Abstract
Pathophysiological events involved in the onset of chronic venous ulceration (CVU) are inflammation, activation of polymorphonucleates (PMNs) and secretion of proteases such as matrix metalloproteinases (MMPs), which degrade extracellular matrix (ECM) that is a support for vascular and tissutal wall. MMPs, neutrophil gelatinase‐associated lipocalin (NGAL) and inflammatory cytokines are overexpressed in CVUs and they could play a central role in pathophysiological mechanisms of skin lesion and delayed wound healing. Bioflavonoids, such as diosmin and other compounds, appear to have several provessel function activities including anti‐inflammatory, antioxidant and phlebotonic effects and are widely used in the treatment of chronic venous disease (CVD)‐related problems.
In this article, we evaluated the effects of Axaven®, a new nutraceutical on both clinical and molecular parameters in patients with CVUs.
During the study period, 83 patients with CVUs of both sexes were enrolled and divided into two groups: group A (treated group): 25 females and 19 males (median age is 67·7 years) received standard treatment (compression therapy and surgical correction of superficial venous incompetence) + Axaven® once a day for 8 months as adjunctive treatment.
Group B (control group): 24 females and 15 males (median age is 65·2 years) were treated only with basic treatment according to their clinical conditions.
In our study, the administration of Axaven® in patients with CVUs was able to decrease inflammatory cytokines, MMPs and NGAL, inducing an improvement of both symptoms with an increase of the speed of wound healing.
Keywords: Chronic venous ulceration, Diosmin, MMPs, Nutraceutical, Patients
Introduction
Chronic venous ulceration (CVU) is a consequence of the pathophysiologic evolution of chronic venous disease (CVD) 1, 2, 3, 4, 5, 6, 7, 8, 9. Pathophysiological events involved in the onset of CVU are inflammation, activation of polymorphonucleates (PMNs) and secretion of proteases 10. The biological substrate is represented by the extracellular matrix (ECM), a complex network of macromolecules that is a support for vascular and tissutal wall and is dynamically maintained by the action of matrix metalloproteinases (MMPs; which degrade ECM proteins) and their inhibitors [(tissue inhibitors of MMPs and tissue inhibitors of metalloproteinases (TIMPs)]. MMPs are involved in many different vascular 11, 12, 13 and non‐vascular diseases 14, 15, which have chronic inflammation as a common denominator. MMPs are overexpressed in CVUs and they could play a central role in pathophysiological mechanisms of skin lesion and delayed wound healing 16, 17, 18. Moreover, we documented an association between neutrophil gelatinase‐associated lipocalin (NGAL) and MMP‐9 18. Moreover, we have also reported that doxycycline and minocycline are able to improve CVU through the action on MMPs 19, 20, as according to guidelines 21, the standard of care for CVUs is local wound care and the application of compression therapy. Recently, Belczak et al. 22 documented that some nutraceuticals, such as diosmin + hesperidin, aminaphthone and coumarin + troxerutin are able to improve the quality of life in patients with CVD.
Diosmin is a flavonoid (gamma‐benzopyrone) that naturally present in citrus 23 and has vasotonic, antioedematous, lymphotropic, anti‐inflammatory and antioxidant effects 24, 25. Several studies documented the efficacy and the safety of diosmin in the treatment of CVD (i.e. venous disease, chronic venous insufficiency and haemorrhoidal disease) 26, 27, 28, 29, 30. The association of diosmin (450 mg) + hesperidin (50 mg) administered every 12 hours appears to have the greatest clinical benefits in patients with venous disease 31, 32. Recently, several nutraceutical products with diosmin + hesperidin have been commercialised in Italy, and between these, a new nutraceutical is Axaven® an association between: diosmin 1000 mg, hesperidin 100 mg, rutin 300 mg, astaxanthin 5 mg, horse chestnut 50 mg, blueberry 160 mg and althea 100 mg. The effects of each component have been well‐reported in literature; rutin is a flavonoid with effects on capillary permeability and oedema (swelling). It has been reported that oxerutins (derivatives of rutin) are able to improve symptoms and quality of life in patients with CVD 33, 34. Similarly, in 2011 Giacalone et al. documented that blueberry containing polyphenols have antioxidant property 35. Astaxanthin is a potent antioxidant drug with a power that is 550‐fold higher with respect to vitamin E and is able to reduce the effects reactive oxygen activation and of NF‐kB 36. The primary active constituent found in horse chestnut seed extract is aescin, other constituents include bioflavonoids (quercetin and kaempferol), proanthocyanidin A2 (an antioxidant) and the coumarins fraxin and aesculin 37. Aescin from horse chestnut has been shown to have antioedematous, anti‐inflammatory and venotonic properties that may be attributable to decreased vascular permeability 38. The effects of rutin have been reported in in vitro study where Li et al. documented its capacity to act on endothelial nitric oxide synthase. Trehalose is a non‐reducing disaccharide in which two glucose units are linked by an α,α‐1,1‐glycosidic bond. Trehalose has protective action against reactive oxygen species and thanks to its safety, it may be used to treat chronic inflammation 39, 40, 41. Althea is a gastroprotective agent that could be used to reduce the gastrointestinal toxicity induced by the administration of previously described nutraceutics.
Therefore, the aim of this study is to evaluate the effects of Axaven® on both clinical and molecular parameters in patients with CVUs.
Material and methods
We performed an open‐label, parallel group study in four clinical centres, between January 2013 and December 2013. This study was approved by the Institutional Review Board – Independent Ethics Committee (IRB‐IEC) of Interuniversity Center of Phlebolymphology – International Research and Educational Program in Clinical and Experimental Biotechnology – Headquarters at University Magna Graecia of Catanzaro and before the beginning of the study, all participants were informed about the aim, procedures, risks and benefits of the study and they gave an informed consent in writing.
Patients
Patients eligible for this study were of both sexes, older than 20 years with a clinical and instrumental diagnosis of venous ulcer, presence of venous reflux flow, ankle brachial pressure index (ABPI) > 0·5 and <0·8, ulcer duration > 6 weeks and ulcer size 2·5–10 cm2 and >50% granulation tissue on the wound bed.
Patients with diabetes mellitus, rheumatoid arthritis, malignancy, blood disorders, systemic disease, no current episode of ulceration, wound infection, ABPI < 0·5 (patients with severe arterial disease at presentation were considered for arterial imaging with a view to revascularisation) or > 0·8, an ankle pressure < 60 mm Hg, presence of necrotic tissue on the wound bed, use of medications that may impair wound healing, pain at rest, sensory loss (neuropathy), cardiac insufficiency and media calcinosis were excluded from the study.
CVUs are included in clinical–aetiology–anatomy–pathophysiology (CEAP) classification stage C6 42; superficial and deep vein systems, severity of venous reflux were evaluated by duplex ultrasound and computed haemodynamic mapping, as previously described 8, 43, 44.
Experimental protocol
In all patients at the time of admission, the medical history was recorded and clinical examination, laboratory findings and duplex ultrasonography were performed. Blood samples were collected in all enrolled patients at the time of admission (T = 0) and at 1 month (T = 1), 4 months (T = 2) and 8 months (T = 3; end of the study) , in order to evaluate the plasma levels of MMPs and cytokines through enzyme‐linked immunosorbent assay (ELISA) test. During the surgery, biopsies of the ulcers were taken and frozen (−80°C) for western blot evaluation of MMPs expression. Healing was assessed by means of direct ulcer tracing onto clear plastic sheet and subsequent computerised planimetry, as previously described 8, 43, 44. Healing was calculated by subtracting the final ulcer area from the initial area and dividing by the number of weeks that the patient had been observed to obtain the total area healed per week.
ELISA test
In order to evaluate the plasma levels of MMP‐1, MMP‐2, MMP‐8, MMP‐9, NGAL, vascular endothelial growth factor (VEGF), tumour necrosis factor (TNF)‐alpha, interleukin (IL)‐6 and IL‐8, blood samples were collected at different times (see Experimental protocol) in agreement with our previous studies 11, 12, 13, 16, 17, 18, 19, 20.
Western blot evaluation
Wounds were subsequently biopsied under a 1% lidocaine local anaesthesia and with full sterile precautions. The biopsy was made at a point equidistant from the centre and edge of the ulcer. Our experience with biopsies in this patient population indicates that it is well‐tolerated by the subject and does not influence healing outcomes in venous ulcers. Biopsy was immediately placed into a sterile collection container and sent for quantitative (microbiology) culture.
The biopsies obtained at the time of wound bed preparation (T = 3) were lysed for Western blot analysis in 2 ml of tissue protein extraction reagent (25 mM Bicine, 150 mM sodium chloride pH = 7·6; Thermo Scientific; Cambridge, UK). Protein concentrations were determined and lysates were stored at 80°C. For Western blot, protein extracts were separated on a 12·5% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes as previously described. Immunoblotting was performed using anti‐MMP‐1, MMP‐2, MMP‐8 and MMP‐9 monoclonal antibodies as recently described, and results have been expressed as arbitrary units 11, 12, 13, 16, 17, 18, 19, 20. All experiments were performed in triplicate.
Statistical analysis
All data are expressed as mean ± standard error medium (SEM). Student's t‐test was performed in order to analyse the difference between each group with their control. Analysis of variance (ANOVA) was used to evaluate the differences between the groups. Differences identified by ANOVA were pinpointed by unpaired Student's t‐test. The threshold of statistical significance was set at P < 0·05. SPSS (SPSS Inc., Chicago, IL) software were used for the statistical analyses. We defined this study as exploratory, therefore we did not determine a power calculation. In this light, these results could only be labelled as exploratory.
Results
Patients
During the study period, 83 patients with CVUs of both sexes were enrolled and divided into two groups (Table 1):
Table 1.
Characteristics of patients
| Group A | Group B | |
|---|---|---|
| Characteristic | Patients treated with Axaven (%) | Patients without Axaven (%) |
| Age range | 48–82 | 48–87 |
| Median age | 67·7 | 65·2 |
| Sex | ||
| Male | 19 (43·18%) | 15 (38·46%) |
| Female | 25 (56·81%) | 24 (61·54%) |
| Familyhistory for Venous Disease | 27 (61·36%) | 24 (61·54%) |
| Venous insufficiency | ||
| Superficial | 11 (25%) | 13 (33·33%) |
| Superficial and deep | 33 (75%) | 26 (66·67%) |
| Previous stripping or phlebectomy | 18 (40·91%) | 14 (35·90%) |
| Overweight (BMI, 25–29·9 kg/m2) | 28 (63·64%) | 19 (48·72%) |
| Obesity (BMI, ≥30 kg/m2) | 9 (20·45%) | 12 (30·77%) |
| Smoking | 19 (43·18%) | 29 (74·36%) |
| Arterial hypertension | 27 (61·36%) | 21 (53·85%) |
| Dyslipidemia | 31 (70·45%) | 23 (58·97%) |
| Mean length of ulcer (cm) | 5·1 | 6·9 |
| Ulcer area (cm2) | 10·2 | 10·5 |
| Total | 44 (100%) | 39 (100%) |
Group A (treated group): 25 females and 19 males (median age is 67·7 years): standard treatment (compression therapy and surgical correction of superficial venous incompetence) + Axaven® once a day for 8 months (end of study) as adjunctive treatment. [Correction added on 20 March 2015, after first online publication: The number of patients, 36 patients (Group A: 9 females and 7 males; Group B 11 females and 9 males) were wrong and have been changed to 83 patients (Group A: 25 females and 19 males; Group B: 24 females and 15 males) in the results section.]
Group B (control group): 24 females and 15 males (median age is 65·2 years) treated with basic CVUs therapy (compression therapy and surgical correction of superficial venous incompetence 4, 45, 46, 47 according to their clinical conditions.
All patients completed the follow‐up. Compliance with elastic stockings was optimal in both groups and no side effects related to Axaven® treatment appeared in group A.
Wound healing
For wound healing evaluation, we considered high‐healing ulcers as those with a healing speed rate ≥ 1 cm2/week and slow‐healing ulcers as those with healing speed rate < 1 cm2/week (Table 2).
Table 2.
Healing of CVUs
| Group A | Group B | |
|---|---|---|
| Median ulcer area for each time point | 9·08 cm2 (T1); 6·34 cm2 (T2) | 9·92 cm2 (T1); 7·18 cm2 (T2) |
| Mean area healed/week (cm2/week) | 1·20 (T1); 1·4 (T2) | 0·97 (T1); 1·17 (T2) |
Healing evaluation
Healing rates at 12 months (Table 2) was 83·80% for group A and 60·56% for group B. The mean total ulcer area rate of healing for group A was 1·3 cm2/week and 0·87 cm2/week for group B. Moreover, the recurrences of ulcers were significantly higher (P < 0·01) in group B with respect to group A, 59·15% and 26·76%, respectively.
ELISA test
Using ELISA test, we documented significantly lower levels of MMP‐1, MMP‐2, MMP‐9, NGAL (P < 0·01) and MMP‐8 (P < 0·05) in plasma fluid of patients treated with Axaven® (group A) with respect to not treated patients (group B; Figures 1 and 2) in a time‐dependent pattern.
Figure 1.
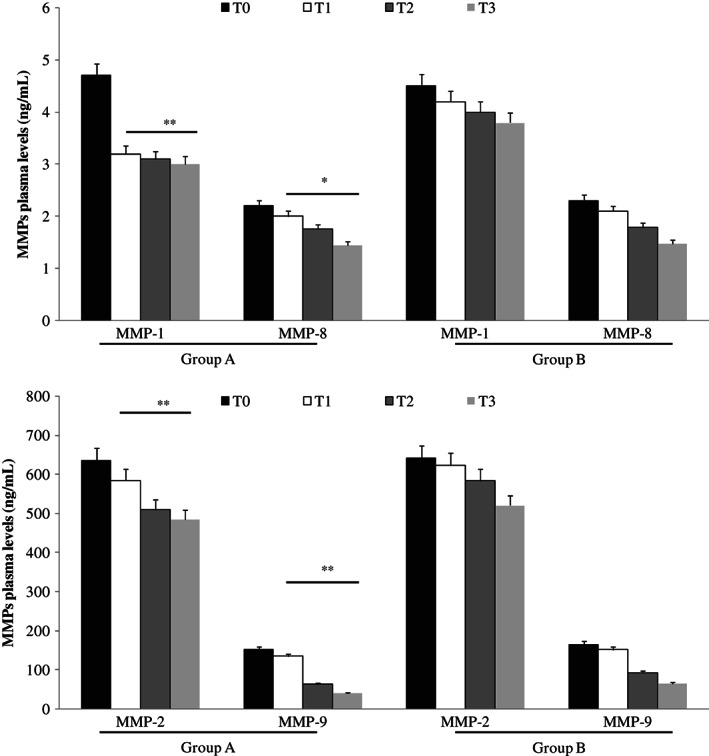
Enzyme‐linked immunosorbent assay (ELISA) test evaluation of matrix metalloproteinase (MMP)‐1, MMP‐8 (up), MMP‐2 and MMP‐9 (down) plasma levels, at different times, in patients with chronic venous ulcerations (CVUs) treated (group A) or not (group B) with Axaven® once daily for 8 months (end of study). Data are expressed as mean ± SEM. *P < 0·05; **P < 0·01. T = 0: admission; T = 1: 1 month; T = 2: 4 months; T = 3: 8 months.
Figure 2.
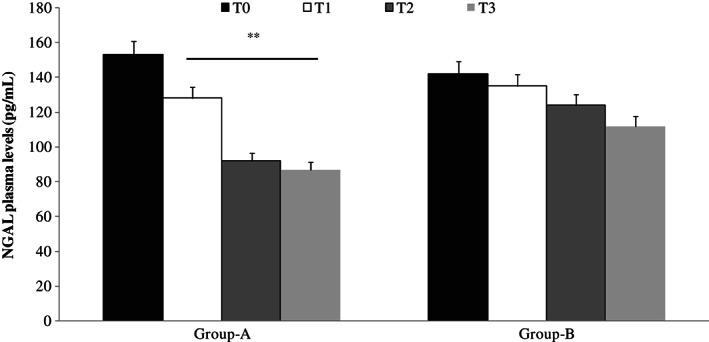
Enzyme‐linked immunosorbent assay (ELISA) test evaluation of neutrophil gelatinase‐associated lipocalin (NGAL) plasma levels, at different times, in patients with chronic venous ulcerations (CVUs) treated (group A) or not (group B) with Axaven® once daily for 8 months (end of study). Data are expressed as mean ± SEM. *P < 0·05; **P < 0·01. T = 0: admission; T = 1: 1 month; T = 2: 4 months; T = 3: 8 months.
Moreover, ELISA test showed lower levels of IL‐6, IL‐8, VEGF and TNF‐alpha in plasma fluid of patients with CVUs treated with Axaven® (group A) with respect to not treated patients (group B; Figures 3 and 4).
Figure 3.
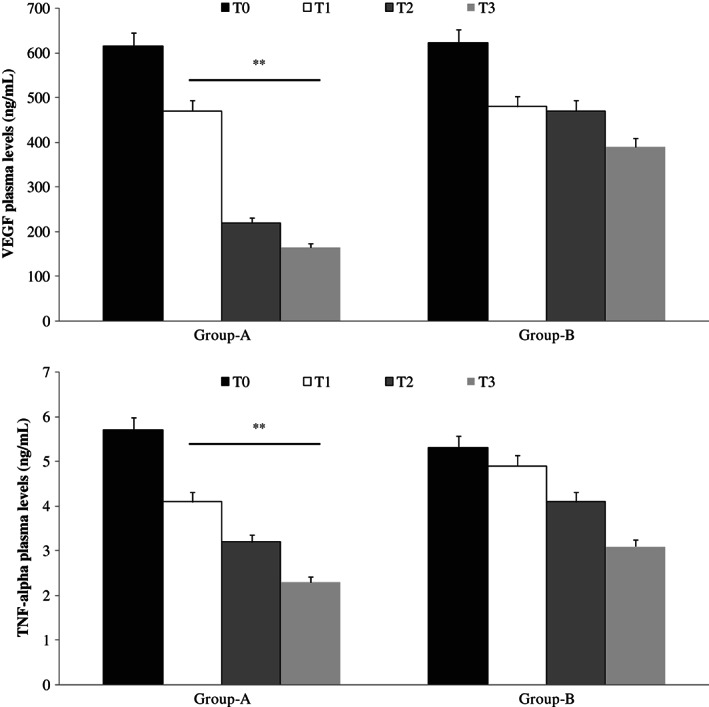
Enzyme‐linked immunosorbent assay (ELISA) plasma evaluation of vascular endothelial growth factor (VEGF; up) and tumour necrosis factor (TNF)‐alpha (down), at different times, in patients with chronic venous ulcerations (CVUs) treated (group A) or not (group B) with Axaven® once daily for 8 months (end of study). Data are expressed as mean ± SEM. *P < 0·05; **P < 0·01. T = 0: admission; T = 1: 1 month; T = 2: 4 months; T = 3: 8 months.
Figure 4.
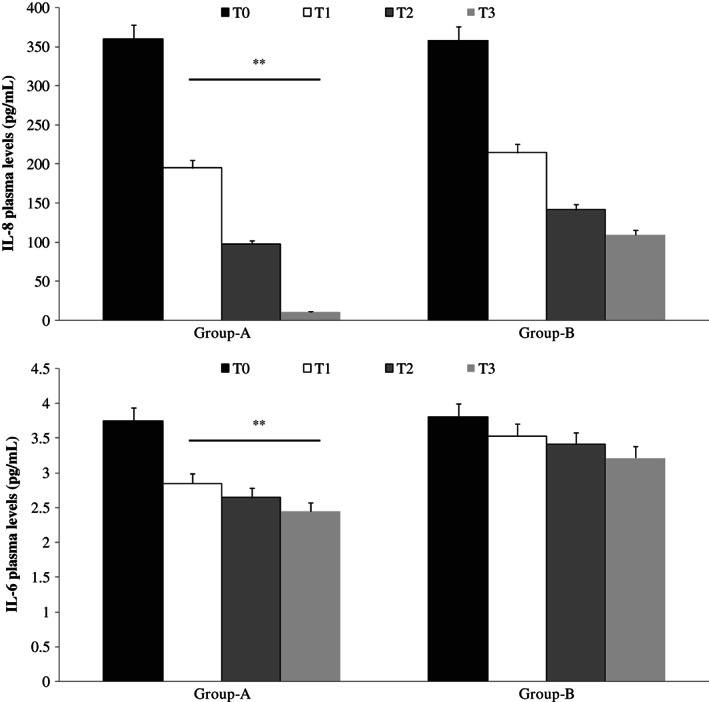
Enzyme‐linked immunosorbent assay (ELISA) plasma evaluation of interleukin‐8 (IL‐8; up) and interleukin‐6 (IL‐6; down), at different times, in patients with chronic venous ulcerations (CVUs) treated (group A) or not (group B) with Axaven® once daily for 8 months (end of study). Data are expressed as mean ± SEM. *P < 0·05; **P < 0·01. T = 0: admission; T = 1: 1 month; T = 2: 4 months; T = 3: 8 months.
Western blot evaluation
Western blot analysis showed a lower expression (P < 0·01) of MMP‐1, MMP‐2, MMP‐9, NGAL (P < 0·01) and MMP‐8 (P < 0·05) in patients treated with Axaven® (group A), with respect to not treated patients (group B; Figure 5).
Figure 5.
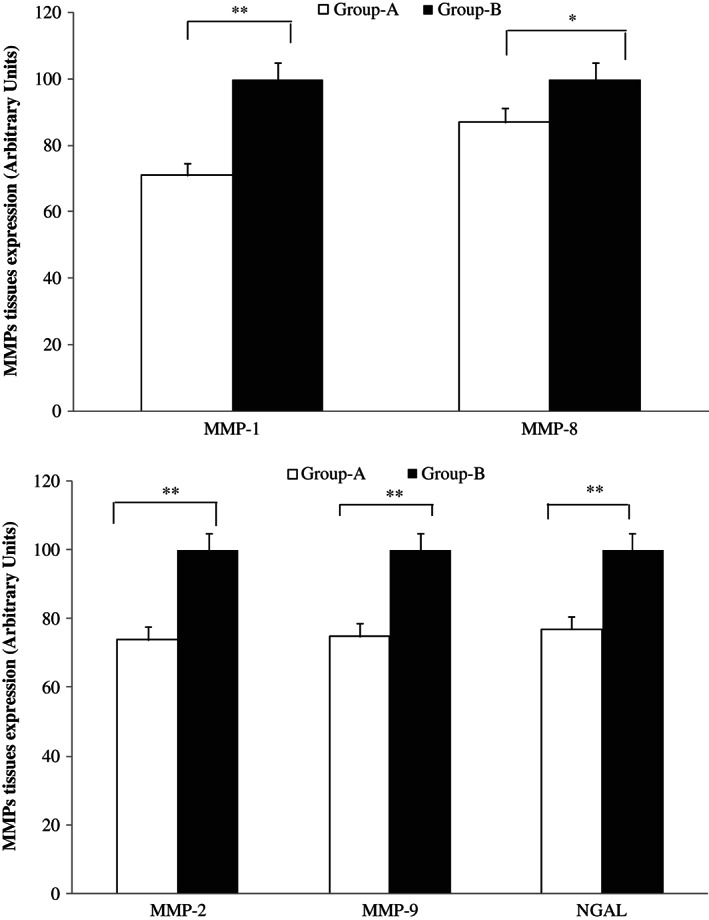
Western blot evaluation of matrix metalloprotease (MMP) expression in wound tissues taken at the time of surgery. MMP‐1 and MMP‐8 (up) and MMP‐2, MMP‐9 and neutrophil gelatinase‐associated lipocalin (NGAL; down) in patients with chronic venous ulcerations (CVUs) treated (group A) or not (group B) with Axaven® once daily for 8 months (end of study). Data are expressed as arbitrary unit, where the higher value has been considered as 100.
Discussion
In this article, we evaluated the effects of a new nutraceutical in patients with CVUs. In agreement with literature data and with good clinical practice 48, 49, all patients were treated with external compression. A previous meta‐analysis of randomised prospective studies in 723 patients with venous ulcers documented that the administration of flavonoid fractions improved the effect of conventional treatment (compression and local care) 50.
Flavonoids are phenolic substances isolated from a wide range of vascular plants with antioxidant, anti‐inflammatory, antimicrobial, antiviral, vasodilating and antiallergic properties 51, 52, 53, 54. The most commonly used flavonoid is diosmin (30,5,7‐trihydroxy‐40‐methoxyflavone‐7‐rutinoside), a naturally occurring O‐rutinoside flavone 55, which may be obtained by dehydrogenation of the corresponding flavanone glycoside, hesperidin that is abundant in citrus 56. Both anti‐inflammatory and antioxidant activity of diosmin appear to be related to the inhibition of production and release from leucocytes of chemical mediators of inflammation (histamine, bradykinin, serotonin and protease). The phlebotonic action is related to the inhibition of monoamine oxidases, the enzymes involved in the degradation of noradrenaline that increase and stimulate the alpha‐1 adrenergic receptor inducing increase in blood pressure 57. Drug formulations currently in use are constituted by different concentrations of diosmin, which are added to other flavonoids of plant origin such as hesperidin or horse chestnut seed extract 32, 50, 58.
In our study, the administration of Axaven® in patients with CVUs was able to decrease inflammatory cytokines, MMPs and NGAL inducing an improvement of both symptoms with an increase of the speed of wound healing. According to literature and our previous studies, the involvement of growth factors 59, 60 and leucocytes 13, 16, 17, 18, 19, 61, 62 in the development of venous ulceration has opened up new areas of investigation. Thus, elevated levels of ILs and VEGF in patients with CVUs in this study are attributable to the action of PMNs and the consequent inflammatory nature of the venous disease. Several studies have shown that activation of inflammatory cells alters the balance of the ECM, favouring the action of MMPs 11, 12, 13, 16, 17, 18, 19, 20, 60. Increased MMPs activity is associated with the pathophysiology of various diseases, especially in inflammatory diseases 63.
MMPs are enzymes able to degrade the basement membranes as well as the ECM, thus liberating VEFG and may be involved in wound healing. The results obtained from our study have shown elevated levels of MMPs, in particular MMP‐1 and MMP‐8 that are involved in delayed healing of venous leg ulcers 17 and MMP‐2 and MMP‐9 that are involved in the pathogenesis of the disease 11, 12, 13, 16, 17, 18, 19, 20, 60. However, patients treated with Axaven® have shown not only an improvement in the clinical condition of the ulcers, but also a more noticeable reduction in the levels of MMPs mentioned above. These effects are related to the action of single constituent other than diosmin + hesperidin. In particular, several studies have documented the effects of astaxanthin (3,30‐dihydroxy‐b,b‐carotene‐4,40‐dione), present in a wide variety of plants, algae and sea foods 64, on oxidative damage and inflammation 65, 66, whereas in vitro study have documented that blueberry is able to inhibit MMPs activity 67. Similarly, it has been documented that rutin derivates are able to decrease the activation of TNF‐alpha and IL‐8 68. Therefore, in agreement with literature data we suppose that the effects of diosmin and classics bioflavonoids (rutin, hesperidin) increased the action of astaxanthin 69, 70, 71 and blueberry that are present in Axaven® with a greater decrease in the tissue expression of MMPs and in the levels of cytokines, MMPs and NGAL in plasma. NGAL is a protein belonging to the lipocalin family and is expressed by activated neutrophils and has the ability to positively modulate the activity of MMP‐9 in particular by forming the NGAL/MMP‐9 complex, thus protecting MMP‐9 from proteolytic degradation 18. Moreover, in our study we did not record any side effects that could be related to the presence of althea and the low dosage of other substance in the formulation. However, it is important to underline that the low time of observation (8 months) and the low number of patients enrolled may represent a limitation of this study.
In conclusion, we can affirm that Axaven® could represent a good choice in patients with CVUs, even if other studies in a large group of patients may be performed in order to confirm these observations.
Acknowledgements
The authors received no funding. The authors declare no conflict of interest.
[Correction added on 20 March 2015, after first online publication: The number of patients, 36 patients (Group A: 9 females and 7 males; Group B 11 females and 9 males) were wrong and have been changed to 83 patients (Group A: 25 females and 19 males; Group B: 24 females and 15 males) in the abstract.]
References
- 1. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Molinari V, Montemurro R, de Franciscis S. A genetic study of chronic venous insufficiency. Ann Vasc Surg 2012;26:636–42. [DOI] [PubMed] [Google Scholar]
- 2. de Franciscis S, De Sarro G, Longo P, Buffone G, Molinari V, Stillitano DM, Gallelli L, Serra R. Hyperhomocysteinaemia and chronic venous ulcers. Int Wound J 2013. doi: 10.1111/iwj.12042 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serra R, Buffone G, Costanzo G, Montemurro R, Perri P, Damiano R, de Franciscis S. Varicocele in younger as risk factor for inguinal hernia and for chronic venous disease in older: preliminary results of a prospective cohort study. Ann Vasc Surg 2013;27:329–31. [DOI] [PubMed] [Google Scholar]
- 4. Serra R, Buffone G, Molinari V, Montemurro R, Perri P, Stillitano DM, Amato B, de Franciscis S. Low molecular weight heparin improves healing of chronic venous ulcers especially in the elderly. Int Wound J 2013. doi: 10.1111/iwj.12071 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gasbarro V, Amato B, Izzo M, Manfrini A, Buffone G, Grande R, Serra R, De Franciscis S. Quercetin and chronic venous ulceration of the lower limbs. Acta Phlebol 2013;14:61–5. [Google Scholar]
- 6. Serra R, Buffone G, de Franciscis A, Mastrangelo D, Vitagliano T, Greco M, de Franciscis S. Skin grafting followed by low‐molecular‐weight heparin long‐term therapy in chronic venous leg ulcers. Ann Vasc Surg 2012;26:190–7. [DOI] [PubMed] [Google Scholar]
- 7. Serra R, Grande R, Buffone G, Costanzo G, Damiano R, de Franciscis S. Chronic venous disease is more aggressive in patients with varicocele. Acta Phlebol 2013;14:57–60. [Google Scholar]
- 8. de Franciscis S, Gasbarro V, Amato B, Buffone G, Grande R, Serra R. Haemodynamic surgery versus conventional surgery in chronic venous disease: a multicenter retrospective study. Acta Phlebol 2013;14:109–14. [Google Scholar]
- 9. de Franciscis S, Grande R, Buffone G, Serra R. Chronic venous ulceration of the lower limbs and thrombosis. Acta Phlebol 2013. In press. [Google Scholar]
- 10. Abd‐El‐Aleem SA, Morgan C, Ferguson MW, McCollum CN, Ireland GW. Spatial distribution of mast cells in chronic venous leg ulcers. Eur J Histochem 2005;49:265–72. [PubMed] [Google Scholar]
- 11. de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, Buffone G, Serra R. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J 2013. doi: 10.1111/iwj.12085 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Franciscis S, Mastroroberto P, Gallelli L, Buffone G, Montemurro R, Serra R. Increased plasma levels of metalloproteinase‐9 and neutrophil gelatinase‐associated lipocalin in a rare case of multiple artery aneurysm. Ann Vasc Surg 2013;27:1185.e5–7. [DOI] [PubMed] [Google Scholar]
- 13. Busceti MT, Grande R, Amato B, Gasbarro V, Buffone G, Amato M, Gallelli L, Serra R, de Franciscis S. Pulmonary embolism, metalloproteinases and neutrophil gelatinase associated lipocalin. Acta Phlebol 2013;14:115–21. [Google Scholar]
- 14. Jung SA, Yang SK, Kim JS, Shim KN, Im SA, Myung SJ, Jung HY, Yu CS, Kim JC, Hong WS, Kim JH, Min YI. The expression of matrix metalloproteinases (MMPs), tissue inhibitor of metalloproteinases (TIMPs) and angiogenesis in relation to the depth of tumor invasion and lymph node metastasis in submucosally invasive colorectal carcinoma. Korean J Gastroenterol 2005;45:401–8. [PubMed] [Google Scholar]
- 15. Yang CC, Lin CY, Wang HS, Lyu SR. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus‐like tissue contribute to knee osteoarthritis progression. PLoS One 2013;8:e79662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serra R, Buffone G, Costanzo G, Montemurro R, Scarcello E, Stillitano DM, Damiano R, de Franciscis S. Altered metalloproteinase‐9 expression as the least common denominator between varicocele, inguinal hernia and chronic venous disorders. Ann Vasc Surg 2013, in press. doi: 10.1016/j.avsg.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 17. Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, Grande R, Serra R, de Franciscis S. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J 2013. doi: 10.1111/iwj.12181[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, de Franciscis S. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen 2013;21:395–401. [DOI] [PubMed] [Google Scholar]
- 19. Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Act Phlebol 2013;14:99–107. [Google Scholar]
- 20. Serra R, Gallelli L, Buffone G, Molinari V, Stillitano DM, Palmieri C, de Franciscis S. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J 2013. doi: 10.1111/iwj.12077[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weller C, Evans S. Venous leg ulcer management in general practice‐practice nurses and evidence based guidelines. Aust Fam Physician 2012;41:331–7. [PubMed] [Google Scholar]
- 22. Belczak SQ, Sincos IR, Campos W, Beserra J, Nering G, Aun R. Venoactive drugs for chronic venous disease: A randomized, double‐blind, placebo‐controlled parallel‐design trial. Phlebology 2013[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg 2011;41:117–25. [DOI] [PubMed] [Google Scholar]
- 24. Dholakiya SL, Benzeroual KE. Protective effect of diosmin on LPS‐induced apoptosis in PC12 cells and inhibition of TNF‐a expression. Toxicol In Vitro 2011;25:1039–44. [DOI] [PubMed] [Google Scholar]
- 25. Carpentier PH, Mathieu M. Evaluation of clinical efficacy of a venotonic drug: lessons of a therapeutic trial with hemisynthesis diosmin in “heavy legs syndrome”. J Mal Vasc 1998;23:106–12. [PubMed] [Google Scholar]
- 26. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids. Angiology 2002;53:245–56. [DOI] [PubMed] [Google Scholar]
- 27. Alvarez N, Vicente V, Martínez C. Synergistic effect of diosmin and interferon‐alpha on metastatic pulmonary melanoma. Cancer Biother Radiopharm 2009;24:347–52. [DOI] [PubMed] [Google Scholar]
- 28. Manuel y KB, Vertommen J, De Leeuw I. The effect of flavonoid treatment on the glycation and antioxidant status in Type 1 diabetic patients. Diabetes Nutr Metab 1999;12:256–63. [PubMed] [Google Scholar]
- 29. Parker‐Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, Murphy T, Legradi G, Tan J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL‐6/MIA associated autism. J Neuroimmunol 2009;217:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirienko AI, Bogachev VI, Zolotukhin IA, Golovanova OV. Semisynthetic diosmin (phlebodia 600) for therapy of lower limb chronic venous insufficiency. Angiol Sosud Khir 2006;12:73–5. [PubMed] [Google Scholar]
- 31. Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol 2009;7:303–8. [DOI] [PubMed] [Google Scholar]
- 32. Smith PC. Daflon 500 mg and venous leg ulcer: new results from a meta‐analysis. Angiology 2005;56(Suppl 1):S33–9. [DOI] [PubMed] [Google Scholar]
- 33. Cesarone MR, Belcaro G, Pellegrini L, Ledda A, Vinciguerra G, Ricci A, Di Renzo A, Ruffini I, Gizzi G, Ippolito E, Fano F, Dugall M, Acerbi G, Cornelli U, Hosoi M, Cacchio M. Venoruton vs Daflon: evaluation of effects on quality of life in chronic venous insufficiency. Angiology 2006;57:131–8. [DOI] [PubMed] [Google Scholar]
- 34. Petruzzellis V, Troccoli T, Candiani C, Guarisco R, Lospalluti M, Belcaro G, Dugall M. Oxerutins (Venoruton): efficacy in chronic venous insufficiency ‐ a double‐blind, randomized, controlled study. Angiology 2002;53:257–63. [DOI] [PubMed] [Google Scholar]
- 35. Giacalone M, Di Sacco F, Traupe I, Topini R, Forfori F, Giunta F. Antioxidant and neuroprotective properties of blueberry polyphenols: a critical review. Nutr Neurosci 2011;14:119–25. [DOI] [PubMed] [Google Scholar]
- 36. Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol 2008;101:58D–68. [DOI] [PubMed] [Google Scholar]
- 37. Bombardelli E, Morazzoni P. Aesculus hippocastanum L. Fitoterapia 1996;67:483–511. [Google Scholar]
- 38. Mrwa U, Güth K, Haist C, Troschka M, Herrmann R, Wojciechowski R, Gagelmann M. Calcium‐requirement for activation of skinned vascular smooth muscle from spontaneously hypertensive (SHRSP) and normotensive control rats. Life Sci 1986;38:191–6. [DOI] [PubMed] [Google Scholar]
- 39. Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology 2003;13:17R–27. [DOI] [PubMed] [Google Scholar]
- 40. Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci 2009;18:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen F, Fukuse T, Hasegawa S, Bando T, Hanaoka N, Kawashima M, Sakai H, Hamakawa H, Fujinaga T, Nakamura T, Wada H. Effective application of ET‐Kyoto solution for clinical lung transplantation. Transplant Proc 2004;36:2812–5. [DOI] [PubMed] [Google Scholar]
- 42. Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification . Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248–52. [DOI] [PubMed] [Google Scholar]
- 43. Fragomeni G, Merola A, Serra R, de Franciscis S, Amato F. A nonlinear lumped parameters model to analyze the dynamics of venous reflux. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1407–10. [DOI] [PubMed] [Google Scholar]
- 44. de Franciscis S, Fragomeni J, Caruso MV, de Franciscis A, Perri P, Serra R. A new photographic computerized measurement system for chronic wound assessment. Acta Phlebol. 2013. In press. [Google Scholar]
- 45. Howard DP, Howard A, Kothari A, Wales L, Guest M, Davies AH. The role of superficial venous surgery in the management of venous ulcers: a systematic review. Eur J Vasc Endovasc Surg 2008;36:458–65. [DOI] [PubMed] [Google Scholar]
- 46. Alden PB, Lips EM, Zimmerman KP, Garberich RF, Rizvi AZ, Tretinyak AS, Alexander JQ, Dorr KM, Hutchinson M, Isakson SL. Chronic venous ulcer: minimally invasive treatment of superficial axial and perforator vein reflux speeds healing and reduces recurrence. Ann Vasc Surg 2013;27:75–83. [DOI] [PubMed] [Google Scholar]
- 47. Gloviczki P, Gloviczki ML. Evidence on efficacy of treatments of venous ulcers and on prevention of ulcer recurrence. Perspect Vasc Surg Endovasc Ther 2009;21:259–68. [DOI] [PubMed] [Google Scholar]
- 48. Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, Lohr JM, McLafferty RB, Meissner MH, Murad MH, Padberg FT, Pappas PJ, Passman MA, Raffetto JD, Vasquez MA, Wakefield TW, Society for Vascular Surgery , American Venous Forum . The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011;53(5 Suppl):2S–48. [DOI] [PubMed] [Google Scholar]
- 49. Gloviczki P, Gloviczki ML. Guidelines for the management of varicose veins. Phlebology 2012;27(Suppl 1):2–9. [DOI] [PubMed] [Google Scholar]
- 50. Coleridge‐Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta‐analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg 2005;30:198–208. [DOI] [PubMed] [Google Scholar]
- 51. Yang B, Kotani A, Arai K, Kusu F. Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci 2001;17:599–604. [DOI] [PubMed] [Google Scholar]
- 52. Amić D, Davidović‐Amić D, Beslo D, Rastija V, Lucić B, Trinajstić N. SAR and QSAR of the antioxidant activity of flavonoids. Curr Med Chem 2007;14:827–45. [DOI] [PubMed] [Google Scholar]
- 53. Gomes A, Fernandes E, Lima JL, Mira L, Corvo ML. Molecular mechanisms of anti‐inflammatory activity mediated by flavonoids. Curr Med Chem 2008;15:1586–605. [DOI] [PubMed] [Google Scholar]
- 54. Pietta PG. Flavonoids as antioxidants. J Nat Prod 2000;63:1035–42. [DOI] [PubMed] [Google Scholar]
- 55. Barreca D, Laganà G, Bruno G, Magazù S, Bellocco E. Diosmin binding to human serum albumin and its preventive action against degradation due to oxidative injuries. Biochimie 2013;95:2042–9. [DOI] [PubMed] [Google Scholar]
- 56. Campanero MA, Escolar M, Perez G, Garcia‐Quetglas E, Sadaba B, Azanza JR. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography‐atmospheric pressure chemical ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J Pharm Biomed Anal 2010;51:875–81. [DOI] [PubMed] [Google Scholar]
- 57. Ramelet AA. Clinical benefits of Daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology 2001;52(Suppl 1):S49–56. [DOI] [PubMed] [Google Scholar]
- 58. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev 2012;11:CD003230. [DOI] [PubMed] [Google Scholar]
- 59. Higley HR, Ksander GA, Gerhardt CO, Falanga V. Extravasation of macromolecules and possible trapping of transforming growth factor‐beta in venous ulceration. Br J Dermatol 1995;132:79–85. [DOI] [PubMed] [Google Scholar]
- 60. Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J 2014. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shoab SS, Scurr JH, Coleridge‐Smith PD. Plasma VEGF as a marker of therapy in patients with chronic venous disease treated with oral micronised flavonoid fraction ‐ a pilot study. Eur J Vasc Endovasc Surg 1999;18:334–8. [DOI] [PubMed] [Google Scholar]
- 62. Stvrtinova V, Jahnova E, Weissova S, Horvathova M, Ferencik M. Inflammatory mechanisms involving neutrophils in chronic venous insufficiency of lower limbs. Bratisl Lek Listy 2001;102:235–9. [PubMed] [Google Scholar]
- 63. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 1999;274:21491–4. [DOI] [PubMed] [Google Scholar]
- 64. Riccioni G, Speranza L, Pesce M, Cusenza S, D'Orazio N, Glade MJ. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012;28:605–10. [DOI] [PubMed] [Google Scholar]
- 65. Karppi J, Rissanen TH, Nyyssönen K, Kaikkonen J, Olsson AG, Voutilainen S, Salonen JT. Effects of astaxanthin supplementation on lipid peroxidation. Int J Vitam Nutr Res 2007;77:3–11. [DOI] [PubMed] [Google Scholar]
- 66. Manago S, Yonehara S. TNF family (TNF alpha, beta, FasL, CD40L). Nihon Rinsho 2005;63(Suppl 4):202–6. [PubMed] [Google Scholar]
- 67. Matchett MD, MacKinnon SL, Sweeney MI, Gottschall‐Pass KT, Hurta RA. Blueberry flavonoids inhibit matrix metalloproteinase activity in DU145 human prostate cancer cells. Biochem Cell Biol 2005;83:637–43. [DOI] [PubMed] [Google Scholar]
- 68. Berg K, Andersen H, Owen TC. The regulation of rhinovirus infection in vitro by IL‐8, HuIFN‐alpha, and TNF‐alpha. APMIS 2004;112:172–82. [DOI] [PubMed] [Google Scholar]
- 69. Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond) 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suzuki Y, Ohgami K, Shiratori K, Jin XH, Ilieva I, Koyama Y, Yazawa K, Yoshida K, Kase S, Ohno S. Suppressive effects of astaxanthin against rat endotoxin‐induced uveitis by inhibiting the NF‐kappa B signaling pathway. Exp Eye Res 2006;82:275–81. [DOI] [PubMed] [Google Scholar]
- 71. Speranza L, Pesce M, Patruno A, Franceschelli S, de Lutiis MA, Grilli A, Felaco M. Astaxanthin treatment reduced oxidative induced pro‐inflammatory cytokines secretion in U937: SHP‐1 as a novel biological target. Mar Drugs 2012;10:890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


