Abstract
Covering the reconstructed area with a healthy soft‐tissue envelope is a major challenge after limb‐sparing surgery in patients with malignant bone and soft‐tissue tumours. Negative pressure wound therapy (NPWT) of open wounds hastens healing and minimises the requirement for complex reconstructive soft‐tissue surgery. The aim of this study was to investigate the effectiveness and safety of NPWT in bone and soft‐tissue malignant tumour patients with postoperative wound complications. Between January 2006 and November 2009, at a single institution, 13 patients with malignant bone and soft‐tissue tumours who had undergone wide resection were retrospectively analysed. NPWT was performed in all patients to temporarily close the soft‐tissue defects. After obtaining the culture negativity and normal infection markers, definitive soft‐tissue reconstruction was performed to close the wound with primary suturisation in two patients, split thickness grafts in four patients, full thickness grafts in two patients, rotational flaps in three patients and free flaps in two patients. Mean duration of hospitalisation was 20 (range 8–48) days and mean follow‐up period was 57·3 (range 50–74) months. There was no tumour recurrence or skip metastasis in the follow‐up period. In addition, there was no periprosthetic infection or complication associated with NPWT. In conclusion, NPWT therapy seems to be a safe and effective option in the management of local wound problems and secondary surgical site infections after musculoskeletal tumour surgery.
Keywords: Musculoskeletal tumour, Negative pressure wound therapy, Soft‐tissue reconstruction, Vacuum‐assisted closure, Wound‐related complication
Introduction
One of the most important problems after limb salvage surgery in patients with bone and soft‐tissue malignant tumours is covering the reconstruction zone with a healthy soft‐tissue envelope 1. In such patients, surgical site infection is a devastating complication, which can cause periprosthetic infection and even loss of the extremity. Prolonged surgery, blood transfusion, extra‐compartmental resections, massive prosthetic or allograft reconstructions, adjuvant chemotherapy and radiotherapy, poor nutritional status and postoperative local wound problems such as skin necrosis at the wound edges markedly increase the risk of infection 2, 3.
Negative pressure wound therapy (NPWT) subjects the wound to continuous or intermittent sub‐atmospheric pressures
over special foams, which facilitates the removal of exudates or pus 4. The main principle is to reduce the edema and bacterial load while improving the local blood flow, angiogenesis, epithelisation and formation of granulation tissue to enable wound closure and to reduce the requirement of soft‐tissue reconstructive operations 5, 6, 7.
The use of NPWT after tumour surgery is controversial and assumed as contraindicated by many clinicians 8. The main doubt is about the angiogenic effect stimulating tumour seeding or recurrence. The aim of this study was to investigate the effectiveness and safety of NPWT in patients with bone and soft‐tissue malignant tumours who have postoperative wound complications or in whom primary closure is not possible.
Materials and methods
Between January 2006 and November 2009, at a single institution, 13 patients with wound‐related complications after limb salvage surgery on malignant bone and soft‐tissue tumours were included. Histologic diagnoses were osteosarcoma in five patients, giant cell tumour in three patients, chondrosarcoma in three patients and liposarcoma in two patients. The defects resulting from wide resections were reconstructed with either modular endoprosthesis or allograft/internal fixation combinations. A subfascial Hemovac drain was placed routinely. Extended antibiotic prophylaxis was administered with cefazolin until the drains were removed. NPWT (V.A.C. Therapy®, KCI USA, Inc, San Antonio, TX) was performed immediately in six patients and after local wound problems in seven patients. Initially, the negative pressure was set at 50 mmHg in continuous mode; when the exudation decreased markedly, it was changed to 75 mmHg in intermittent mode. The foam and dressings were changed every 48–72 hours in the operating room under sterile conditions. At every such instance, local debridement was performed and wound cultures were obtained. In addition, the infection markers [erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP)] were obtained on a weekly basis. Definitive skin closure was performed with either primary suturation or split‐/full‐thickness skin grafting.
Data regarding diagnosis, number of NPWT applications, clinical or laboratory evidence for infection, time of hospitalisation, follow‐up period, associated complications and tumour recurrence were recorded for each patient.
In the patients' follow‐up visits after their discharge from hospital, clinical signs of wound problems, infection markers, and presence of tumour recurrence or metastases were recorded.
Results
The patient demographics, diagnosis, tumour localisations, the number of NPWT applications and follow‐up period were summarised in Table 1. The average age of the patients included in the study was 37 (range 15–74) years. There were ten males and three females. Histological analyses revealed that wide resections were achieved with clear resection margins in all patients. NPWT was applied immediately in six patients who had undergone resections around the knee (distal femur and proximal tibia). We routinely performed medial gastrocnemius rotational flap to cover the endoprosthesis and skin defects were closed with NPWT if primary closure was impossible (Figure 1). In the remaining seven patients, NPWT was applied at an average of 6·5 days because of local wound problems such as necrosis, dehiscence and serous drainage. The average number of NPWT applications was five (range, three to nine). After the maturation of granulation tissue, skin defects were closed with either primary suturation 2, split‐thickness graft 4, full‐thickness graft 2 or rotational flaps 3. In two patients with proximal tibial reconstructions, a free latissimus dorsi flap was performed because the medial gastrocnemious flap and granulation tissue obtained by NPWT could not cover the entire reconstruction zone.
Table 1.
Detailed patient and tumour characteristics
| Patient | Age (years) | Gender | Diagnosis | Tumour localisation | Resection zone | Reconstruction | Initial application (days) | Number of NPWT application | Time of hospitalisation (days) | Time of F/U (months) | Definitive skin closure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | M | Osteosarcoma | Proximal femur | Wide | Modular prosthesis | 6 | 3 | 8 | 51 | Primary |
| 2 | 15 | M | Osteosarcoma | Proximal tibia | Wide | Modular prosthesis | Immediate | 4 | 17 | 59 | Free flap + ST graft |
| 3 | 66 | F | Liposarcoma | Gluteal region | Wide | — | 3 | 9 | 48 | 54 | Rotational flap |
| 4 | 35 | M | Giant cell tumour | Distal femur | Wide | Modular prosthesis | 5 | 7 | 26 | 62 | Primary |
| 5 | 15 | M | Osteosarcoma | Distal femur | Wide | Modular prosthesis | Immediate | 4 | 15 | 63 | Free flap + ST graft |
| 6 | 74 | M | Chondrosarcoma | Pelvis | Wide | Custom made prosthesis | 10 | 4 | 16 | 56 | Rotational flap |
| 7 | 23 | F | Osteosarcoma | Proximal tibia | Wide | Modular prosthesis | 4 | 7 | 24 | 53 | ST graft |
| 8 | 29 | M | Chondrosarcoma | Proximal tibia | Wide | Modular prosthesis | Immediate | 3 | 11 | 61 | FT graft |
| 9 | 48 | F | Giant cell tumour | Distal femur | Wide | Modular prosthesis | Immediate | 5 | 13 | 56 | ST graft |
| 10 | 54 | M | Liposarcoma | Proximal femur | Wide | — | 11 | 6 | 28 | 50 | FT graft |
| 11 | 18 | M | Osteosarcoma | Tibial diaphysis | Wide | Allograft + internal fixation | Immediate | 3 | 19 | 74 | ST graft |
| 12 | 34 | M | Giant cell tumour | Pelvis | Wide | Vascularised fibula | 7 | 8 | 25 | 52 | Rotational flap |
| 13 | 44 | M | Chondrosarcoma | Proximal tibia | Wide | Modular prosthesis | Immediate | 3 | 11 | 55 | ST graft |
F, female; FT, full thickness; M, male; NPWT, negative pressure wound therapy; ST, split thickness; F/U, follow‐up.
Figure 1.
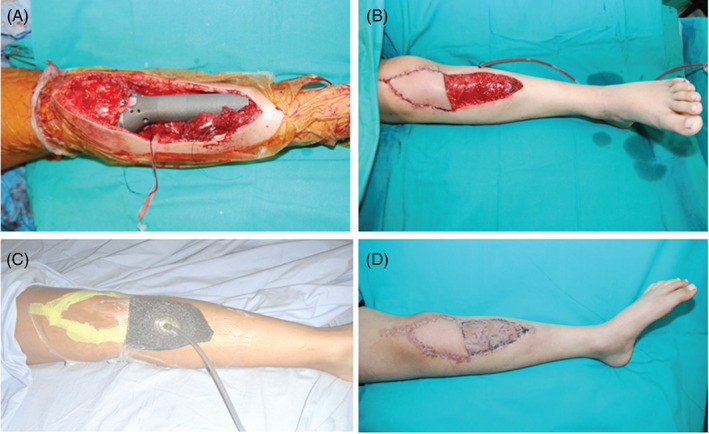
A 15‐year‐old male with osteosarcoma in the proximal tibia underwent wide resection and reconstruction with modular endoprosthesis (A). A medial gastrocnemius rotational flap was used to cover the tumour prosthesis and a free flap was placed over the proximal wound (B). NPWT was used for the distal wound, which could not be closed (C). After maturation of granulation tissue, definitive skin closure was performed using a split‐thickness skin graft (D).
Twelve patients healed successfully without any complications. A superficial wound infection developed in the gluteal region in one patient with liposarcoma. Methicillin‐resistant Staphylococcus aureus was detected in the patient's wound cultures. This patient was treated with serial debridement, NPWT application and parenteral antibiotics (vancomycin). There was no deep infection in any patient. The average duration of hospitalisation was 20 (range, 8–48) days. There was no local tumour recurrence or skip metastasis after an average 57·3 (range 50–74) months of follow‐up period.
Discussion
Wide resection of malignant bone and soft‐tissue tumours results in large tissue defects, which necessitate skeletal and soft‐tissue reconstruction to maintain the integrity of extremity functions. Generally, the closure of soft‐tissue defects after skeletal reconstruction is difficult and requires advanced soft‐tissue reconstruction procedures. The main problems after primary closure of such defects are wound dehiscence, necrosis of overlying soft tissues and infection 9, 10.
In wound healing, the surrounding healthy tissue repairs the defect with homeostasis mechanisms. Healing is promoted by several factors, such as healthy tissue perfusion and oxygenation, fine wound edges without necrosis, a dry wound bed without pus collection, regular dressings and good communication with the patient. NPWT facilitates wound healing by limiting seropurulent exudate collection, reducing bacterial load, promoting angiogenesis and local blood flow, improving granulation tissue and decreasing tissue edema 6. NPWT is being used extensively in several types of wounds. While most of the reports are case series and systematic reviews 11, 12, there are only a few randomised studies on the use of NPWT in diabetic foot, chronic ulcers and skin grafts 13, 14, 15. In orthopaedic practice, NPWT has been used to facilitate healing of surgical incisions and high‐energy wounds in the extremities 16, 17, 18.
The use of NPWT after resection of musculoskeletal tumours is controversial. The presence of malignancy in the area of NPWT application is accepted as a contraindication by many clinicians 8. The main issue is that angiogenic mechanisms and local growth factors may trigger tumour recurrence or dissemination. However, there is no evidence‐based knowledge supporting this argument. On the other hand, there are several studies demonstrating the effectiveness of NPWT in wound healing after resection of musculoskeletal tumours 9, 10, 19, 20, 21. In a clinical study, Sakellariou et al. reported shorter time of hospitalisation, lower complication rates, a relative reduction in infection rates and need for further surgery, and a lower total cost of wound‐healing treatment in comparison to conventional wound treatment methods 22. However, there is no information on tumour recurrence or dissemination in these studies. In this study, after 57 months of follow‐up period, no local recurrence, skip metastasis, tumoural dissemination or distant metastases were encountered. In addition, The advantages of NPWT with regard to hospitalisation times and reduction of the number and complexity of surgical interventions for soft‐tissue reconstructions were observed. We suggest that NPWT can be safely used in patients with musculoskeletal tumours, if there is confirmation of wide resection with clear resection margins on histologic evaluation.
Complications of NPWT include on‐going pain, hypertrophy of granulation tissue over the foam and skin, and damage to important adjacent vessels 20, 23. Fortunately, none of these complications were experienced in these series.
There were several limitations to this study such as the retrospective design and absence of a control group. In addition, there was a very limited study population included in the study. From a positive perspective, the follow‐up period of 57 months is considerable in a series of patients with malignant bone and soft‐tissue tumours.
Conclusions
The studies on NPWT in the treatment of soft‐tissue infections and the wound problems of non‐tumour patients have shown promising results. Primary closure of soft‐tissue defects and wound‐related problems after wide resection of bone and soft‐tissue tumours can be challenging and can increase treatment costs. In earlier studies, it was thought that NPWT is contraindicated in tumour surgery. We suggest that wound‐related problems after wide resection of musculoskeletal tumours can be safely and effectively managed with NPWT, after confirmation of the wide resection with clear resection margins. Nevertheless, future studies with a prospective design and adequate patient numbers are needed to verify these results.
In this study, all medical and surgical treatments were performed at Hacettepe University School of Medicine Hospitals.
References
- 1. Peat BG, Bell RS, Davis A, O'Sullivan B, Mahoney J, Manktelow RT, Bowen V, Catton C, Fornasier VL, Langer F. Wound healing complications after soft‐tissue sarcoma surgery. Plast Reconstr Surg 1994;93:980–7. [DOI] [PubMed] [Google Scholar]
- 2. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre‐operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol 2002;28:75–9. [DOI] [PubMed] [Google Scholar]
- 3. Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, Fletcher CD, Fletcher JA, Ladanyi M, Meltzer P, O'Sullivan B, Parkinson DR, Pisters PW, Saxman S, Singer S, Sundaram M, van Oosterom AT, Verweij J, Waalen J, Weiss SW, Brennan MF. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res 2003;9:1941–56. [PubMed] [Google Scholar]
- 4. Webb LX. New techniques in wound management: vacuum assisted wound closure. J Am Acad Orthop Surg 2002;10:303–11. [DOI] [PubMed] [Google Scholar]
- 5. Wongworawat MD, Schnall SB, Holtom PD, Moon C, Schiller F. Negative pressure dressings as an alternative technique for the treatment of infected wounds. Clin Orthop Relat Res 2003;414:45–8. [DOI] [PubMed] [Google Scholar]
- 6. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment. Animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 7. Kilpadi DV, Stechmiller JK, Childress B. Composition of wound fluid from pressure ulcers treated with negative pressure wound therapy using V.A.C. therapy in home health of extended care patients: a pilot study. Wounds 2006;18:119–26. [Google Scholar]
- 8. Wallis L. FDA warning about negative pressure wound therapy. Am J Nurs 2010;110:16. [Google Scholar]
- 9. Arbeit JM, Hilaris BS, Brennan MF. Wound complications in the multimodality treatment of extremity and superficial truncal sarcomas. J Clin Oncol 1987;5:480–8. [DOI] [PubMed] [Google Scholar]
- 10. Siegel HJ, Long JL, Watson KM, Fiveash JB. Vacuum‐assisted closure for radiation associated wound complications. J Surg Oncol 2007;96:575–82. [DOI] [PubMed] [Google Scholar]
- 11. Lambert KV, Hayes P, McCarthy M. Vacuum‐assisted closure: a review of development and current applications. Eur J Vasc Endovasc Surg 2005;29:219–26. [DOI] [PubMed] [Google Scholar]
- 12. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 13. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuumassisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37. [DOI] [PubMed] [Google Scholar]
- 14. Gupta S, Baharestani MM, Baranoski S, de Leon J, Engel SJ, Mendez‐Eastman S, Niezgoda JA, Pompeo MQ. Guidelines for managing pressure ulcers with negative pressure wound therapy. Adv Skin Wound Care 2004;17(2 Suppl):1–16. [DOI] [PubMed] [Google Scholar]
- 15. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 16. DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, Ward WG, Teasdall RG. The use of vacuum assisted closure therapy for the treatment of lower extremity wounds with exposed bone. Plast Reconstr Surg 2001;108:1184–91. [DOI] [PubMed] [Google Scholar]
- 17. Herscovici D Jr, Sanders RW, Scaduto JM, Infante A, DiPasquale T. Vacuum‐assisted wound closure (VAC therapy) for the management of patients with high‐energy soft tissue injuries. J Orthop Trauma 2003;17:683–8. [DOI] [PubMed] [Google Scholar]
- 18. Parrett BM, Matros E, Pribaz JJ, Orgill DP. Lower extremity trauma: trends in the management of soft‐tissue reconstruction of open tibia‐fibula fractures. Plast Reconstr Surg 2006;117:1315–22. [DOI] [PubMed] [Google Scholar]
- 19. Bickels J, Kollender Y, Wittig JC, Cohen N, Meller I, Malawer MM. Vacuum assisted closure after resection of musculoskeletal tumors. Clin Orthop Relat Res 2005;441:346–50. [DOI] [PubMed] [Google Scholar]
- 20. Senchenkov A, Petty PM, Knoetgen J 3rd, Moran SL, Johnson CH, Clay RP. Outcomes of skin graft reconstructions with the use of vacuum assisted closure (VAC(R)) dressing for irradiated extremity sarcoma defects. World J Surg Oncol 2007;29:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delman KA, Johnstone PA. Vacuum‐assisted closure for surgical wounds in sarcoma. J Surg Oncol 2007;96:545–6. [DOI] [PubMed] [Google Scholar]
- 22. Sakellariou VI, Mavrogenis AF, Papagelopoulos PJ. Negative pressure wound therapy for musculoskeletal tumor surgery. Adv Skin Wound Care 2011;24:25–30. [DOI] [PubMed] [Google Scholar]
- 23. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br 2000;82:959–66. [DOI] [PubMed] [Google Scholar]


