Abstract
Gabapentinoids are effective adjunct drugs for reducing postoperative pain. However, the effects of gabapentinoids on wound healing have not been evaluated yet. In this study we evaluated their effects on wound healing. A total of 17 male Wistar‐Albino rats, 250–350 g, were divided into three groups randomly: control group (n = 5, 2 ml saline), gabapentin group (n = 6, 20 mg/kg gabapentin) and pregabalin group (n = 6, 20 mg/kg pregabalin). Until day 13 inflammation scores were significantly lower (P < 0·05) and wound healing was significantly better in the control group when compared with gabapentin and pregabalin groups (P < 0·001). Inflammation scores were significantly lower in pregabalin group when compared with gabapentin group until day 13. But wound healing was significantly better in gabapentin group than in pregabalin group between days 13 and 21. In conclusion when gabapentin and pregabalin were compared, although pregabalin decreases inflammation scores, gabapentin has better results in wound healing.
Keywords: Gabapentin, Pregabalin, Wound healing
Wound healing is a complicated process in which the tissue repairs itself after injury. The four phases of wound healing are hemostasis, inflammation, proliferation and remodelling 1. However, wound healing is a fragile process and is susceptible to failure which can result in the formation of non‐healing chronic wounds. Metabolic diseases such as diabetes mellitus, circulatory diseases, infection, some medications and age may contribute to formation of chronic wounds.
Postoperative pain is one of the most common complaints of surgical patients. According to the literature, pain is treated inadequately in approximately half of the patients 2. Pain is caused by multiple mechanisms. Multimodal analgesia regimen is therefore appropriate for treatment of pain. Drug groups such as opioids, local anaesthetics, non‐steroidal anti‐inflammatory drugs, paracetamol and gabapentinoids are used for pain therapy 3, 4, 5, 6. Gabapentinoids are anticonvulsant drugs. Many researches have been conducted to study their role in acute pain treatment 7, 8, 9. Gabapentin and pregabalin are two major drugs of the gabapentinoid group. They are increasingly used as part of multimodal analgesia at perioperative period. They decrease opioid use and postoperative pain 10, 11. However, the effects of gabapentinoids on wound healing have not been evaluated yet. In this study we evaluate their effects on wound healing.
Material and methods
Afyonkarahisar University Animal Ethics Committee approved this experimental study (Protocol: 211‐13, 05·03·2013). All the experimental manipulations and postoperative care were administered in concordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 17 male Wistar‐Albino rats, aged 5 months and weighing between 250 and 350 g were divided into three groups randomly: control group (n = 5), gabapentin group (n = 6) and pregabalin Group (n = 6). All the rats were housed in individual cages at 25°C with alternating 12 hour light–dark cycles. All had free access to standard laboratory diet and water. The rats were premedicated with 10 mg/kg intraperitoneal (ip) Xylazine HCl (Rompun vial, 23·32 mg/ml, Bayer Turkish Chemistry Industry Ltd.C., Istanbul, Turkey), and anaesthetised with ip 100 mg/kg Ketamin HCl (Ketalar vial, 50 mg/ml, Eczacibasi Medicine and Commerce A.C., Istanbul, Turkey).
The dorsal surface hair of all rats was shaved bilaterally and the rats were disinfected. No prophylactic or therapeutic antibiotic was administered; 2 cm skin incision was made to the right side of the dorsum of all the rats and then sutured with 2‐0 prolene (Dogsan,Trabzon, Turkey) and two mattress sutures in sterile conditions; 1.5 cm diameter round‐shaped, full thickness skin patch was removed from the left side of the dorsum of all the rats and dressed with sterile gauzes. All the surgical procedures were performed under aseptic conditions by the same surgeon; 20 mg/kg of gabapentin (Neurontin®; Pfizer, New York) and 20 mg/kg of pregabaline (Lyrica®; Pfizer) diluted with 2 ml saline was administered orally to the gabapentin and pregabaline groups, respectively, by orogastric tube, and only saline was administered to the control group 1 day before the surgery and 9 days after the surgery, once a day. Sutures at the incision sites of all the rats were removed at day 9 and sampling was carried out from the scar tissue under anaesthesia (with same anaesthesia protocol) for histopathological evaluation. Rats were euthanised with ip thiopental 150 mg/kg and tissue sampling was carried out from scar tissue at day 21 (Figures 1, 2, 3). Evaluation of the round‐shaped scar tissue healing was done by copying the scar shape on acetate and calculating the surface area at days 1, 3, 5, 7, 9, 11, 13, 15, 17, 19 and 21.
Figure 1.
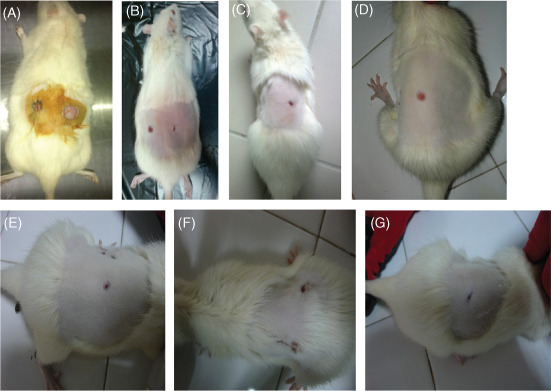
(A) Day 0, control group; (B) day 9, pregabalin group; (C) day 9, control group; (d) day 9 gabapentin group; (E) day 21, control group; (F) day 21 pregabalin group and (g) day 21, gabapentin group.
Figure 2.
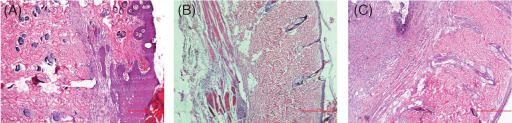
Histopathological sections on day 9. (A) Control group: normal epidermis; (B) pregabalin group: epidermal thinning and moderate inflammation and (C) gabapentin group: epidermal thinning, severe inflammation and connective tissue accumulation.
Figure 3.
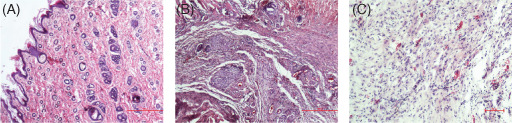
Histopathological sections on day 21. (A) Control group: normal tissue; (B) pregabalin group: moderate inflammation, connective tissue accumulation and vascularisation and (C) gabapentin group: mild vascularisation.
Pathological method
Tissue samples were fixed in 10% formaldehyde solution for 24 hours. They were processed and embedded in paraffin blocks and were cut into 5 µm sections with a microtome. After deparaffinisation, sections were prepared for staining with haematoxylin and eosin. A semi‐quantitative evaluation was performed in light microscopy at 10× and 40× by two distinct pathologists blinded to the study groups. During the pathological assessment epidermis and dermis were evaluated. Epidermis was evaluated regarding thinning and inflammation [grade 0 = normal (−), grade 1 = mild (+), grade 2 = moderate (++) and grade 3 = severe (+++)]. Dermis was evaluated for the thinning and existence of inflammatory cells in addition to new vessel formation, accumulation of connective tissue matrix and collagen fibres [grade 0 = normal (−), grade 1 = mild (+), grade 2 = moderate (++) and grade 3 = severe (+++)].
Statistical analysis
Data were analysed with the SPSS 16·0 (SPSS Inc, for Windows) software package. A one‐way analysis of variance (ANOVA) test was used to compare the wound areas between groups. Test significance levels within and between groups were checked using Duncan's test. For the scores and non‐normally distributed variables, comparison between groups was carried out by the Kruskal–Wallis test. Descriptive results are expressed as means ± standard deviation. For all comparative tests, a value of P < 0·05 was considered significant.
Results
No animals died in control, gabapentin and pregabalin groups during study period. The thinning, inflammation, new vessel formation, accumulation of connective tissue matrix and collagen fibres were significantly lower in control group compared with pregabalin and gabapentin groups on day 10 in the dermis and epidermis (P < 0·05). These were also significantly lower in pregabalin group than gabapentin group on day 10 in the dermis and epidermis (P < 0·05). Although there were no significant differences between groups on day 21, the scores were lower in control group than other groups (Table 1).
Table 1.
Control, pregabalin and gabapentin groups wound surface area changes at measurement days*
| Day | Control (n = 5) Wound surface area (cm2) | Pregabalin (n = 6) Wound surface area (cm2) | Gabapentin (n = 6) Wound surface area (cm2) |
|---|---|---|---|
| 0 | 1·77 | 1·77 | 1·77 |
| 1 | 1·50 ± 0·08a | 1·73 ± 0·09b | 1·54 ± 0·09a |
| 3 | 0·95 ± 0·10b | 1·17 ± 0·22a | 1·24 ± 0·27a |
| 5 | 0·81 ± 0·11a | 0·97 ± 0·32b | 0·82 ± 0·15a |
| 7 | 0·71 ± 0·08 | 0·80 ± 0·22 | 0·79 ± 0·14 |
| 9 | 0·35 ± 0·08b | 0·41 ± 0·16a | 0·42 ± 0·09a |
| 11 | 0·24 ± 0·04 | 0·30 ± 0·08 | 0·33 ± 0·09 |
| 13 | 0·17 ± 0·06b | 0·24 ± 0·06a | 0·27 ± 0·07a |
| 15 | 0·07 ± 0·04a | 0·20 ± 0·07b | 0·17 ± 0·06c |
| 17 | 0·03 ± 0·02 | 0·08 ± 0·05 | 0·07 ± 0·03 |
| 19 | 0·003 ± 0·005 | 0·05 ± 0·21 | 0·01 ± 0·02 |
| 21 | 0 | 0·02 ± 0·02 | 0·003 ± 0·005 |
The values with different letters (a, b and c) in the same line show statistical significance (P < 0·001).
Healing of the round‐shaped scar wounds was also evaluated by copying the scar shape on acetate and by calculating the surface area and decreased surface area at days 1, 3, 5, 7, 9, 11, 13, 15, 17, 19 and 21 in the three groups (Table 2). According to these calculations wound healing in the control group was significantly better than pregabalin (PGB) and gabapentin (GBP) groups at days 3, 9 and 13 (P < 0·001). At day 5 wound healing in the control and GBP groups was significantly better than PGB group (P < 0·001). At day 21 control group scar tissue was healed completely; however, in PGB and GBP groups, scar tissues did not close completely but these disclosures were not statistically significant. On the other hand, healing in GBP group was better than PGB group at days 13, 15, 17 and 19.
Table 2.
Mean (SD) histopathological scores of the groups at days 9 and 21*
| Day 9 | Day 21 | |||
|---|---|---|---|---|
| Epidermis | Dermis | Epidermis | Dermis | |
| Control (n = 5) | 0·2 ± 0·4a | 0·4 ± 0·8a | 0·2 ± 0·4 | 0·4 ± 0·5 |
| Pregabalin (n = 6) | 0·6 ± 0·5ab | 1·8 ± 0·7b | 0·6 ± 1·2 | 1·16 ± 1·16 |
| Gabapentin (n = 6) | 1 ± 0b | 2·8 ± 0·4c | 0·3 ± 0·5 | 0·8 ± 0·4 |
The different letters (a, b and c) in the same column show statistical significance (P < 0·05).
Discussion
The permanent or temporary loss of existent physiological properties due to the loss or failure of integrity of the skin and mucosal structures is called a wound. Healing period begins from the second the trauma happens and goes on for days, months even years. The healing capacity of a wound depends on its depth, and the health and nutritional status of the organism 12. Davidson reported that after exposure to the anaesthesia and surgery combination, many of the immune system functions deteriorated 13. There are many medications used during the perioperative period, for example, intravenous anaesthetics, inhalational anaesthetics and local anaesthetics, opioids, non‐steroids, muscle relaxants, corticosteroid, antiemetics and gabapentinoids. Their effect on the healing capacity is a very important subject. Local anaesthetics, opioids, corticosteroids and some of the anaesthetics were evaluated for their effects on wound healing. Although gabapentinoids are commonly used for postoperative pain relief during the perioperative period, their effect on wound healing is still unknown.
Healing process is influenced by the degree of inflammation, which is the key step in wound healing. Inflammation is a natural reaction to injury and it is essential for tissue repair. Inflammatory reactions are initiated by multiple factors such as trauma, infection, metabolic dysfunction and surgery. Inflammation is the first acute response of the tissue to the trauma. This phase is characterised by increased vascular permeability, chemotaxis of the cell from the circulation to the wound site, local release of cytokines and growth factor and activation of migration of cells 14. Although the wound healing process is divided into four phases it is a continuous process and phases overlap each other. The presence of more mature capillary vessels in the vicinity of the wound and large amount of collagen fibre provides better tissue oxygenation and nutrition which is adequate for wound healing process 15. So the drugs that effect any of these phases have influence on wound healing.
Inhalational anaesthetics are major anaesthetic drugs for general anaesthesia. Their effects on wound healing and inflammation have been evaluated by several researchers. Yang et al. reported that inhalational anaesthetics, especially isoflurane, cause abnormal calcium release from the endoplasmic reticulum via excessive activation of inositol 1,4,5‐trisphosphate (IP3) receptors so they may induce cell damage 16. Also isoflurane, halothane and enflurane attenuate the inflammatory response by decreasing release of proinflammatory cytokines 17. Lee et al. reported that the exposure to sevoflurane over 4 hours affects early period of wound healing, leading to delayed wound size reduction, and decreased expression of transforming growth factor‐β1(TGF‐β1) and basic fibroblast growth factor (bFGF) on the wound surface 18. They also found that 8 hours exposure to sevoflurane caused delayed wound healing compared with the oxygen 18. Consequently it can be assumed that the patient who has been subjected to general anaesthesia with inhalational anaesthetics is under risk of delayed wound healing as the exposure time gets prolonged. So we used ketamin and xylazin for anaesthesia of the animals in this study to avoid the effects of inhalational anaesthetics on inflammation and wound healing.
Local anaesthetics is another drug group which is a touchstone for anaesthesia practice; their effects on surgical wound healing were also evaluated 19, 20. There are conflicting results in the literature about local anaesthetics on wound healing. Chiang et al. reported that local anaesthetics have proinflammatory effects and cause delayed resolution of inflammation 21. Lidocain can reduce inflammatory responses and protect tissues from local injury in certain settings; on the other hand, it impairs polymorphonuclear apoptosis and macrophage phagocytosis, thus delaying the resolution of inflammation 21 and cause a delay in wound healing. However Eroglu et al. evaluated the effects of lidocaine/prilocaine cream on wound healing and concluded that it had no adverse effect on incisional wound model 22. Also Nykanen et al. concluded that eutectic mixture of lidocaine/prilocaine cream does not affect wound healing adversely and is comparable to 1% lidocaine infiltration in a single blind prospective rat model 23. Zeren et al. reported that levobupivacaine impairs wound healing during the early period and has positive effect during later periods of wound healing 24. Feder et al. showed that lidocaine, bupivacaine and ropivacaine had concentration‐dependent cytotoxic effect on fibroblasts 25.
Opioids are also used frequently during intraoperative and postoperative period for pain relief. They can interfere during the different stages of inflammation, decrease inflammation via vasoconstriction and decrease neuropeptide release 26. Corticosteroids, a frequently used drug group at perioperative period, decrease inflammation, thus affecting cell migration, proliferation and angiogenesis 27. And as a result corticosteroids cause delayed wound healing by inhibiting the inflammatory phase and collagen synthesis 28.
Gabapentin and pregabalin have been used in the treatment of postsurgical pain, neuropathic pain, epilepsy, spasticity and anxiety. In recent years gabapentin and pregabalin have been used widely for acute postsurgical pain treatment. They were found to be effective in reducing acute postoperative pain 10, 11. Chang et al. have reported gabapentin to be a safe and efficacious drug for the treatment of postoperative pain 29. They help to decrease opioid use and reduce postoperative pain 10, 11. Pregabalin is also considered an alternative drug for opioid dependence, and prevent hyperalgesia 30. And additionally perioperative administration of gabapentinoids is effective in reducing incidence of chronic postsurgical pain 31. Gabapentin and pregabalin have been proposed to have mechanism of action of gabapentinoids and inhibit α (2)/δ subunit of inactivated voltage‐dependent calcium channels. Also gabapentin inhibits glutamate release, increases the activity of voltage gated N‐methyl‐d‐aspartate receptors and inhibits the activity of voltage‐gated potassium channels 32, 33. However, it is still not clear if these mechanisms play a role in analgesic effects of gabapentinoids.
Pregabalin decreases calcium influx thus decreasing secretion of excitatory neurotransmitters, such as glutamate, noradrenalin and substance P 34. Yong Ha et al. evaluated pregabalin in a rat model of spinal cord injury and concluded that it has neuroprotective effect and this effect is attributed to its anti‐inflammatory and antinociceptive effects 35. But the mechanism of the anti‐inflammatory effect is still unknown. Substance P is a potent vasodilator. It can cause degranulation of mast cells and induce chemotaxis of neutrophils and lymphocytes 36. The inhibition of substance P by gabapentinoids may be one of the causes of their anti‐inflammatory effect.
Gabapentin treatment increased peritoneal macrophage migration and carrageenan induced paw oedema, thus suggesting that gabapentin is a proinflammatory factor 37. The inflammation scores are higher in the gabapentin group in our study also. So it can be deduced that gabapentinoids can interfere at different stages in the inflammatory cascade. In the literature, gabapentin and pregabalin are used for treatment of pain in a wide variety of dose ranges 29, 37, 38, 39. We studied the effects of 20 mg/kg dose and found that it has a negative effect on wound healing. Higher doses must be evaluated in future studies.
Postoperative day 9 is the proliferative phase of the wound healing; collagen and fibroblast synthesis increased during this period 40. So the sutures were removed during this period (7–10 days) 41. Day 21 is the remodelling phase of the wound healing 40. So the first sampling was performed at day 9 and second one at day 21.
Inflammation scores were significantly lower and wound healing was significantly better in the control group than pregabalin and gabapentin groups until day 13. Inflammation scores were significantly lower in pregabalin group when compared with gabapentin group until day 13. This may be the cause of elongation of inflammatory phase of wound healing and the consequent delay in wound healing process. Camara et al. reported that gabapentin accentuates nerve and peripheral inflammatory response, which could be mainly due to an independent central nervous system‐mediated mechanism and raise some concerns about inflammatory side effects when used clinically 37.
Although gabapentinoids have been widely used for postoperative acute pain treatment, they especially pregabalin should be avoided in patients with poor wound healing risk, or if fast healing is essential. In such patients retinoic acid, which significantly increases the hydroxyproline content at normal levels, may improve the healing process 28.
This study has some limitations. Rat skin morphology and characteristics do not completely simulate the human skin as wound contraction occurs more rapidly than epithelisation 38. Rats have loose skin and this property allows wound contraction to play a significant role in closing rat skin wounds, and finally faster wound contraction than epithelisation results in faster healing time of rat wounds 38. And the environment of human wounds cannot be imitated in experimental animals absolutely.
Conclusion
Our results suggest that although gabapentinoids are effective drugs for postoperative acute pain treatment they should be avoided especially in patients with poor wound healing risk. Gabapentin delayed wound healing prominently during first 10 days but pregabalin effect is seen between day 10 and 21 and it has more prominent negative effect on wound healing. Additional studies are needed on different doses of gabapentinoids and their effect at the molecular level on inflammation, collagen structure and wound tension strength.
Author contributions
TBS helped design the study, conduct the study, analyse the data and wrote the manuscript. ZKS helped design the study and analyse the data. MK helped with all experimental examinations. AS analysed all the histopathological data.
Acknowledgements
Authors declared no conflict of interest. All the funding was obtained by the authors.
References
- 1. Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998;176(2A Suppl):26S–38. [DOI] [PubMed] [Google Scholar]
- 2. Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician 2001;63:1979–84. [PubMed] [Google Scholar]
- 3. Carstensen M, Moller AM. Adding ketamine to morphine for intravenous patient controlled analgesia for acute postoperative pain: a qualitative review of randomized trials. Br J Anaesth 2010;104:401–6. [DOI] [PubMed] [Google Scholar]
- 4. Bonnet F, Marret E. Postoperative pain management and outcome after surgery. Best Pract Res Clin Anaesthesiol 2007;21:99–107. [DOI] [PubMed] [Google Scholar]
- 5. Wood S. Postoperative pain 1: understanding the factors affecting patients' experiences of pain. Nurs Times 2010;106:10–3. [PubMed] [Google Scholar]
- 6. Chelly JE, Ploskanych T, Dai F, Nelson JB. Multimodal analgesic approach incorporating paravertebral blocks for open radical retropubic prostatectomy: a randomized double‐blind placebo‐controlled study. Can J Anaesth 2011;58:371–8. [DOI] [PubMed] [Google Scholar]
- 7. Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol 2007;20:456–72. [DOI] [PubMed] [Google Scholar]
- 8. Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg 2007;105:1805–15. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, Ho K‐Y, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta‐analysis. Br J Anaesth 2011;106:454–62. [DOI] [PubMed] [Google Scholar]
- 10. Kong VK, Irwin MG. Gabapentin A: multimodal perioperative drug? Br J Anaesth 2007;99:775–86. [DOI] [PubMed] [Google Scholar]
- 11. Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg 2007;104:1545–56. [DOI] [PubMed] [Google Scholar]
- 12. Morin RJ, Tomaselli NL. Interactive dressings and topical agents. Clin Plast Surg 2007;34:643–58. [DOI] [PubMed] [Google Scholar]
- 13. Davidson JM. Animal models for wound repair. Arch Dermatol Res 1998;290(Suppl):S1–11. [DOI] [PubMed] [Google Scholar]
- 14. Witte MB, Barbul A. Wound healing. Surg Clin North Am 1997;77:509–28. [DOI] [PubMed] [Google Scholar]
- 15. Drucker M, Cardenas E, Azitri P, Valenzuela A. Experimental studies on effect of lidocaine on wound healing. World J Surg 1998;22:394–8. [DOI] [PubMed] [Google Scholar]
- 16. Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008;109:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helmy SA, Al‐Attiyah RJ. The effects of halothane and isoflurane on plasma cytokine levels. Anaesthesia 2000;55:904–10. [DOI] [PubMed] [Google Scholar]
- 18. Lee HJ, Kwon JY, Shin SW, Baek SH, Choi KU, Jeon YH, Kim WS, Bae JH, Choi HJ, Kim HK, Baik SW. Effects of sevoflurane on collagen production and growth factor expression in rats with an excision wound. Acta Anaesthesiol Scand 2010;54:885–93. [DOI] [PubMed] [Google Scholar]
- 19. Drucker M, Cardenas E, Arizti P, Valenzuela A, Gamboa A. Experimental studies on the effect of lidocaine on wound healing. World J Surg 1998;22:394–7. [DOI] [PubMed] [Google Scholar]
- 20. Morris T, Tracey J. Lignocaine: its effects on wound healing. Br J Surg 1977;64:902–3. [DOI] [PubMed] [Google Scholar]
- 21. Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impacts on resolution of inflammation. PLoS One 2008;3:e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eroglu E, Eroglu F, Agalar F, Altuntas I, Sutcu R, Ozbasar D. The effect of lidocaine/prilocaine cream on experimental wound healing model. Eur J Emerg Med 2001;8:199–201. [DOI] [PubMed] [Google Scholar]
- 23. Nykanen D, Kissoon N, Rieder M, Armstrong R. Comparison of a topical mixture of lidocaine and prilocaine (EMLA) versus 1% lidocaine infiltration on wound healing. Pediatr Emerg Care 1991;7:15–7. [DOI] [PubMed] [Google Scholar]
- 24. Zeren S, Kesici S, Kesici U, İsbilir S, Turkmen UA, Ulusoy H, Karpuz V, Ozcan O, Polat E, Ipcioglu OM, Sari MK. Effects of levobupivacaine on wound healing. Anesth Analg 2013;116:495–9. [DOI] [PubMed] [Google Scholar]
- 25. Fedder C, Beck‐Schimmer B, Aguirre J, Hasler M, Roth‐ Z'graggen B, Urner M, Kalberer S, Schliker A, Votta‐Velis G, Bonvini JM, Graetz K, Borgeat A. In vitro exposure of human fibroblasts to local anaesthetics impairs cell growth. Clin Exp Immunol 2010;162:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stein C, Küchler S. Targeting inflammation and wound healing by opioids. Trends Pharmacol Sci 2013;34:303–12. [DOI] [PubMed] [Google Scholar]
- 27. Leibovich SJ, Ross R. The role of macrophage in wound repair: a study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 28. Durmus M, Karaaslan E, Ozturk E, Gulec M, Iraz M, Edali N, Ersoy MO. The effects of single dose dexamethasone on wound healing in rats. Anesth Analg 2003;97:1377–80. [DOI] [PubMed] [Google Scholar]
- 29. Chang CY, Challa CK, Shah J, Eloy JD. Gabapentin in acute postoperative pain management. Biomed Res Int 2014;2014:7. DOI: 10.1155/2014/631756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurokawa K, Shibasaki M, Mizuno K, Ohkumo S. Gabapentin blocks methamphetamine‐induced sensitization and conditioned place preference via inhibition of α (2)/δ‐1 subunits of the voltage gated calcium channels. Neuroscience 2011;176:328–35. [DOI] [PubMed] [Google Scholar]
- 31. Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using Gabapentin and Pregabalin: A combined systemic review and meta‐analysis. Anesth Analg 2012;115:428–42. [DOI] [PubMed] [Google Scholar]
- 32. Sills GJ. The mechanisms of action gabapentin and pregabalin. Curr Opin Pharmacol 2006;120:421–33. [DOI] [PubMed] [Google Scholar]
- 33. Cheng VY, Bonin RP, Chiu MW, Newell JG, MacDonald JF, Orser BA. Gabapentin increases a tonic inhibitory conductance in hypocampal pyramidal neurons. Anesthesiology 2006;105:325–33. [DOI] [PubMed] [Google Scholar]
- 34. Ling B, Coudore F, Decalonne L, Eschalier A, Authier N. Comparative antiallodynic activity of morphine, pregabalin and lidocaine in a rat model of neuropathic pain produced by one oxaliplatin injection. Neuropharmacology 2008;55:724–8. [DOI] [PubMed] [Google Scholar]
- 35. Ha K‐Y, Kim Y‐H, Rhyu K‐W, Kwon S‐E. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J 2008;1:864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teresiak‐Mikołajczak E, Czarnecka‐Operacz M, Jenerowicz D, Silny W. Neurogenic markers of the inflammatory process in atopic dermatitis: relation to the severity and pruritus. Postepy Dermatol Alergol 2013;30:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Câmara CC, Ramos HF, da Silva AP, Araújo CV, Gomes AS, Vale ML, Barbosa AL, Ribeiro RA, Brito GA, Costa CM, Oriá RB. Oral gabapentin treatment accentuates nerve and peripheral inflammatory responses following experimental nerve constriction in Wistar rats. Neurosci Lett 2013;556:93–8. [DOI] [PubMed] [Google Scholar]
- 38. Miyazaki R, Yamamato T. The efficacy of morphine, pregabalin, gabapentin, and duloxetine on mechanical allodynia is different from that on neuroma pain in the rat neuropathic pain model. Anesth Analg 2012;115:182–8. [DOI] [PubMed] [Google Scholar]
- 39. Hahm TS, Ahn HJ, Ryu S, Gwak MS, Choi SJ, Kim JK, Yu JM. Combined carbamazepine and pregabalin therapy in a rat model of neuropathic pain. Br J Anaesth 2012;109:968–74. [DOI] [PubMed] [Google Scholar]
- 40. Dorsett‐Martin WA. Rat models of skin wound healing: a review. Wound Repair Regen 2004;12:591–9. [DOI] [PubMed] [Google Scholar]
- 41. Jones JS, Gartner M, Drew G, Pack S. The shortland vertical mattress stitch: evaluation of a new suture technique. Am J Emerg Med 1993;11:483–5. [DOI] [PubMed] [Google Scholar]


