Abstract
The aim of this study was to evaluate the results of treatment of venous lower limbs ulcers through the topical application of polynucleotides and hyaluronic acid gel (PNHA): Nucliaskin S™ (Mastelli srl, San Remo, Italy). This study was carried out in 39 consecutive patients who were randomly allocated to two groups: group I (20 patients) received treatment with PNHA (topical gel application two times a week, for a total of 6 weeks); group II (19 patients) received only hyaluronic acid (HA) topical application. All patients received a surgical debridement of the ulcerative lesions before topical treatment with PNHA or HA. Pre‐treatment data indicated the area of ulceration. The number of healed ulcers and the variation in area of ulceration were considered as endpoints. The endpoints were observed after 45 days from the beginning of treatment. Complete wound healing occurred in 60% of limbs of group I and in 22% of those of group II patients. The average area reduction was 67% versus 34% in patients of group I and II, respectively. No side effects were recorded in both groups. Our experience shows that PNHA has an elevated trophic effect and speeds the healing rate of venous lower limb ulcers. This treatment may be a valid option in clinical practice.
Keywords: Hyaluronic acid, Lower limbs, PDRN, Polynucleotides, Venous ulcers
Introduction
Chronic venous ulcers (CVUs) of lower limbs represent an important challenge from both a medical and a socioeconomic point of view, with a prevalence of 0·25–1·25% in the general population 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and high costs for the patients in terms of sanitary expenditure, invalidity and absence from work.
Several drugs to promote healing of venous lower limb ulcers 11, 12, 13, 14, 15, compression bandage therapy and topical application of special substances or innovative dressing could be useful.
Actually, topical application of hyaluronic acid (HA) topical on the wound bed represents one of the main diffuse local treatments 16.
HA is an unsulfated, unepimerised linear glycosaminoglycan (GAG) and represents a major constituent of the extracellular matrix (ECM) of the skin, joints, eye and many other tissues and organs. HA has extraordinary hydrophilic, rheological, and signalling properties, and it is dynamically involved in many biological processes, such as embryogenesis, inflammation, tissue turnover and wound healing. HA plays a key role in each phase of wound healing by stimulating cell migration, differentiation and proliferation as well as by regulating ECM organisation and metabolism 17, 18.
More recently, various studies have demonstrated the effects of DNA fractions, such as nucleotides and nucleosides, on wound healing. In particular, polydeoxyribonucleotides (PDRNs) act on adenosine A2A receptors and stimulate endothelial cell proliferation, migration and secretion of Vascular Endothelial Growth Factor (VEGF), reduce inflammation and improve granulation tissue proliferation. PDRNs also act as a stimulant on cell lines such as osteoblasts, fibroblasts, preadipocytes, and collagen 19, 20, 21, 22, 23.
The aim of this study is to evaluate the effects of a new medical device, derived from an original combination of polynucleotides and hyaluronic acid (PNHA) in the form of a gel for topical application, the Nucliaskin S™ (Mastelli srl, San Remo, Italy), on healing of CVUs.
Materials and methods
We performed an open‐label, parallel group study in two clinical centres, between December 2012 and December 2013. This study was approved by the Institutional Review Board – Independent Ethics Committee (IRB‐IEC) before the beginning of the study, all participants were informed about the aim, procedures, risks and benefits of the study and informed consent was obtained from them in writing.
Patients eligible were older than 20 years with a clinical and instrumental diagnosis of venous ulcer (class 6 of CEAP Classification), presence of venous reflux flow, ankle brachial pressure index (ABPI) >0·9, size 2·5–10 cm2 and >50% granulation tissue on the wound bed.
The exclusion criteria included presence of rheumatoid arthritis, active infection, systemic disorders, haematologic diseases, malignancy, necrotic tissue on the wound bed, neuropathy, medial calcinosis, rest pain and arterial disease (ABPI < 0·8). Non‐ compliant patients and patients who showed intolerability to PNHA and HA were not enrolled in this study.
As described previously 24, all patients underwent lower limb ultrasonography to confirm a venous insufficiency, which also determines the cause of the ulcers. Venous district evaluation was performed with the patient in an orthostatic position to detect the presence of obstructive lesions (total or partial) and valvular insufficiency in the femoral, popliteal and leg veins. Ulcers were observed at each visit and the data on each trophic lesion was recorded in a computerised database. The size of the ulcers was calculated from contour sheets at a central location by manual planimetry followed by digital scanning and analysis software 25.
All patients were subjected to the most appropriate treatment (defined as basic treatment) and HA or PNHA application as adjuvant treatment. The PHNA (Nucliaskin S™) was added to a pre‐filled sterile syringe containing 2 ml of an isotonic gel at pH 7·2, consisting of polynucleotides, HA, mannitol, monobasic sodium phosphate dihydrate, dibasic sodium phosphate dihydrate and water for injections.
The type of surgery, when it was accepted, was chosen according to the anatomical level of vein incompetence: superficial venous surgery [Cure Conservatrice et Haemodinamique de l'Insuffisance Veineuse en Ambulatorie (CHIVA) was performed for the correction of superficial venous reflux] and/or subfascial endoscopic perforating surgery (SEPS) after computed haemodynamic mapping. Elastic bandaging was applied at the end of the surgical procedure.
All patients received a surgical debridement of the ulcers to eliminate necrotic and/or infected tissues on the wound bed.
The endpoints of the study were represented by the percentage of complete wound healing after 45 days (6 weeks) of observation and the reduction in the extension of the ulcers from the beginning of the study to the last clinical examination.
Results
A total of 39 patients (27 females and 12 males, aged between 47 and 83 years) with similar comorbidities, risk factors, demographic characteristics and venous disease were enrolled in this study (Table 1).
Table 1.
| Group I (20 patients) | Group II (19 patients) | P value | |
|---|---|---|---|
| Mean age | 58·4 | 56·7 | |
| Sex | |||
| Male, n | 5 | 7 | 0·423 |
| Female, n | 15 | 12 | 0·423 |
| Comorbidities | |||
| Hypertension | 7 | 3 | 0·17 |
| Smoking | 11 | 5 | 0·07 |
| Obesity | 6 | 3 | 0·29 |
| Chronic obstructive disease | 4 | 1 | 0·168 |
| Diabetes mellitus | 3 | 2 | 0·67 |
| Venous insufficiency | |||
| Partial or complete deep thrombosis | 1 | 0 | 0·311 |
| Partial or complete superficial thrombosis | 0 | 0 | – |
| Sapheno‐femoral incompetence | 11 | 7 | 0·255 |
| Sapheno‐popliteal incompetence | 3 | 2 | 0·676 |
| Superficial vein valvular incompetence | 9 | 7 | 0·604 |
| Deep vein valvular incompetence | 8 | 6 | 0·583 |
| Perforating vein valvular incompetence | 5 | 4 | 0·769 |
| Ulcer characteristics | |||
| Average weeks from onset | 7 | 8 | |
| Ulcer area in cm2 | 15·5 | 14·3 | |
PNHA, polynucleotides and hyaluronic acid gel.
Group I: patients treated with PNHA. Group II: patients treated with HA alone.
The threshold of statistical significance was set at P < 0·025.
Patients were randomised into two main groups:
Group I (20 patients) received ulcer cleaning with saline solution and topical application of PNHA as adjuvant treatment, after which the wound was covered by standard sterile dressing; this procedure was performed 2 days a week for six consecutive weeks. Patients of group II (19 patients) received ulcer cleaning with saline solution and topical application of HA as adjuvant treatment daily, with the wound subsequently being covered by sterile dressing.
In all patients, wound area measurements were performed at baseline (T = 0) and after 15 (T = 1), 30 (T = 2) and 45 days (T = 3) of treatment.
We observed a progressive healing and reduction of ulcer size in both groups. In particular, a complete ulcer healing occurred in 60% of limbs of group I and in 22% of limbs of group II patients at 45 days. The average area reduction was 67% versus 34% in patients of group I and II, respectively. In group I, PNHA decreased the inflammation of perilesional areas, increased wound contraction and epithelialisation, decreased scar tissue size, and increased alignment and organisation of the regenerated scar tissue (Figures 1, 2, 3, 4).
Figure 1.
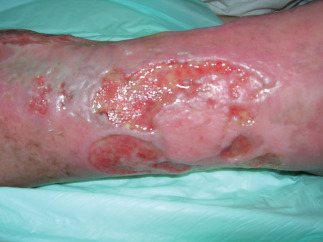
Example of venous ulcer at admission (T = 0).
Figure 2.
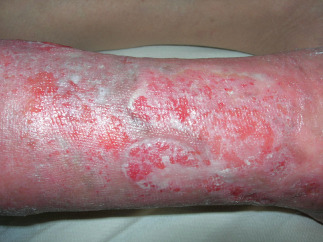
Example of venous ulcer after 15 days of polynucleotides and hyaluronic acid gel (PNHA) application (T = 1).
Figure 3.
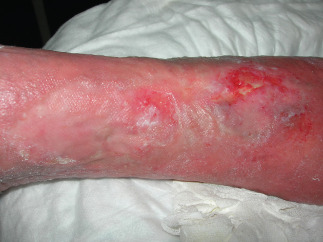
Example of venous ulcer after 30 days of polynucleotides and hyaluronic acid gel (PNHA) application (T = 2).
Figure 4.
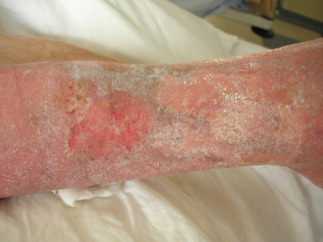
Example of venous ulcer after 45 days of polynucleotides and hyaluronic acid gel (PNHA) application (T = 3).
No side effects were recorded in both groups of patients. The main results are presented in Table 2.
Table 2.
| Group I | Group II | P value | |
|---|---|---|---|
| Complete healing at 45 days (% of patients) | 12 patients (60%) | 4 patients (22%) | 0·135 |
| Average area reduction at 45 days (%) | 67% | 34% | – |
| Adverse events | – | – | – |
PNHA, polynucleotides and hyaluronic acid gel.
Group I: patients treated with PNHA. Group II: patients treated with HA alone.
The threshold of statistical significance was set at P < 0·025.
Discussion
Studies have demonstrated the benefits of DNA fractions in accelerating the wound healing process 26. They stimulate nucleic acid synthesis and bind to purinergic receptors. In particular, PDRN, a DNA active fraction obtained by Mastelli through an original extraction method, acts on adenosine A2A receptors and stimulates endothelial cell proliferation, migration and secretion of VEGF; reduces inflammation; and improves granulation tissue proliferation. It also acts as a stimulant on cell lines such as osteoblasts, fibroblasts, preadipocytes, and collagen 26.
In previous studies, PDRN was used as an angiogenesis stimulator and wound healing agent in diabetic mice 27; in cases of thermal injury 28, it improves blood flow in peripheral artery occlusive disease in rats 29; and in in vitro models, it enhances the growth rate of human fibroblasts and osteoblasts 30. PDRN was also used in plastic and dermatologic surgery, and recently in urology, for its regenerative properties; in ischemic skin flaps for its restorative effects 31, and for its ability to improve intratesticular vascularisation 32. In addition, PDRN has been shown to stimulate corneal epithelium regeneration after photorefractive keratectomy 33 and to accelerate wound healing in graft donor sites 34. PDRN has protective and regenerative effects on UV‐irradiated mouse cell cultures 35 and UV‐irradiated dermal fibroblasts 36. PDRN has shown proliferation effects in human preadipocytes, which represent the richest reservoir of human adult stem cells 37. Recently, the results of a double blind, randomised clinical trial on 216 patients demonstrated the efficacy of PDRN in improving the healing rate of diabetic foot ulcers 38.
We have evaluated the effects of a new bioactive medication, which combines for the first time two of the most effective substances for the regeneration and wound healing process: PNHA. The results of our study during follow‐up highlight the effectiveness of PNHA on venous ulcer healing. In fact, patients of group I underwent a faster reduction in ulcer size and a faster healing of the ulcers: healing occurred in 60% of lower limbs of group I versus 22% of limbs of group II patients. The average reduction in the ulcer area was 67% and 34% in patients of groups I and II, respectively.
The limitation of this study is the lack of clinical observation beyond the period of 45 days (T3), and because of this very short period of follow‐up, we do not have any information on the ulcer recurrence rate after the treatment.
In our experience, PNHA modulated the inflammation, which is the main cause of the physiopathology of CVUs 39, 40, 41, 42, increased wound contraction and epithelialisation, decreased scar tissue size, and increased alignment and organisation of the regenerated scar tissue. There is some evidence to suggest that local application of PNHA is more effective in venous lower limb ulcer healing than isolated HA local application. These findings indicate that the association of polynucleotide (PN) and HA may be key to faster wound repair in patients with CVUs of the lower limbs.
Acknowledgement
The authors declare that they have no competing interests. This work received no funding.
References
- 1. Nelzen O, Bergqvist D, Lindhagen A. The prevalence of chronic lower‐limb ulceration has been under estimated: results of a validate population questionnaire. Br J Surg 1996;83:255–8. [PubMed] [Google Scholar]
- 2. Baker SR, Stacey MC, Jopp‐McKay AG, Hoskin SE. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864–7. [DOI] [PubMed] [Google Scholar]
- 3. de Franciscis S, De Sarro G, Longo P, Buffone G, Molinari V, Stillitano DM, Gallelli L, Serra R. Hyperhomocysteinaemia and chronic venous ulcers. Int Wound J 2013; doi: 10.1111/iwj.12042 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Molinari V, Montemurro R, de Franciscis S. A genetic study of chronic venous insufficiency. Ann Vasc Surg 2012;26:636–42. [DOI] [PubMed] [Google Scholar]
- 5. Serra R, Buffone G, Molinari V, Montemurro R, Perri P, Stillitano DM, Amato B, de Franciscis S. Low molecular weight heparin improves healing of chronic venous ulcers especially in the elderly. Int Wound J 2013; doi: 10.1111/iwj.12071 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serra R, Grande R, Buffone G, Costanzo G, Damiano R, De Franciscis S. Chronic venous disease is more aggressive in patients with varicocele. Acta Phlebol 2013;14:57–60. [Google Scholar]
- 7. Serra R, Buffone G, Costanzo G, Montemurro R, Perri P, Damiano R, de Franciscis S. Varicocele in younger as risk factor for inguinal hernia and for chronic venous disease in older: Preliminary results of a prospective cohort study. Ann Vasc Surg 2013;27:329–31. [DOI] [PubMed] [Google Scholar]
- 8. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Vitagliano T, Greco M, de Franciscis S. Skin grafting followed by low‐molecular‐weight heparin long‐term therapy in chronic venous leg ulcers. Ann Vasc Surg 2012;26:190–7. [DOI] [PubMed] [Google Scholar]
- 9. de Franciscis S, Gasbarro V, Amato B, Buffone G, Grande R, Serra R. Haemodynamic surgery versus conventional surgery in chronic venous disease: a multicenter retrospective study. Acta Phlebol 2013;14:109–14. [Google Scholar]
- 10. de Franciscis S, Grande R, Buffone G, Serra R. Chronic venous ulceration of the lower limbs and thrombosis. Acta Phlebol 2014. In press. [Google Scholar]
- 11. De Caridi G, Massara M, Stilo F, Spinelli F, Grande R, Butrico L, de Franciscis S, Serra R. Effectiveness of prostaglandin E1 in patients with mixed arterial and venous ulcers of lower limbs. Int Wound J 2014; doi: 10.1111/iwj.12334 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Act Phlebol 2013;14:99–107. [Google Scholar]
- 13. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2013; doi: 10.1111/iwj.12077 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serra R, Grande R, Butrico L, Buffone G, Caliò FG, Squillace A, Rizzo BA, Massara M, Spinelli F, Ferrarese AG, De Caridi G, Gallelli L, de Franciscis S. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J 2014; doi: 10.1111/iwj.12240 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serra R, Gallelli L, Conti A, De Caridi G, Massara M, Spinelli F, Buffone G, Caliò FG, Amato B, Ceglia S, Spaziano G, Scaramuzzino L, Ferrarese AG, Grande R, de Franciscis S. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des Devel Ther 2014;8:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Price RD, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing: assessment of clinical evidence. Am J Clin Dermatol 2005;6:393–402. [DOI] [PubMed] [Google Scholar]
- 17. Olczyk P, Komosińska‐Vassev K, Winsz‐Szczotka K, Kuźnik‐Trocha K, Olczyk K. Hyaluronan: structure, metabolism, functions, and role in wound healing. Postepy Hig Med Dosw (Online) 2008;62:651–9. [PubMed] [Google Scholar]
- 18. Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 1999;7:79–89. [DOI] [PubMed] [Google Scholar]
- 19. Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol 2002;160:2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s‐linked) receptors. J Exp Med 1997;186:1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altavilla D, Squadrito F, Polito F, Irrera N, Calò M, Lo Cascio P, Galeano M, La Cava L, Minutoli L, Marini H, Bitto A. Activation of adenosine A2A receptors restores the altered cell‐cycle machinery during impaired wound healing in genetically diabetic mice. Surgery 2011;149:253–61. [DOI] [PubMed] [Google Scholar]
- 22. Bitto A, Oteri G, Pisano M, Polito F, Irrera N, Minutoli L, Squadrito F, Altavilla D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J Clin Periodontol 2013;40:26–32. [DOI] [PubMed] [Google Scholar]
- 23. Chung KI, Kim HK, Kim WS, Bae TH. The effects of polydeoxyribonucleotide on the survival of random pattern skin flaps in rats. Arch Plast Surg 2013;40:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fragomeni G, Merola A, Serra R, de Franciscis S, Amato F. A nonlinear lumped parameters model to analyze the dynamics of venous reflux. Conf Proc IEEE Eng Med Biol Soc 2008;2008:1407–10. [DOI] [PubMed] [Google Scholar]
- 25. de Franciscis S, Caruso MV, de Franciscis A, Perri P, Serra R. A new photographic computerised measurement system for chronic wound assessment. Acta Phlebol 2014;15:13–8. [Google Scholar]
- 26. Squadrito F, Bitto A, Altavilla D, Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino C, Minutoli L, Saitta A, Vaccaro M, Cucinotta D. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial. J Clin Endocrinol Metab 2014;99:746–53. [DOI] [PubMed] [Google Scholar]
- 27. Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calò M, Lo Cascio P, Stagno d'Alcontres F, Squadrito F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen 2008;16:208–17. [DOI] [PubMed] [Google Scholar]
- 28. Bitto A, Galeano M, Squadrito F, Minutoli L, Polito F, Dye JF, Clayton EA, Calò M, Venuti FS, Vaccaro M, Altavilla D. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit Care Med 2008;36:1594–602. [DOI] [PubMed] [Google Scholar]
- 29. Bitto A, Polito F, Altavilla D, Minutoli L, Migliorato A, Squadrito F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J Vasc Surg 2008;48:1292–300. [DOI] [PubMed] [Google Scholar]
- 30. Sini P, Denti A, Cattarini G, Daglio M, Tira ME, Balduini C. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem Funct 1999;17:107–14. [DOI] [PubMed] [Google Scholar]
- 31. Polito F, Bitto A, Galeano M, Irrera N, Marini H, Calò M, Squadrito F, Altavilla D. Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps. J Vasc Surg 2012;55:479–88. [DOI] [PubMed] [Google Scholar]
- 32. Arena S, Minutoli L, Arena F, Nicotina PA, Romeo C, Squadrito F, Altavilla D, Morgia G, Magno C. Polydeoxyribonucleotide administration improves the intra‐testicular vascularization in rat experimental varicocele. Fertil Steril 2012;97:165–8. [DOI] [PubMed] [Google Scholar]
- 33. Lazzarotto M, Tomasello EM, Caporossi A. Clinical evaluation of corneal epithelialization after photorefractive keratectomy in patients treated with polydeoxyribonucleotide (PDRN) eye drops: a randomised, double‐blind, placebo‐controlled trial. Eur J Ophthalmol 2004;14:284–9. [PubMed] [Google Scholar]
- 34. Rubegni P, De Aloe G, Mazzatenta C, Cattarini L, Fimiani M. Clinical evaluation of the trophic effect of polydeoxyribonucleotide (PDRN) in patients undergoing skin explants. A Pilot Study. Curr Med Res Opin 2001;17:128–31. [PubMed] [Google Scholar]
- 35. Guizzardi S, Martini D, Bacchelli B, Valdatta L, Thione A, Scamoni S, Uggeri J, Ruggeri A. Effects of heat deproteinate bone and polynucleotides on bone regeneration: an experimental study on rat. Micron 2007;38:722–8. [DOI] [PubMed] [Google Scholar]
- 36. Belletti S, Uggeri J, Gatti R, Govoni P, Guizzardi S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB‐exposed dermal fibroblasts. Photodermatol Photoimmunol Photomed 2007;23:242–9. [DOI] [PubMed] [Google Scholar]
- 37. Raposio E, Guida C, Coradeghini R, Scanarotti C, Parodi A, Baldelli I, Fiocca R, Santi PL. In vitro polydeoxyribonucleotide effects on human preadipocytes. Cell Prolif 2008;41:739–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Squadrito F, Bitto A, Altavilla D, Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino C, Minutoli L, Saitta A, Vaccaro M, Cucinotta D. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial. J Clin Endocrinol Metab 2014;99:E746–53. [DOI] [PubMed] [Google Scholar]
- 39. Serra R, Buffone G, Costanzo G, Montemurro R, Scarcello E, Stillitano DM, Damiano R, de Franciscis S. Altered metalloproteinase‐9 expression as the least common denominator between varicocele, inguinal hernia and chronic venous disorders. Ann Vasc Surg 2014;28:705–9. [DOI] [PubMed] [Google Scholar]
- 40. Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, Grande R, Serra R, de Franciscis S. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J. 2013; doi: 10.1111/iwj.12181 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen 2013;21:395–401. [DOI] [PubMed] [Google Scholar]
- 42. Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J 2014; doi: 10.1111/iwj.12225 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


