Abstract
The potential for adipose‐derived stem cells to differentiate into keratinocyte‐like cells has recently been receiving attention, stemming from the hypothesis that a bioengineered skin may be manufactured from these readily available mesenchymal stem cells. This study was conducted to evaluate the influence of human keratinocyte non‐contact coculture on hADSCs. Human epidermal keratinocytes and hADSCs obtained by lipoaspiration were cultured in keratinogenic growth media, which were divided into the following groups: human adipose‐derived stem cell (hADSC) monoculture, non‐contact coculture of hADSCs and human keratinocytes and keratinocyte monoculture. Cell proliferation was assessed, and keratogenicity was analysed through immunocytochemistry and polymerase chain reaction of early, intermediate and late keratogenic markers. hADSCs cocultured with keratinocytes displayed enhanced proliferation compared with the monoculture group. After a 7‐day coculture period, immunohistochemistry and polymerase chain reaction findings revealed the presence of specific keratinocyte markers in the coculture group. This study demonstrates that hADSCs cocultured with keratinocytes have the capacity to transdifferentiate into keratinocyte lineage cells, and suggests that adipose tissue may be a source of keratinocytes that may further be used in structuring the bioengineered skin.
Keywords: Adipose‐derived stem cells, Adult stem cells, Coculture, Differentiation, Keratinocytes
Introduction
Tissue engineering and regenerative medicine is a multidisciplinary science that has recently focused on the use of stem cells to repair or regenerate tissues and organs 1. Human mesenchymal stem cells (MSCs) possess multipotent differentiation capabilities and are a potent source of multiple paracrine factors. The best characterised human source of MSCs is the bone marrow 2. However, because obtaining bone marrow‐derived stem cells is an invasive procedure requiring either spinal or general anaesthesia, and the isolation yield is low, alternative reservoirs of MSCs have been extensively studied. Human adipose tissue, usually waste products of liposuction or abdominoplasty, therefore sparing deliberate donor injury, is a highly complex tissue that consists of mature adipocytes, preadipocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, resident macrophages and lymphocytes, and can deliver human adipose‐derived stem cells (hADSCs) 3.
Studies on bone marrow‐derived MSCs have demonstrated their differentiation into adipocytes, hepatocytes, chondrocytes, neuronal cells, myoblasts, osteoblasts and keratinocytes 4, 5. The mechanism of each type of conversion is suspected to involve direct cell to cell interaction, and paracrine‐regulated soluble factors, but is incompletely understood. Epidermal keratinocytes, a major player in wound healing, have been shown to direct bone marrow‐derived MSCs into epithelial, myofibroblast or neural morphology through direct cell to cell contact or non‐contact cocultures 6. Although a less invasive source of stem cells, the differentiation of human adipose tissue‐derived stem cells into epithelial cell types has not been studied as extensively. This study investigates the effect of human epidermal keratinocytes on the growth and differentiation of ADSCs.
Materials and methods
Ethical statement
This study was approved by the Institutional Review Board of the Catholic University of Korea. All human tissue samples were obtained with written informed consent from the patients. All data were analysed anonymously and according to the principles in the Declaration of Helsinki 1975 (revised in 2008).
Isolation of human keratinocytes and cell culture
Human keratinocytes were obtained from the remnant tissue of split‐thickness skin graft donor sites of otherwise healthy patients who had skin defects due to trauma. Split‐thickness skin of 0·008 in. thickness that included all of the epidermis and part of the dermis was harvested from the thigh. This tissue was incubated for 2 hours in dispase II (1·25 U/ml, Roche, Switzerland) in 5% CO2 at 37°C in order to separate the epidermis from the dermal components. After separation, the epidermis was finely chopped, incubated in 1× trypsin‐EDTA (Gibco‐BRL, Grand Island, NY) at 5% CO2, 37°C for 15 minutes. Foetal bovine serum (FBS, Gibco‐BRL) was added, and the mixture was vortexed for 10 minutes at room temperature (22°C). After filtering through a 0·2‐µm filter, the suspension was centrifuged at 2094 g for 5 minutes. The supernatant was removed, and the remaining pellet portion was dispersed in CnT‐57 culture medium. The cells were transfered to six‐well Transwell coculture plates at a concentration of 500 000 cells/well on CnT‐57 (CELLnTEC, Bern, Switzerland) keratinogenic media, and this culture media was changed every 3 days. CnT‐57 is a serum‐free kerationcyte progenitor cell‐targeted culture media with low calcium concentration (0·07 mM) and low bovine pituitary extract (BPE, 6 µg/ml). It consists of a protein‐free basal medium that contains amino acids, minerals, vitamins and organic compounds, and four supplements, one of these being BPE.
Isolation of hADSCs and cell culture
Human adipose tissue, in the form of lipoaspirated fat, was obtained from otherwise healthy patients. The tissue was washed in phosphate buffered saline (PBS; Wisent Inc., St. Bruno, Quebec, Canada), and digested with 0·1% collagenase type I (Sigma, St. Louis, MO) for 30 minutes in a 37°C Celltibator (Medi‐Kahn, Seoul, Republic of Korea). The digested sample was washed twice in Dulbecco's Modified Eagles Medium (DMEM) solution containing 10% FBS and 1% antibiotic/antimycotic, then centrifuged at 2094 g for 3 minutes. The stromal vascular fraction (SVF) was sifted through a 100 µm strainer (BD, San Jose, CA) and centrifuged at 2094 g for 3 minutes. The pellets were then resuspended in cell media, cell counts were performed and cells were dispersed at a concentration of 100 000 cells/well on a six‐well Transwell coculture plate. The plate media was a solution of DMEM (Gibco‐BRL) containing 10% FBS and 1% antibiotic/antimycotic solution (Gibco‐BRL). The cells were cultured at 5% CO2, at a temperature of 37°C. The media was changed every 3 days. Sub‐cultures were initiated when the initial cell cultures (P0) reached 80–90% confluence. The hADSCs obtained for the study were from passage 3 (P3).
Culture groups
Culture groups are depicted in Figure 1. One group of passage 3 hADSCs was transfered to the CnT‐57 media as a keratinocyte negative control. This group was designed to evaluate the effect of keratinogenic media itself on hADSC growth and transdifferentiation and was designated as group 1. A group of human keratinocytes cultured only in CnT‐57 media was designated as group 3. For group 2, the coculture group of keratinocytes and hADSCs, the human keratinocytes cultured in the six‐well plates (insert wells) were grown to 90% confluence, and then inserted into the Transwell coculture system (Transwell®, Corning, NY) with the passage 3 hADSCs which had grown to 70–80% confluence. The medium in all the groups was changed every 3 days.
Figure 1.
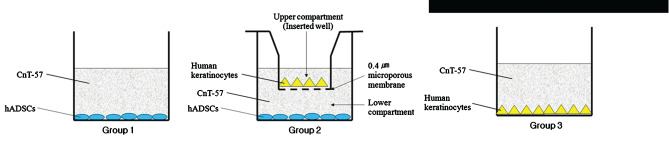
Schematic representation of the culture groups. Group 2 is a Transwell coculture system. CnT‐57, CnT‐57 keratinogenic media; hADSC, human adipose‐derived stem cells.
Analysis of cell proliferation
In order to evaluate the growth rate of the cells, the number of cells was observed on day 1, day 3 and day 7 of coculture using the CCK‐8 kit (Cell Count Kit‐8; Dojindo, Santa Clara, CA) as described previously. The CCK‐8 kit solution was mixed with DMEM at a ratio of 1:9, and after the removal of the inset wells, 400 µl was added to each well. The wells were incubated for 2 hours under 5% CO2 at 37°C, and 100 µl of supernatant was transferred to a 96‐well plate. The plates were analysed under 450 mm wavelength using a microplate reader (SoftMax Pro5; Molecular Devices, Sunnyvale, CA).
Analysis of cell differentiation
Immunocytochemistry
Immunocytochemistry (ICC) to assess expression of differentiation markers was performed on day 7 after the coculture was initiated. The insert wells were removed from the six‐well plates, washed with PBS and fixed in 2% paraformaldehyde for 10 minutes. The samples were washed in PBS three more times, blocked for 60 minutes in 0·5% normal goat serum (S‐1000, Vector, Burlington, Ontario, Canada), then washed again in PBS three times each for 5 minutes. Primary antibodies outlined in Table 1 were diluted in PBS at 1 µg/ml. Antibodies for progressive stages of keratinocyte differentiation were chosen. Cytokeratin 10 and involucrin antibodies were mouse monoclonal immunoglobulin (Ig) G antibodies, and filaggrin antibodies were rabbit polyclonal IgG antibodies. Primary antibodies against cytokeratin 10, involucrin and filaggrin were applied onto the plates, incubated for 24 hours at 4°C and then washed three times in PBS for 5 minutes each. Goat anti‐rabbit Chromeo 488 (green) secondary antibodies were diluted at 1:1000 in PBS, goat anti‐mouse Chromeo 642 (red) secondary antibodies were diluted at 1:1000 in PBS and incubated for 60 minutes at room temperature. The plates were washed three times in PBS, and 4′,6‐diamidino‐2‐phenylindole (DAPI) (Invitrogen, Carlsbad, CA) was added, which provided counterstaining for the nuclei. After washing three times with PBS, the wells were mounted with PBS, and were visualised with a fluorescent microscope (Axiovert 200, Zeiss, Oberkochen, Germany).
Table 1.
Primary antibodies
| Antibody | Manufacturer | Species | Dilution |
|---|---|---|---|
| Filaggrin | Abcam, Inc. (Cambridge, MA) | Rabbit polyclonal | 1 µg/ml |
| Cytokeratin 10 | Abcam, Inc. (Cambridge, MA) | Rabbit polyclonal | 1 µg/ml |
| Involucrin | Abcam, Inc. (Cambridge, MA) | Mouse monoclonal | 1 µg/ml |
| Anti‐rabbit | Abcam, Inc. (Cambridge, MA) | Chromeo 488 | 1/1000 |
| Anti‐mouse | Abcam, Inc. (Cambridge, MA) | Chromeo 642 | 1/1000 |
Reverse transcriptase polymerase chain reaction
Reverse transcriptase polymerase chain reaction (RT‐PCR) of the differentiation markers was performed on day 7 after coculture. Total ribonucleic acid (RNA) was extracted from the samples using the Alphagene RNA mini kit (Alphagene Co. Ltd, Seongnam‐si, Republic of Korea). One microgram of total RNA was converted to complementary deoxyribonucleic acid (cDNA) using 200 U M‐MLV reverse transcriptase (Invitrogen). For the amplification process, sense and anti‐sense primers depicted in Table 2 were used on an AB Prism 7000 PCR System (Applied Biosystems, Foster City, CA). Each assay was performed in triplicate.
Table 2.
Sense and anti‐sense primers used for reverse transcriptase polymerase chain reaction (RT‐PCR)
| Species | Marker | Sense primer sequence | Anti‐sense primer sequence |
|---|---|---|---|
| Human | Keratin 5 | CAGAGCCACCTTCTGCGTCCTG | GCTGAAGCTACGACTGCCCCC |
| Human | Involucrin | TCCTCCAGTCAATACCCATCAG | CAGCAGTCATGTGCTTTTCCT |
| Human | Filaggrin | TGAAGCCTATGACACCACTGA | TCCCCTACGCTTTCTTGTCCT |
Statistical analysis
Statistical analysis of differences in each experimental group was carried out using the Student's t‐test, with significance set at P < 0·05.
Results
Characterisation of stem cells
Approximately 30 g of human fat tissue was processed according to the protocol described above. The cells took a spindle‐like shape throughout the culture period, with a size of approximately 80–100 µm × 200 µm. They were positive for CD90, CD105, CD13 and CD73 and negative for CD34, CD45, CD146 and HLA‐DR. In adipogenic conditions, the isolated cells expressed oil droplets and stained positive for Oil Red O (Figure 2).
Figure 2.
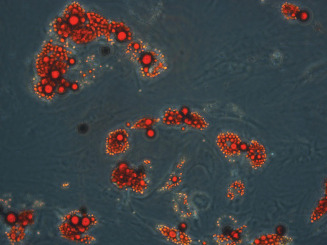
Isolation and characterisation of human adipose‐derived stem cells (hADSC). ADSCs were cultured in adipogenic conditions for 2 weeks, then stained with Oil Red O, showing positive uptake in the cell microdroplets (×200).
hADSC proliferation
As shown in Figure 3, an increase in hADSC proliferation was observed in the group cocultured with human keratinocytes (group 2). This increase was significant compared to the hADSC monoculture group (group 1), which demonstrated decrease in cell count (P = 0·0003 at day 1, P < 0·0001 at day 7). Culture and cell count was performed in triplicate.
Figure 3.
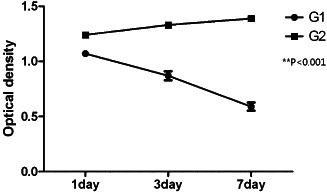
Human adipose‐derived stem cell (hADSC) proliferation is maintained in the cocultured group. Cell count at days 1, 3 and 7 after coculture initiation is shown (G1, group 1; G2, group 2).
hADSC expression of keratinocyte markers
After 7 days of growth, each group of cells was evaluated for progressive keratinocyte markers. Antibodies for cytokeratin 10 (early marker), involucrin (early terminal marker) and filaggrin (terminal marker) were applied for ICC staining. hADSCs cultured in DMEM and otherwise indentical conditions were also stained to compare the effects of keratinogenic media. RT‐PCR was performed for keratin 5, involucrin and filaggrin.
As shown in Figure 4, hADSCs cultured alone, whether in DMEM or CnT‐57, did not express cytokeratin 10, whereas hADSCs in the cocultured group stained positive. Involucrin and filaggrin were found to stain positive in the cocultured group, and the hADSCs cultured in CnT‐57.
Figure 4.
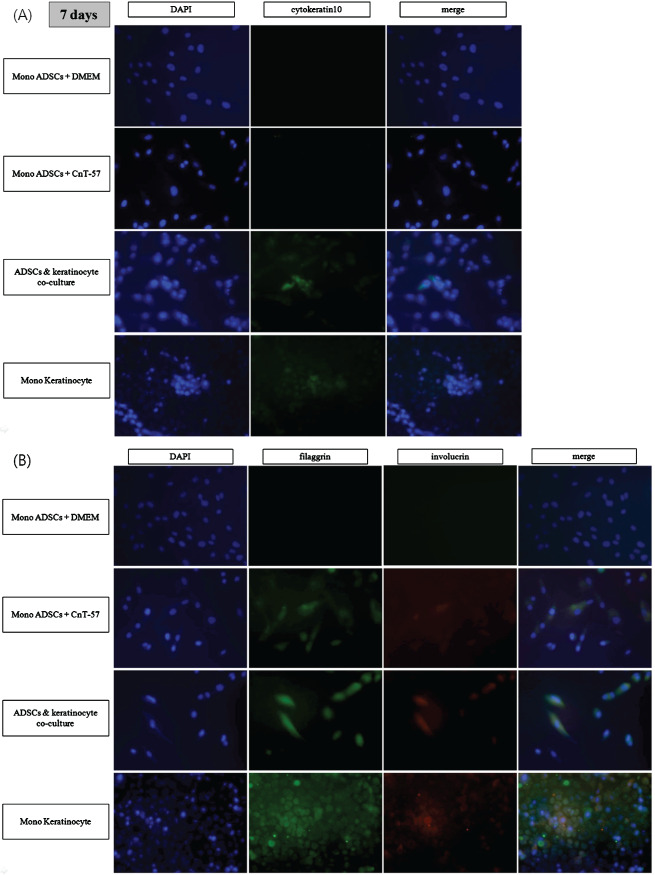
Immunocytochemistry (ICC) staining of human adipose‐derived stem cells (hADSCs) in all groups for cytokeratin 10 (A), involucrin and filaggrin (B). hADSCs express keratinocyte markers by day 7 (×100). ADSCs seeded in Transwell plates and cocultured with keratinocytes in keratinocyte growth medium (CnT‐57) were fixed and stained on day 7 for immunocytochemistry (ICC) analysis. 4′,6‐diamidino‐2‐phenylindole (DAPI) was used for counterstaining. These images are representative of triplicate independent experiments. (A) Staining for cytokeratin 10 is positive in the cocultured group and the keratinocyte‐only group. No staining is seen in ADSC‐only group, whether the media is Dulbecco's Modified Eagles Medium (DMEM) or CnT‐57. (B) Staining for involucrin and filaggrin is slightly positive in the ADSC‐only group cultured in keratinogenic media (CnT‐57), with more uptake in the hADSC and keratinocyte coculture group.
hADSCs cocultured with keratinocytes showed increased expression of keratin 5, involucrin and filaggrin at the mRNA level when compared with the hADSC‐only group, as shown in Figure 5. Expression of keratin 5 was 0·807 ± 0·377 in group 2 and 0·508 ± 0·169 in group 1 (P = 0·139). Expression of involucrin was 0·621 ± 0·048 in group 2 and 0·242 ± 0·028 in group 1 (P < 0·001), and expression of filaggrin was 1·133 ± 0·068 in group 2 and 0·322 ± 0·161 in group 1 (P < 0·001).
Figure 5.
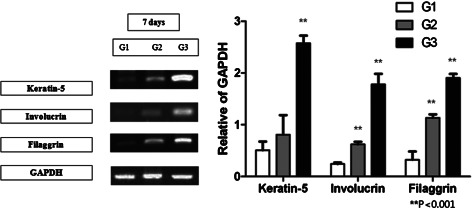
RT‐PCR for all groups for keratin 5, involucrin and filaggrin. Human adipose‐derived stem cells (hADSCs) express keratinocyte markers by day 7. Expression of all the three markers was increased in the coculture group compared with the ASC‐only group, and statistically significant for involucrin and filaggrin [keratin 5 – 0·807 ± 0·377 in group 2 versus 0·508 ± 0·169 in group 1 (P = 0·139); involucrin – 0·621 ± 0·048 in group 2 versus 0·242 ± 0·028 in group 1 (P < 0·001); filaggrin – 1·133 ± 0·068 in group 2 versus 0·322 ± 0·161 in group 1 (P < 0·001)].
Discussion
Human MSCs have the potential to differentiate into a variety of different cell types. The factors that decide their outcome are the subject of multiple studies. The microenvironment seems to play a key role in inducing differentiation, and the exact mechanisms controlling each process are being elucidated 6, 7. Although most differentiation is to tissue of the same mesenchymal origin, ‘transdifferentiation’ to ectodermal or endodermal lineages have also been induced. Previous reports have stated that transdifferentiation is initiated by changes in the cell microenvironment, with a coculture method or a conditioned media containing differentiation factors 8. Studies have demonstrated epithelial transdifferentiation in human bone marrow‐derived MSCs cocultured with keratinocytes, and Sivamani et al. found that keratinocyte proximity and contact played a major role in determining the outcome of bone marrow‐derived stem cells 6, 9, 10, 11, 12.
However, focus has recently migrated to hADSCs that cause less donor morbidity and have a much higher yield. Studies have shown that an average of 5 × 103 stem cells can be isolated from 1 g of adipose tissue, which is 500 times more cells than from an equivalent amount of bone marrow 13. hADSCs are known to differentiate into many mesenchymal tissue types, but they are also capable of neurogenic (ectoderm), hepatic or endothelial (endoderm) transdifferentiation 14. In 2013, Chavez‐Munoz et al. reported transdifferentiation of hADSCs to keratinocyte‐like cells, and also engineered a stratified epidermis 15.
This study was based on the hypothesis that hADSCs are capable of transdifferentiating into the keratinocyte lineage when cocultured with keratinocytes. Results show that hADSCs maintain steady increase in cell count when cocultured with keratinocytes, whereas cells in the hADSC‐only group decreased with time. This difference is probably an effect of a keratinocyte paracrine factor that helps sustain hADSC survival in the serum‐free keratinocyte growth media. Identification of this factor requires further investigation. We have demonstrated that hADSC has the capacity to transdifferentiate into the keratinocyte lineage using a coculture system, expressing specific keratinocyte markers. Cytokeratin 10, expressed in all suprabasal layers, and keratin 5, expressed from the basal layer, are early markers 16. Filaggrin is an intermediate marker, expressed by well‐differentiated keratinised cells and is essential for the regulation of epidermal homeostasis. Involucrin is expressed in the early stages of terminal differentiation synthesised by keratinising epithelia 17. After a culture period of 7 days, the cocultured hADSCs showed the presence of all these markers, demonstrating transdifferentiation potential. Quantification of the expression levels of each marker at different time periods is yet to be studied. One thing to note is that the hADSCs cultured in the keratinocyte growth medium CnT‐57 were also positive for filaggrin and involucrin, although at a lower level in both protein and mRNA. CnT‐57, a progenitor cell‐targeted serum‐free media consists of a protein‐free basal medium, BPE, and three supplements. It is designed for optimal growth and proliferation of keratinocytes, and is not a differentiation media. However, the supplements contain growth factors including fibroblast growth factor (FGF)‐1 and endothelial growth factor (EGF), which may have induced a low expression of such keratinocyte markers. Analysis of these ADSCs to see whether they also expressed markers of different lineages was not performed.
The keratinocyte is only one of many cell types that populate the skin, other resident cell types being endothelial cells, melanocytes and fibroblasts. This cell is the main player in wound healing, and autografts or allografts of cultured keratinocytes have been used to accelerate epithelial coverage in the treatment of burn patients 18. Transdifferentiation of hADSCs into the keratinocyte lineage may be the first step in building a bioengineered skin from a readily accessible stem cell source, and opens the potential for a tissue engineered solution for extensive wound coverage.
Acknowledgements
This research was supported by a Seoul R&BD Program by the Seoul Government of Korea (No. SS110011C0211601) and also by a grant from the Korea Food and Drug Administration (No. 10172KFDA993). This study was approved by the Institutional Review Board of the Catholic University of Korea (KCMC06BR067).
References
- 1. Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng 2000;122:570–5. [DOI] [PubMed] [Google Scholar]
- 2. Al Battah F, De Kock J, Ramboer E, Heymans A, Vanhaecke T, Rogiers V, Snykers S. Evaluation of the multipotent character of human adipose tissue‐derived stem cells isolated by Ficoll gradient centrifugation and red blood cell lysis treatment. Toxicol In Vitro 2011;25:1224–30. [DOI] [PubMed] [Google Scholar]
- 3. Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori H, Hanaoka J, Iwahashi S, Saito Y, Iwaguro H. Human adipose‐derived stem cells: potential clinical applications in surgery. Surg Today 2011;41:18–23. [DOI] [PubMed] [Google Scholar]
- 4. Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 2004;6:532–9. [DOI] [PubMed] [Google Scholar]
- 5. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 2004;113:1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sivamani RK, Schwartz MP, Anseth KS, Isseroff RR. Keratinocyte proximity and contact can play a significant role in determining mesenchymal stem cell fate in human tissue. FASEB J 2011;25:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two‐stage hypothesis for regulation of MSC fate. Sci STKE 2005;294:pe37. [DOI] [PubMed] [Google Scholar]
- 8. Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong BF, Hu K, Cui FZ, Wang Y, Zhang X. Transdifferentiation of human adipose‐derived stem cells into urothelial cells: potential for urinary tract tissue engineering. Cell Tissue Res 2012;347:737–46. [DOI] [PubMed] [Google Scholar]
- 9. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–7. [DOI] [PubMed] [Google Scholar]
- 10. Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 2003;100:2397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dai Y, Li J, Li J, Dai G, Mu H, Wu Q, Hu K, Cao Q. Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns 2007;33:418–28. [DOI] [PubMed] [Google Scholar]
- 12. Chun‐mao H, Su‐yi W, Ping‐ping L, Hang‐hui C. Human bone marrow‐derived mesenchymal stem cells differentiate into epidermal‐like cells in vitro. Differentiation 2007;75:292–8. [DOI] [PubMed] [Google Scholar]
- 13. Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 2013;15:434–48. [DOI] [PubMed] [Google Scholar]
- 14. Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 2014;163:399–408. [DOI] [PubMed] [Google Scholar]
- 15. Chavez‐Munoz C, Nguyen KT, Xu W, Hong SJ, Mustoe TA, Galiano RD. Transdifferentiation of adipose‐derived stem cells into keratinocyte‐like cells: engineering a stratified epidermis. PLoS One 2013;8:e80587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leigh IM, Purkis PE, Whitehead P, Lane EB. Monospecific monoclonal antibodies to keratin 1 carboxy terminal (synthetic peptide) and to keratin 10 as markers of epidermal differentiation. Br J Dermatol 1993;129:110–9. [DOI] [PubMed] [Google Scholar]
- 17. Watt FM. Involucrin and other markers of keratinocyte terminal differentiation. J Invest Dermatol 1983;81:100s–3s. [DOI] [PubMed] [Google Scholar]
- 18. Auxenfans C, Shipkov H, Bach C, Catherine Z, Lacroix P, Bertin‐Maghit M, Damour O, Braye F. Cultured allogenic keratinocytes for extensive burns: a retrospective study over 15 years. Burns 2014;40:82–8. [DOI] [PubMed] [Google Scholar]


