth area in the gauze‐covered plate was 93·2% of the covered area, while that in the Acticoat 7‐covered plate was estimated to be 15%, which indicated that Acticoat 7 prevented approximately 85% bacteria from leaking out.
Figure 2.
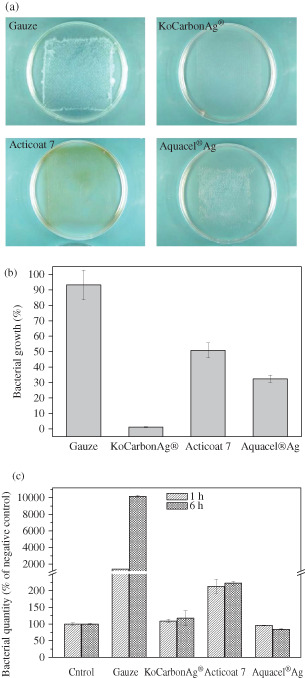
Retaining assay. (A) Representative photographs of growth of Pseudomonas aeruginosa beneath indicated dressings. (B) Percentage area of P. aeruginosa growth beneath indicated dressings. (C) The quantity of P. aeruginosa growth beneath indicated dressings by WST‐1 assay.
The results of blockage and retention tests also represent the antimicrobial activity of these silver‐coated dressings. The results of the WST‐1 Assay indicated that KoCarbonAg® and Aquacel® Ag eliminated almost all the bacteria that escaped from the dressing. However, Acticoat 7, which contains the highest concentration of silver (1·7 mg/cm2) among these three dressings, could not eliminate all the bacteria that escaped the dressing.
Wound healing of silver‐containing dressing
Thus, the above data indicate that KoCarbonAg® and Aquacel® Ag have better capacity to block, retain and kill microbes than Acticoat 7 and gauze. To understand the effect of these factors on the outcome of wound healing, we analysed the closure areas of infected wounds in a rat model. The results indicated that the factors mentioned above played important roles in wound healing. Throughout the experimental period, all the silver‐containing dressings resulted in a greater reduction in the wound area than gauze (Figures 3 and 4), which barely prevented bacterial growth/contamination in the wound area. However, these three factors only partly affect wound healing. Regardless, KoCarbonAg® and Aquacel® Ag showed similar capacities in preventing bacterial growth/contamination in the wound area, with KoCarbonAg® exhibiting a greater reduction of the wound area than Aquacel® Ag. In addition, although Aquacel® Ag showed better bacterial inhibition than Acticoat 7, these two dressings showed a similar reduction in the wound area throughout the experimental period (Figures 3 and 4). This result suggested that once 85% of the bacterial growth was inhibited (minimal percentage of inhibition among these three silver‐containing dressings), the remaining small amount of replicating bacteria in the wound area did not reach the level of critical colonisation, and thus did not interfere with wound healing. Besides reducing bacterial burden, KoCarbonAg® actively promoted wound healing to accelerate the healing process.
Figure 3.
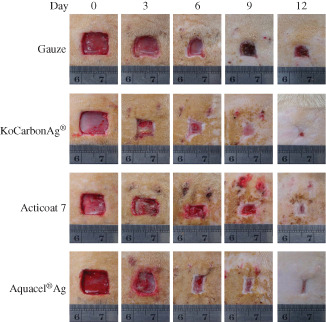
Representative photographs of wound on postoperative days 3, 6, 9 and 12 with the treatment of indicated silver‐containing dressings.
Figure 4.
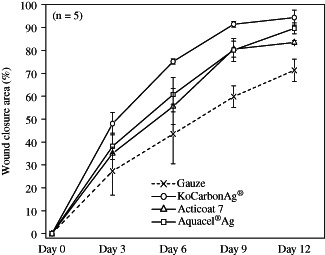
The percentage of infected wound closure treated with silver‐containing dressings (n = 5).
Histologic analysis and western blotting analysis of wound tissue
To determine whether KoCarbonAg® promoted collagen synthesis during wound healing, tissue samples from wounds treated with gauze, KoCarbonAg®, Aquacel® Ag and Acticoat 7 were investigated using Masson's trichrome staining after 3 days (Figure 5). Results of Masson's trichrome staining showed that KoCarbonAg®‐ and Aquacel® Ag‐treated wounds showed higher collagen synthesis (red arrows). Moreover, collagen fibres were aligned more tightly in the wound treated with KoCarbonAg®. Acticoat 7‐treated wounds showed less and loose collagen fibres than KoCarbonAg®‐ and Aquacel® Ag‐treated wounds. In contrast, gauze‐treated wounds showed loose and sporadic collagen fibres. To quantify the amount of collagen produced, western blotting was performed using anti‐collagen‐1 antibody (Figure 6A). On day 2, collagen‐1 fibres in the wound area treated with KoCarbonAg® showed 2·11‐fold increase, whereas those in the wounds area treated with Aquacel® Ag, Acticoat 7 and gauze showed 1·81‐, 1·03‐, and 1·36‐fold increase, respectively (Figure 6B). These results indicated that KoCarbonAg® promoted wound healing by increasing collagen‐1 synthesis.
Figure 5.
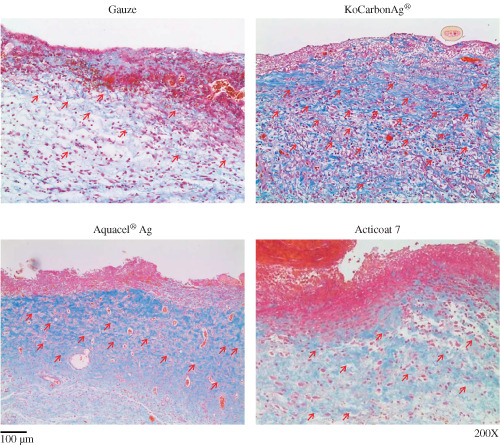
Histologic image of the wound treated with indicated dressing on day 3 by Masson's Trichrome staining (200×).
Figure 6.
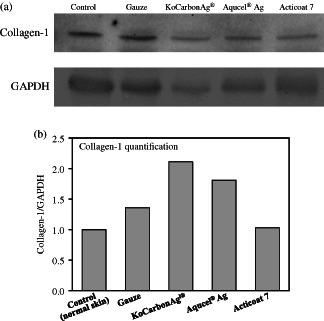
Effect of collagen‐1 protein level on wounds applied by gauze, KoCarbonAg®, Aquacel® Ag and Acticoat 7 at day 2. (A) Autoradiography for western blot analysis using collagen‐1 and GAPDH antiboby. (B) Quantitative data of collagen‐1 protein expression after correcting for GAPDH protein.
Discussion
Most chronic wounds are characterised by a prolonged inflammatory phase. Proinflammatory cytokines induced by bacteria decrease the level of growth factors and subsequently delay the entry of the wound into the proliferation phase. In vitro data indicate that silver‐containing dressings block bacterial entry from the external environment and retain bacteria in the dressing to inhibit bacterial growth on the wounds. Silver may play a role in these two events but is not a decisive factor because dressings with low silver concentration, such as KoCarbonAg® and Aquacel® Ag, have better inhibition capacities. In the animal model, silver dressings promoted wound healing by blocking external bacteria, retaining bacteria in the dressing and exerting antimicrobial activity. Reduction in bacterial burden prevents the accumulation of proinflammatory cytokines and endotoxins, thus promoting the production of growth factors such as TGF‐β, which is important for the entry into the proliferation phase. Of the three dressings, KoCarbonAg® exhibited the greatest reduction in healing time. This may be partly because of the characteristic of its constituent material, that is, ACF. ACF has higher microporosity and substantial internal surface area, indicating that it has excellent capacity to absorb gases and chemicals, including endotoxins 19, 20, 21, 22, 23. Therefore, besides inhibiting bacterial growth in the wound, KoCarbonAg® may also adsorb deleterious endotoxins from the wound bed to decrease their interference in wound healing.
Furthermore, ACF emits far infrared radiation (FIR) that promotes wound healing by increasing the proliferation of fibroblasts and production of collagen in the wound 24, 25. Moreover, FIR promotes wound healing by increasing skin microcirculation that is believed to be the mechanism of action of FIR therapy for treating different wounds 26, 27. To confirm the FIR‐emitting ability of KoCarbonAg®, emissivity of 2–22 µm at 25°C, according to the JSC‐3 inspection method, was tested by the Tze‐Chiang Foundation of Science & Technology, Taiwan. Their results showed that the FIR emissivity rate of KoCarbonAg® was 90% (data upon request), indicating that KoCarbonAg® emitted FIR. Thus, wound dressings containing ACF, such as KoCarbonAg®, accelerate wound healing and induce the expression of collagen‐1 compared with gauze and other commercial dressings. Thus, the better wound healing associated with KoCarbonAg® might be attributed to FIR emitted by ACF in the dressing.
Conclusion
Wound healing is a complicated process involving many factors that interact reciprocally. Control of bacterial burden is critical in the early phase of wound healing. In the proliferation phase, active promotion of wound healing is a prominent factor. In this study, we showed that the silver‐containing dressing KoCarbonAg® not only reduced bacterial burden in the inflammatory phase but also promoted wound healing in the proliferation phase by promoting the production of collagen‐1. Although an ideal dressing might not exist, choosing a multifunctional dressing might be cost‐effective and helpful to both patients and clinicians.
Acknowledgements
The authors would like to thank Enago (http://www.enago.tw) for the English language review.
References
- 1. Jagetia GC, Rajanikant GK. Acceleration of wound repair by curcumin in the excision wound of mice exposed to different doses of fractionated γ radiation. Int Wound J 2012;9:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Monaco JL, Lawrence WT. Acute wound healing an overview. Clin Plast Surg 2003;30:1–12. [DOI] [PubMed] [Google Scholar]
- 3. Berlanga‐Acosta J. Diabetic lower extremity wounds: the rationale for growth factors‐based infiltration treatment. Int Wound J 2011;8:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1–28. [DOI] [PubMed] [Google Scholar]
- 5. Madden JW, Peacock EE. Studies on the biology of collagen during wound healing. Rate of collagen synthesis and deposition in cutaneous wounds in the rat. Surgery 1968;64:288–94. [PubMed] [Google Scholar]
- 6. Cutting KF, Harding KG. Criteria to identify wound infection. J Wound Care 1994;3:198–201. [DOI] [PubMed] [Google Scholar]
- 7. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–6. [DOI] [PubMed] [Google Scholar]
- 8. Murphy RC, Robson MC, Heggers JP, Kadowaki M. The effect of microbial contamination on musculocutaneous and random flaps. J Surg Res 1986;41:75–80. [DOI] [PubMed] [Google Scholar]
- 9. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77:637–50. [DOI] [PubMed] [Google Scholar]
- 10. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 11. Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg 1990;17:485–92. [PubMed] [Google Scholar]
- 12. Lin YH, Lin JH, Wang SH, Ko TH, Tseng GC. Evaluation of silver‐containing activated carbon fiber for wound healing study: in vitro and in vivo . J Biomed Mater Res B Appl Biomater 2012;100:2288–96. [DOI] [PubMed] [Google Scholar]
- 13. Yoo HJ, Kim HD. Synthesis and properties of waterborne polyurethane hydrogels for wound healing dressings. J Biomed Mater Res B Appl Biomater 2008;85:326–33. [DOI] [PubMed] [Google Scholar]
- 14. Fujiwara T, Hosokawa K, Kubo T. Comparative study of antibacterial effects and bacterial retentivity of wound dressings. Eplasty 2013;13:31–40. [PMC free article] [PubMed] [Google Scholar]
- 15. Lee WR, Park JH, Kim KH, Kim SJ, Park DH, Chae MH, Suh SH, Jeong SW, Park KK. The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair Regen 2009;17:505–10. [DOI] [PubMed] [Google Scholar]
- 16. Balakrishnan B, Mohanty M, Fernandez AC, Mohanan PV, Jayakrishnan A. Evaluation of the effect of incorporation of dibutyryl cyclic adenosine monophosphate in an in situ‐forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2006;27:1355–61. [DOI] [PubMed] [Google Scholar]
- 17. Lin YH, Feng CL, Lai CH, Lin JH, Chen HY. Preparation of epigallocatechin gallate‐loaded nanoparticles and characterization of their inhibitory effects on Helicobacter pylori growth in vitro and in vivo . Sci Technol Adv Mater 2014;15:045006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin YH, Lin JH, Chou SC, Chang SJ, Chung CC, Chen YS, Chang CH. Berberine‐loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: in vitro and in vivo study . Nanomedicine (Lond) 2015;10:57–71. [DOI] [PubMed] [Google Scholar]
- 19. Lee J, Kim J, Hyeon T. Recent progress in the synthesis of porous carbon caterials. Adv Mater 2006;18:2073–94. [Google Scholar]
- 20. Lin JH, Ko TH, Lin YH, Pan CK. Various treated conditions to prepare porous activated carbon fiber for application in supercapacitor electrodes. Energy Fuels 2009;23:4668–77. [Google Scholar]
- 21. Pegues AS, Sofer SS, McCallum RE, Hinshaw LB. The removal of 14C labeled endotoxin by activated charcoal. Int J Artif Organs 1979;2:153–8. [PubMed] [Google Scholar]
- 22. Du XN, Niu Z, Zhou GZ, Li ZM. Effect of activated charcoal on endotoxin adsorption. Part I: an in vitro study. Biomater Artif Cells Artif Organs 1987;15:229–35. [PubMed] [Google Scholar]
- 23. Lin YH, Hsu WS, Chung WY, Ko TH, Lin JH. Evaluation of various silver‐containing dressing on infected excision wound healing study. J Mater Sci Mater Med 2014;25:1375–86. [DOI] [PubMed] [Google Scholar]
- 24. Frost RL, Cash GA, Kloprogge T. ‘Rocky Mountain leather’, sepiolite and attapulgite‐an infrared emission spectroscopic study. Vib Spectrosc 1998;16:173–84. [Google Scholar]
- 25. Toyokawa H, Matsui Y, Uhara J, Tsuchiya H, Teshima S, Nakanishi H, Kwon AH, Azuma Y, Nagaoka T, Ogawa T, Kamiyama Y. Promotive effects of far‐infrared ray on full‐thickness skin wound healing in rats. Exp Biol Med (Maywood) 2003;228:724–9. [DOI] [PubMed] [Google Scholar]
- 26. Yu SY, Chiu JH, Yang SD, Hsu YC, Lui WY, Wu CW. Biological effect of far‐infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed 2006;22:78–86. [DOI] [PubMed] [Google Scholar]
- 27. Inoué S, Kabaya M. Biological activities caused by far‐infrared radiation. Int J Biometeorol 1989;33:145–50. [DOI] [PubMed] [Google Scholar]


