Abstract
Chronic wounds require extensive healing time and place patients at risk of infection and amputation. Recently, a fresh hypothermically stored amniotic membrane (HSAM) was developed and has subsequently shown promise in its ability to effectively heal chronic wounds. The purpose of this study is to investigate the mechanisms of action that contribute to wound‐healing responses observed with HSAM. A proteomic analysis was conducted on HSAM, measuring 25 growth factors specific to wound healing within the grafts. The rate of release of these cytokines from HSAMs was also measured. To model the effect of these cytokines and their role in wound healing, proliferation and migration assays with human fibroblasts and keratinocytes were conducted, along with tube formation assays measuring angiogenesis using media conditioned from HSAM. Additionally, the cell–matrix interactions between fibroblasts and HSAM were investigated. Conditioned media from HSAM significantly increased both fibroblast and keratinocyte proliferation and migration and induced more robust tube formation in angiogenesis assays. Fibroblasts cultured on HSAMs were found to migrate into and deposit matrix molecules within the HSAM graft. These collective results suggest that HSAM positively affects various critical pathways in chronic wound healing, lending further support to promising qualitative results seen clinically and providing further validation for ongoing clinical trials.
Keywords: Amnion, Chronic wound healing, Chronic wounds, Hypothermically stored amniotic membrane, Regenerative healing
Introduction
Diabetic foot ulcers (DFUs) are a major comorbidity of diabetes, often resulting from poorly controlled hyperglycaemia. There are 29·1 million type II diabetics in the USA (9·3% of the total population) 1, and with DFUs at a lifetime incidence rate of 15–25% 2, this complication accounts for health care costs in excess of 9–13 billion dollars annually 3, 4. In many cases, DFUs lead to amputations, which are associated with mortality rates between 30% and 50% at 1 year 5. DFUs are not only difficult to close, owing to the complex diabetic wound environment, but they are also expensive to treat, with the cost of treating a DFU estimated to run in excess of $18 000 per case 6. Improving healing rates and tissue quality of regenerated tissue in patients with these chronic wounds would not only improve patient quality of life but also reduce the overall burden to the health care system.
Under normal conditions, wound healing proceeds through four specifically timed phases: haemostasis, inflammation, repair and remodelling 7. Chronic wounds develop when the healing process becomes stalled, most often in the inflammatory stage, and fails to progress though the subsequent phases of healing 8. Chronic wounds are characterised by a prolonged expression of high levels of pro‐inflammatory cytokines, including tissue necrosis factor‐alpha (TNF‐α), interleukin‐6 (IL‐6) and interleukin‐1 beta (IL‐1β). This imbalance in inflammatory cytokines then leads to a prolonged recruitment period for macrophages, neutrophils and mast cells at the wound site. These cells, in turn, produce high levels of reactive oxidative species (ROS) and proteases.
Of the proteases produced within a chronic wound, matrix metalloproteases (MMPs) are of interest because of their multifunctional role. MMPs are an integral part of the normal wound‐healing process, serving to function in several capacities including eliminating damaged proteins, destroying the provisional extracellular matrix (ECM) and facilitating cell migration and tissue remodelling 9. However, in the chronic wound environment, the influx of cells and the increase in pro‐inflammatory cytokines leads to elevated and prolonged expression of MMPs and down‐regulated expression of MMP inhibitors [tissue inhibitors of matrix metalloproteinases (TIMPs)]. Many studies have shown that levels of MMPs are higher in the exudates of chronic wounds than in those of acute wounds, pointing to a potential mechanism underlying the chronic nature of DFUs 10. In a study by Muller et al. 9 evaluating MMPs longitudinally in DFUs, it was found that despite similar initial levels of MMPs, good healers were characterised by a spike in MMP‐1 at 2 weeks and a marked decrease in MMPs by week 4 (compared to no significant changes in MMP levels over time in poor healers). These studies point to the careful regulation of these proteases as an essential component to the successful healing of chronic wounds as increased levels of proteases collectively cause an excessive break down of ECM, growth factors and growth factor‐specific receptors 8, 11.
A key consequence of the destruction of growth factors and their receptors is a drop in the mitogenic activity of cells and a decrease in the recruitment of additional cells to the wound bed 12, 13. Fibroblasts, which are known to be the building blocks of dermal tissue, are one example of cells affected by the chronic wound environment 14. Fibroblasts from diabetic patients have been shown to have a decreased responsiveness to growth factors 15, resulting in a lower proliferative response and deficiency in reorganising the ECM. The chronic wound environment also detrimentally affects keratinocytes. Comparisons of keratinocytes obtained from healthy skin and DFUs have indicated that there are significant changes in many of the signalling pathways associated with apoptosis, migration and proliferation 16. Keratinocytes analysed along the margins of DFUs have been found to be hyper‐proliferative and lacking in their ability to migrate and completely differentiate 17, 18, 19.
The cellular processes involved in effective wound healing require a significant amount of oxygen, and this oxygen is received through normal blood flow. In many chronic wounds, the oxygen levels needed to proceed with efficient wound healing are unavailable because of underlying ischaemic conditions. Ischaemic conditions are often a consequence of diabetic vascular complications that can affect the blood flow to the wound site 20 and cause deficiencies in blood vessel formation in peripheral tissues 21. Another consequence of the proteolytic environment within chronic wounds is the inhibition of angiogenesis. This occurs through the destruction of ECM proteins and cell adhesion molecules necessary for blood vessel formation and the destruction of angiogenic growth factors 22, consequently affecting the delivery of oxygen to the wound microenvironment, impeding normal healing 23.
Clinically, there are many types of advanced dressings and biological products used to treat difficult‐to‐heal DFUs. One category that has grown in utilisation recently is cellular tissue products, specifically those derived from placental tissues. These membranes in their native configuration serve to surround and protect the foetus; placental‐derived grafts consist of one or more layers of amnion and/or chorion. With consent from the mother, these membranes are collected post‐delivery of the full‐term, healthy baby. These placental‐derived allografts are especially suited for chronic wound healing because they are known to naturally address many of the contributing factors of chronic wound development, including suppressing dysregulated/uncontrolled inflammatory responses, increasing levels of MMP inhibitors in the wound environment, stimulating proliferation and migration of important cell types and promoting angiogenesis 24, 25, 26. Current processing technologies focus on dehydration or cryopreservation of placental‐derived membranes for clinical use; the goal of this paper is to evaluate wound‐healing responses elicited using a novel hypothermically stored amniotic membrane (HSAM).
Methods
HSAM (Affinity®, NuTech Medical®, Birmingham, AL) is a hypothermically stored allograft composed of amniotic membrane derived from human placenta. Placentas were donated with informed consent after planned caesarean sections, and all processing was completed in accordance with the Food and Drug Administration's (FDA) Good Tissue Practices and the American Association of Tissue Banks' standards. All donors were tested to check if they were free of infectious diseases, including human immunodeficiency virus, human T‐lymphotropic virus I/II, hepatitis B and C and syphilis. HSAM is aseptically processed and stored in a proprietary hypothermic storage solution using the Allofresh™ process.
In order to determine the growth factor content present within HSAMs, multiple 1 cm2 samples from nine donors were assessed for a variety of cytokines and growth factors by utilising a quantitative multiplex enzyme‐linked immunosorbent assay (ELISA) proteomics microarray (RayBiotech Inc., Norcross, GA). Growth factors evaluated in this study are thought to be relevant to wound healing and have previously been identified within placental‐derived tissue 25, 26. For the purposes of this evaluation, cytokines have been categorised into general functional areas (detailed in Table 1).
Table 1.
Relevant cytokine categories for wound healing
| Angiogenic |
| Acidic fibroblast growth factor (aFGF) |
| Basic fibroblast growth factor (bFGF) |
| Vascular endothelial growth factor (VEGF) |
| Endocrine gland‐derived vascular endothelial growth factor (EG‐VEGF) |
| Vascular endothelial growth factor D (VEGF‐D) |
| Platelet‐derived growth factor BB (PDGF‐BB) |
| Angiopoietin (ANG) |
| Thrombospondin 1 (TSP‐1) |
| Angiopoietin‐2 (ANG‐2) |
| Placental growth factor (PlGF) |
| Angiopoietin‐like 4 (APL4) |
| Regenerative |
| Epidermal growth factor (EGF) |
| Hepatocyte growth factor (HGF) |
| Galectin‐7 (GAL) |
| Insulin‐like growth factor‐1 (IGF‐I) |
| Insulin‐like growth factor‐2 (IGF‐II) |
| Transforming growth factor beta 1 (TGF‐β1) |
| Transforming growth factor beta 3 (TGF‐β3) |
| Insulin‐like growth factor‐binding protein 1 (IGFBP‐1) |
| Insulin‐like growth factor‐binding protein 5 (IGFBP‐5) |
| Anti‐inflammatory |
| Transforming growth factor alpha (TGF‐α) |
| Tissue Inhibitor of metalloproteinase 1 (TIMP‐1) |
| Tissue Inhibitor of metalloproteinase 2 (TIMP‐2) |
| Interleukin 1 receptor antagonist (IL‐1ra) |
| Interleukin 10 (IL‐10) |
For the proteomics array, HSAM grafts were selected from nine donors unless otherwise specified. Grafts were washed in saline and then homogenised using a Retsch cryomill (Verder Scientific Inc., Newtown, PA). After cryomilling, the tissue was incubated overnight in total protein extraction buffer with a protease inhibitor cocktail (EMD Millipore, Billerica, MA) at 4°C with agitation. Following overnight incubation, the supernatant was removed and loaded into the microarray chambers as per the manufacturer's instructions. The slides were imaged using a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale, CA), and scanned images were imported and analysed using GenePix Pro 7 software (Molecular Devices, Sunnyvale, CA).
To investigate the release of growth factors from HSAM grafts over time, samples were cut into 1·5 × 1·5 cm2 sections and placed in a 48‐well plate in a serum‐free medium. The HSAM samples were cultured under standard conditions for 7 days with gentle rocking. At 4, 12, 24, 48, 72, 96, 120 and 168 hours, the supernatant was collected, sterile filtered and stored at −80°C. At the end of the experiment, cultured HSAM grafts were collected and also stored at −80°C. Culture media and HSAM grafts were then evaluated using a proteomics array using methods described above.
To evaluate the effect of HSAM on cell proliferation, commercially available adult human fibroblasts or keratinocytes (Lonza, Walkersville, MD) were cultured in the presence of assay media conditioned with HSAM. Conditioned media (CM) was obtained by incubating HSAM in assay media for 5 days at 4°C at a concentration of 1 cm2 HSAM membrane per millilitre (ml) of assay media. Following incubation, assay media was filter sterilised prior to use. Media was stored at 4°C for up to 14 days. Cells were then seeded into 48‐well plates at a concentration of 3300 and 10 000 cells per well for fibroblasts and keratinocytes, respectively. CM was added to assay media at concentrations of 50%, 25% and 10% (v/v) at 1, 4, 7 and 10 days of culture immediately following evaluation with AlamarBlue. AlamarBlue assays were conducted according to manufacturer's instructions (Invitrogen, Carlsbad, CA) by incubating cells in 350 µl of a 10% AlamarBlue working solution in Dulbecco's Modified Eagle Medium with 2·5% fetal bovine serum (FBS) for 4 hours under standard culture conditions. Following incubation, fluorescence was measured in a plate reader from 100 µl samples using a fluorescence excitation wavelength of 540–570 nm and fluorescence emission wavelength of 580–610 nm.
Cell migration assays were conducted with adult human dermal fibroblasts, keratinocytes and human umbilical cord endothelial cells (HUVECs) using a standard Boyden chamber assay 27. Three lots of fibroblasts were used, one from ZenBio (ZenBio Inc., Research Triangle Park, NC) and two from Lonza (Lonza, Walkersville, MD). HUVECs were purchased from Lonza and Life Technologies (Life Technologies, Carlsbad, CA). Keratinocytes were purchased from Lonza. Prior to the start of the assay, cells at less than or equal to 80% confluence were serum‐starved overnight. Reservoirs were loaded with assay media alone (negative control), assay media + 10% FBS (positive control) or CM from HSAM at concentrations of 50%, 25% and 10% (v/v). Prior to adding cells to the migration chambers, both the cell inserts and reservoirs were coated with 5 µg/ml Fibronectin (ThermoFisher, Waltham, MA) overnight at room temperature to promote initial cell attachment. Inserts and reservoirs were then washed thrice with PBS. Next, cells were trypsinised and added to the top of the inserts at a concentration of 10 000 (for fibroblasts) or 20 000 (for HUVECs and keratinocytes) cells per insert and then incubated for 24 hours to allow for migration. Non‐migrating cells remaining at the top of the cell inserts were removed with a cotton tip applicator, and cells that had migrated to the bottom of the insert were fixed with a 4% solution of neutral buffered formalin in PBS for 10 minutes prior to staining with a crystal violet solution. Images of the inserts were taken with an inverted microscope (Nikon Eclipse Ti, Tokyo, Japan), and representative images were used to count the number of migrated cells.
Tube formation assays were conducted using the angiogenesis starter kit (Life Technologies) as per the manufacturer's instructions. Two lots of HUVECs (passages 3–5) were used in these experiments (Life Technologies) 28, 29. HUVECs were expanded in Medium 200 with large vessel endothelial supplement (Life Technologies) and were passaged at least once after thawing and prior to beginning the tube formation assay. Once cells had reached 80% confluency, HUVECs were seeded into 48‐well plates with a reduced growth factor basement membrane matrix (Geltrex, Life Technologies) at a concentration of 25 000 cells/cm2. Cells were then cultured overnight with either CM from HSAM or assay media alone (endothelial basal media EBM). CM were obtained as described in detail above. After overnight culture, cells were fixed in methanol for 1 minute, rinsed thrice in distilled water and imaged via phase‐contrast with an inverted microscope (Nikon Eclipse Ti). Images were then imported into ImageJ (NIH, Bethesda, MD), and the average tube length was quantified.
For all quantitative mechanistic assays (proliferation, migration and angiogenesis), statistical analysis was conducted using a one‐way analysis of variance ANOVA with a post‐hoc Tukey test, where P < 0·05 was considered significant. Throughout, * denotes P < 0·05, ** denotes P < 0·01, *** denotes P < 0·001, and **** denotes P < 0·0001. For qualitative imaging, representative images of all groups were taken and presented.
To qualitatively analyse how fibroblasts interacted with HSAM, 50 000 human dermal fibroblasts were seeded onto HSAM grafts (2·5 cm × 2·5 cm). Grafts were then cultured in six‐well plates for 2 weeks under standard culture conditions. Post the 2‐week culture, grafts were fixed in 4% neutral buffered formalin for 24 hours. Following paraffin embedding, 5‐µm thick serial sections were cut from the tissue blocks and floated onto charged glass slides (Super‐Frost Plus, Fisher Scientific, Pittsburgh, PA) and dried overnight at 60° C. Sections were stained for haemotoxylin and eosin (H&E), Masson's trichrome, Verhoeff's stain and Alcian blue. All sections for immunohistochemistry were deparaffinised and hydrated using graded concentrations of ethanol to deionised water. For fibronectin immunohistochemistry, antigen retrieval was performed using a solution of proteinase K (P6556, 1:50 dilution in TE‐CaCl2 buffer pH 8) for 15 minutes at 37°C. For collagen I and III immunohistochemistry, tissues sections were subjected to antigen retrieval using a 0·01 M Tris–1 mM EDTA buffer (pH 9) at 70°C for 20 minutes. Following antigen retrieval, all sections were washed gently in deionised water, then transferred to a 0·05‐M Tris‐based solution (TBST) in 0·15 M NaCl with 0·1% v/v Triton‐X‐100, pH 7·6 (TBST). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 20 minutes. To reduce further non‐specific background staining, slides were incubated with 3% normal goat serum for 30 minutes (Sigma‐Aldrich, St. Louis, MO) at room temperature. All slides then were incubated at 4°C overnight with various antibodies (Fibronectin, PA5‐29578, Thermo Fisher, 1:100; Collagen I, ab34710, Abcam Cambridge, United Kingdom, 1:100; Collagen III, ab7778, Abcam, 1:100). Negative controls were produced by eliminating the primary antibodies from the diluents. After washing with TBST, sections were then incubated with the matching secondary antibodies conjugated with horseradish peroxidase. Diaminobenzidine (DAB; Scy Tek Laboratories, Logan, UT) was used as the chromagen and haematoxylin (Richard‐Allen Scientific, Kalamazoo, MI) as the counterstain. All stained samples were visualised using an inverted Microscope (Nikon Eclipse Ti; Nikon, Melville, NY) with a 40× objective.
Results
Proteomic microarrays confirmed physiologically relevant levels of numerous growth factors and cytokines important for wound healing (Table 1). These growth factors were broken down using general categories to describe their activity, including angiogenic, regenerative and anti‐inflammatory growth factors (Figure 1; average ± standard deviation, n = 9 unless otherwise noted). There is some sample‐to‐sample variability observed in the levels of growth factors and cytokines present in the tissue. This level of variability is expected and has been observed in previous studies of dehydrated human amnion‐chorion membranes (dHACM) 25, 30 and of cryopreserved human amniotic membrane (cHAM) 31, 32, 33. Many of the same growth factors are found to be present in cHAM, dHACM and HSAM. However, differences have been noted that may be caused by several factors, including degradation of growth factors or cytokines occurring as a result of tissue processing (dehydration, lyophilisation and sterilisation) and the layers included in the product (amnion versus amnion and chorion), as well as study‐related factors such as protein extraction methods, methods for evaluation of growth factors and sample size. The most abundant growth factors present in the HSAM were thrombospondin‐1 (TSP‐1), angiopoietin‐like 4 (APL4) and insulin‐like growth factor‐1 (IGF‐1) in nearly all of the samples that were tested, suggesting that while specific levels of growth factors in the tissues may vary to some degree, relative concentrations of these most abundant factors are consistent.
Figure 1.
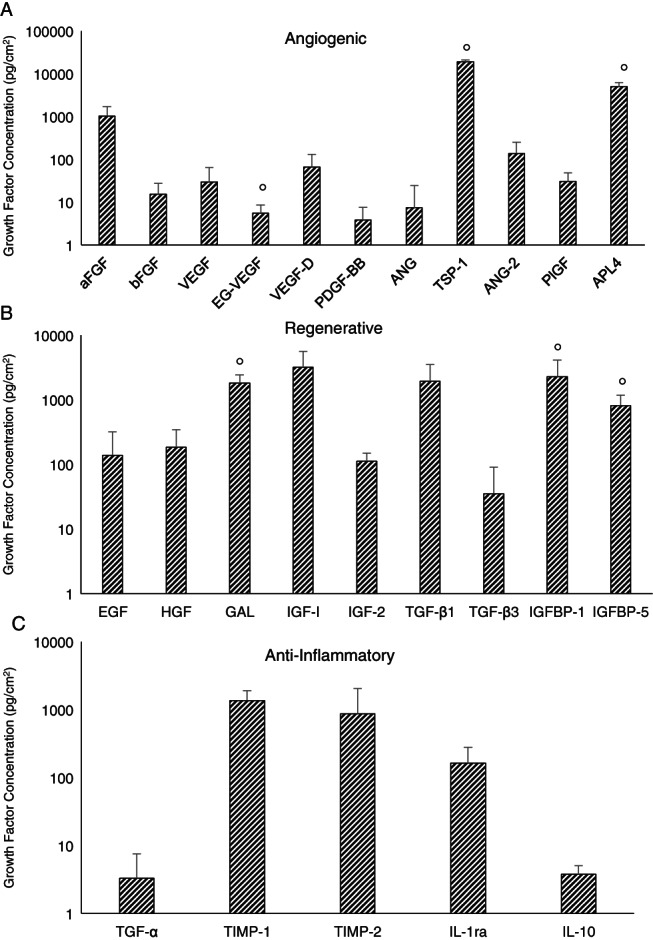
Multiplex enzyme‐linked immunosorbent assay proteomics microarray measuring growth factors and cytokines relating to mechanisms of wound healing, including: (A) angiogenic growth factors, (B) regenerative growth factors and (C) anti‐inflammatory growth factors. For all growth factors and cytokines, results are shown as average ± standard deviation (n = 9, except where marked with n = 4).
Subsequently, we were interested in evaluating the time course for the release of growth factors from HSAMs over a 7‐day period. All growth factors analysed in Figure 1 were included in this analysis, and the total growth factor and cytokine release over 7 days was approximately 70·6 ± 19·9% of the total measured cytokines (Figure 2A). Key growth factors, including basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), interleukin 1 receptor antagonist (IL‐1ra), transforming growth factor beta 3 (TGF‐β3) and TIMP‐1/2, were evaluated and graphed individually (Figure 2B–F). After 7 days, 91·7 ± 5·6% (n = 3) of the bFGF had been released; bFGF is widely known to accelerate wound healing, primarily through it mitogenic effects on cells 34 (Figure 2B). HGF is particularly important to the wound‐healing environment because of its role in stimulating proliferation and cell motility in cells of epithelial origin 35, 36; in our study, 89·9 ± 4·5% (n = 3) of HGF was released from HSAM after 7 days (Figure 2C). IL‐1ra is a natural inhibitor of pro‐inflammatory IL‐1β and is thought to also play a role in reepithelialisation. Of interest, delayed healing has been observed in IL‐1ra‐deficient mice as evidenced by problems with collagen deposition and vascularisation 37. Over the course of 7 days, approximately 92·2 ± 9·9% (n = 3) IL‐1ra was released from HSAM (Figure 2D). After 7 days, approximately 97·8 ± 3·7% (n = 3) of TGF‐β3 in HSAM had been released (Figure 2E). TGF‐β3 is thought to assist in wound healing by reducing fibrosis and scarring at the wound site 38, 39.
Figure 2.
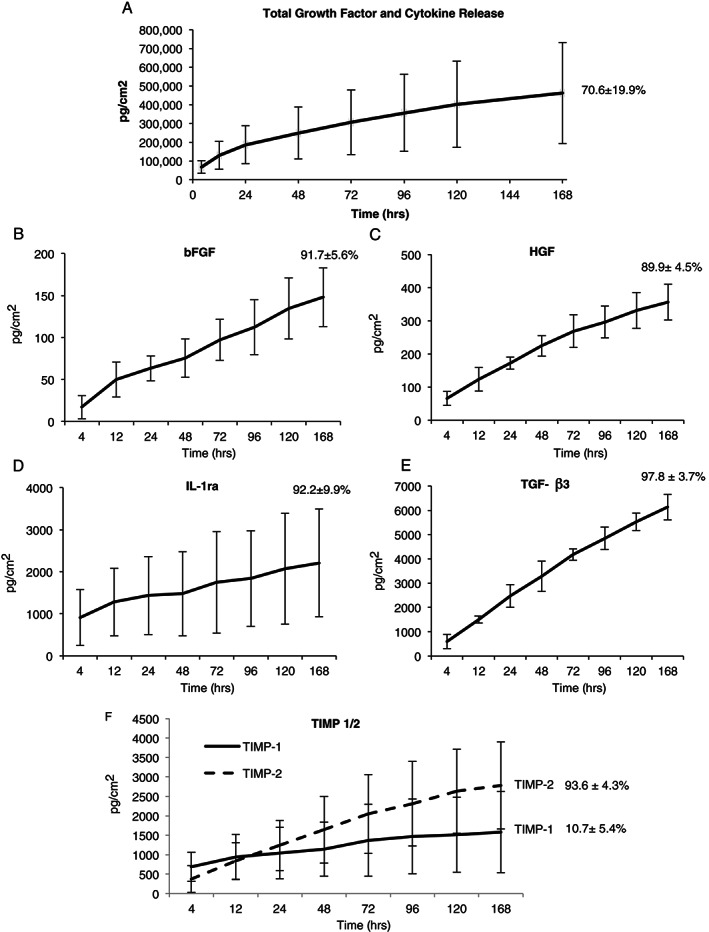
Release of growth factors from hypothermically stored amniotic membrane (A) cumulative release curve for all 25 growth factors listed in Table 1, (B) basic fibroblast growth factor (bFGF), (C) hepatocyte growth factor (HGF), (D) interleukin 1 receptor antagonist (IL‐1ra), (E) transforming growth factor beta 3 (TGF‐β3) and (F) tissue inhibitor of metalloproteinases 1 and 2 (TIMP‐1/2). Cytokine concentrations are shown as average ± standard deviation for all time points, and total % average ± standard deviation released at 7 days is inset for all release curves (n = 3).
Another important component of the wound‐healing process is appropriate regulation of protease activity. Over‐expression of MMPs is thought to be one of the causative factors for chronic wounds; therefore, inhibitors of MMPs (TIMPs) and their availability are of particular interest 40. We found that the majority of TIMP‐2 (93·6 ± 4·3%, n = 3) was released from the membrane by day 7; we also found that TIMP‐1 was released from the membrane. However, only 10·7 ± 5·4% (n = 3) of the total quantity of TIMP‐1 was released by day 7. The low release rate of TIMP‐1 compared to TIMP‐2 may be, in part, explained by the interaction of TIMP‐1 with the β1 integrin 41. Because of its interaction with the ECM, the release profile of TIMP‐1 is particularly likely to be much different in vivo because of interactions of the graft with the chronic wound environment and subsequent break down of the ECM.
In addition to studying the growth factor/cytokine profile and their respective release characteristics, we also investigated the effects released cytokines have on cell types important to wound healing. Specifically, we investigated the effects of cytokines released from HSAM on the behaviour of human dermal fibroblasts, human keratinocytes and human endothelial cells. Fibroblast migration and proliferation is thought to be an indicator of healing in wounds 7, 42, 43. In Figure 3A and B, it was found that fibroblasts proliferate more quickly in media conditioned from HSAM than in assay media alone, which represents the baseline control. Quantitatively, it was found that 50%, 25% and 10% CM significantly increased fibroblast proliferation at 7, 10 and 14 days, although there were no measurable differences between the CM groups of different concentrations. Qualitative images were taken at 14 days, and these images confirmed that HSAM CM groups had a higher density of cells present than the assay media group (Figure 3B).
Figure 3.
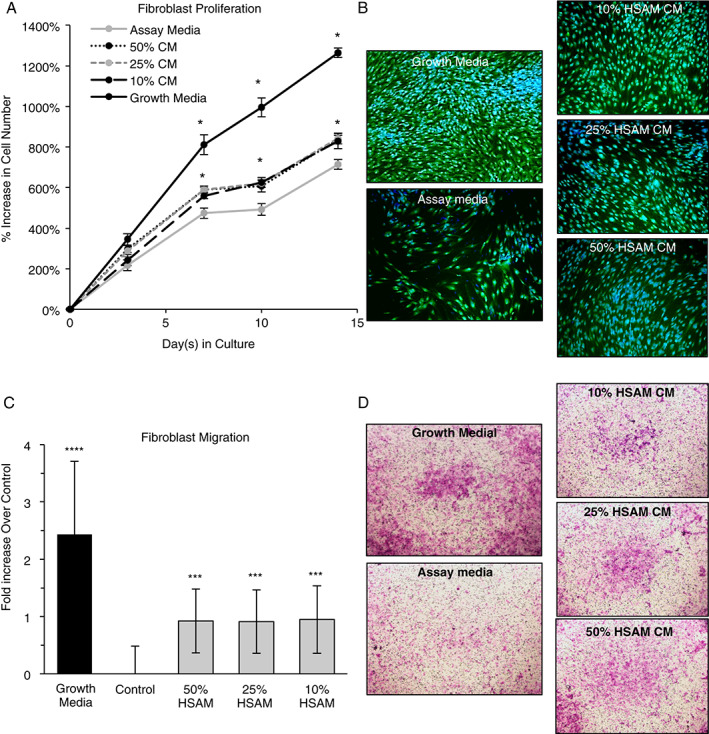
Evaluation of hypothermically stored amniotic membrane (HSAM) effects on fibroblasts: (A) quantitative proliferation assay in assay media alone or HSAM conditioned media (CM) in assay media (with 50%, 25% and 10% CM by volume); (B) qualitative analysis of proliferation assay with CFDA (green) and Dapi (blue) staining at 14 days; (C) quantitative analysis of fibroblast migration over 24 hours in growth media, assay media and 50%, 25% and 10% HSAM CM by volume; and (D) confirmatory images of cell migration with cells stained with crystal violet. For quantitative assays, significance compared to assay media is denoted by: *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001.
Additionally, the migration of fibroblasts through a microporous membrane was evaluated. Fibroblast migration assays showed that the presence of HSAM CM resulted in a significant increase in migration relative to assay media alone. Interestingly, the dose of CM (50%, 25% or 10%) did not affect the increase in fibroblast migration, with all groups showing between a 0·91‐ and 0·95‐fold increase over assay media alone. Crystal violet‐stained cells qualitatively confirmed greater migration in groups with HSAM CM than in assay media alone (Figure 3D).
Human epidermal keratinocytes (HEKs) are essential to wound closure; in the normal healing process, HEKs proliferate and migrate into the wound and form an epithelial layer 44, 45, 46. To model this in vitro, we assessed the effect HSAM CM would have on HEK proliferation and migration (Figure 4). After 7 days, all the CM groups evaluated (10%, 25% and 50% HSAM CM) resulted in significantly increased cell proliferation relative to assay media alone. Migration of keratinocytes is considered an important step in wound healing as they play an integral role in reepithelialisation of the wound bed 47. To model this effect in vitro, HEK migration was evaluated using a Boyden chamber assay (Figure 4B). It was found that 50% and 25% HSAM CM stimulated significantly increased cell migration compared to assay media, 340% and 232%, respectively. Qualitatively, these changes were confirmed with the crystal violet staining of cells attached to the Boyden chamber membrane (Figure 4C).
Figure 4.
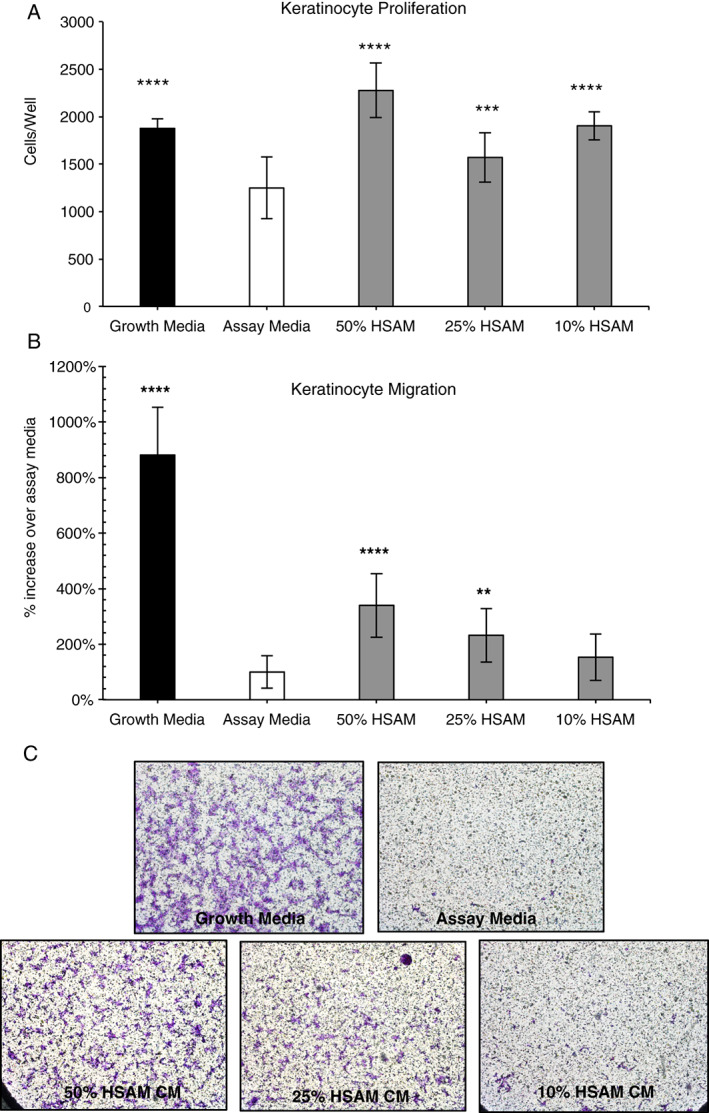
Evaluation of hypothermically stored amniotic membrane (HSAM) effects on human epidermal keratinocytes (HEKs). (A) Quantitative analysis of proliferation of HEKs cultured under various conditions, including growth media, assay media and HSAM conditioned media (50%, 25% and 10%). Migration of HEKs in response to growth media, assay media and 50%, 25% and 10% HSAM CM evaluated quantitatively (B) and qualitatively with crystal violet staining (C). For quantitative assays, significance compared to assay media is denoted by *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001.
Angiogenesis, or the formation of new blood vessels, is essential for wound healing 48. However, this process is often disrupted in chronic wounds. In this study, HSAM CM or assay media was applied to a monolayer of HUVECs, and the formation of tubules was assessed (Figure 5). HSAM CM at concentrations of 50% and 25% significantly increased average tubule length by 104% and 95%, respectively. HSAM CM of 10% and 1% showed no appreciable increases in tubule length. Representative phase‐contrast images are shown in Figure 5B for each of the groups analysed, with qualitative increases in tube formation shown in higher concentrations of HSAM CM.
Figure 5.
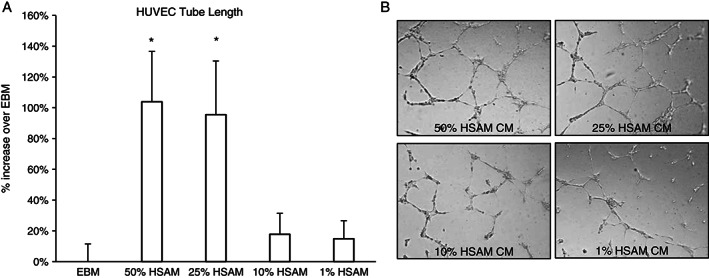
Impact of hypothermically stored amniotic membrane (HSAM) on angiogenesis as measured by tube formation: (A) tube formation was evaluated after culture of HUVECs overnight with or without CM from HSAM (50%, 25%, 10% and 1%), and (B) representative phase‐contrast images of tube formation were taken. Image J was used to quantify tube length, and data are presented as average ± standard deviation. Significance is denoted by *P < 0·05.
To model the interaction of fibroblasts with HSAM, we cultured fibroblasts on HSAM for up to 2 weeks and subsequently evaluated the tissue using H&E, Masson's trichrome, Verhoeff's stain and Alcian blue (Figure 6). Immunohistochemistry was also conducted for collagen I, III and fibronectin (Figure 7). Images show a series of histological results for HSAM with (Figures 6B, D, F, H and 7B, D, F) and without (Figures 6A, C, E, G and 7A, C, E) fibroblasts seeded for up to 2 weeks. Based on the H&E and Masson's trichrome images, it is clear that fibroblasts readily infiltrate and proliferate within the HSAM grafts. Masson's trichrome and Verhoeff's staining showed deposition of collagen and elastin into the HSAM by fibroblasts. While HSAM contains a high quantity of glycosaminoglycans, Alcian blue images do not indicate the deposition of additional glycosaminoglycans by fibroblasts. HSAM was found to contain high amounts of collagen I throughout stromal layer, collagen III specifically localised to the basement membrane layer and the spongy layer and fibronectin within the basement membrane layer and around cells. Fibroblasts cultured on HSAM deposited additional collagen III and Fibronectin throughout the HSAM. While we also expected to see fibroblasts‐producing collagen I, the high levels of native collagen I found throughout the HSAM make it difficult to detect an additional protein deposition.
Figure 6.
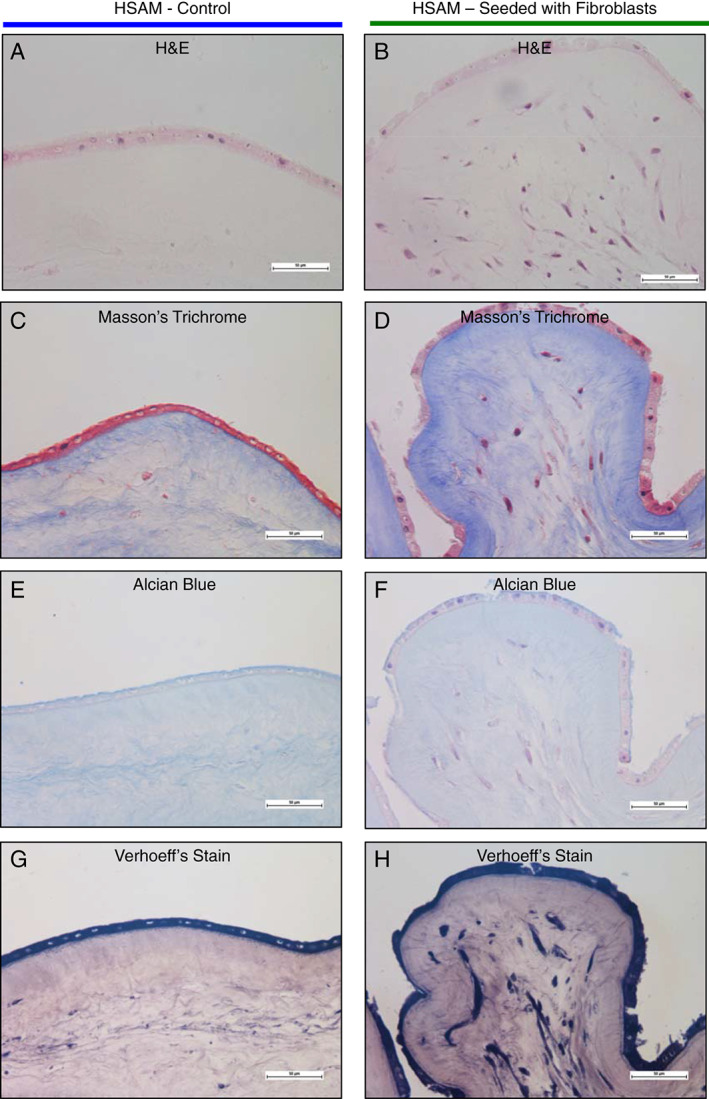
Qualitative evaluation of fibroblast interaction with hypothermically stored amniotic membrane (HSAM) was completed using staining. HSAM was cultured with or without fibroblasts for 2 weeks, and samples were processed and stained with haemetoxylin and eosin (H&E), Masson's trichrome, Alcian blue, or Verhoff's stains. Representative images were taken of H&E (A and B), Masson's trichrome (C and D), Alcian blue (E and F) and Verhoeff's stain (G and H).
Figure 7.
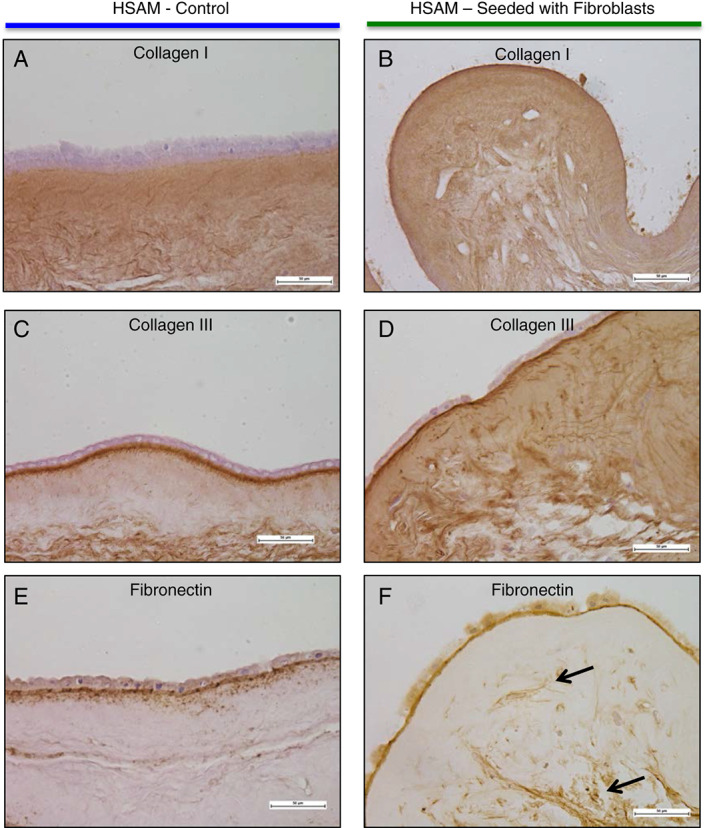
Qualitative evaluation of fibroblast interaction with hypothermically stored amniotic membrane was completed using immunohistochemical stains. Representative images were taken of collagen I (A and B), collagen II (C and D) and fibronectin (E and F). Black arrows point to fibronectin deposited throughout the matrix.
Discussion
The use of placental‐based tissues is proposed to aid in healing through various mechanisms, including reduction in inflammation, enhancement of cell migration into the wound environment, stimulation of cell proliferation, increased production of ECM and improved angiogenesis 25, 49, 50, 51. A significant part of the function of these tissues is attributed to the growth factor content released from the membranes. Selected growth factors and cytokines of interest are shown in Table 1; while this list is not comprehensive of all molecules found in placental‐based tissues, these molecules were selected because of the important role they play in wound healing. Some growth factors and cytokine concentrations are lower than in singular recombinant growth factor therapies used for chronic wounds, but it is important to note that there may be a substantial benefit in the sustained delivery of multiple cytokines with different roles/signalling processes in the healing wound 52, 53.
The management of inflammation is essential to resolving chronic wounds. Amniotic membranes and the cells within these membranes have been shown to elicit an anti‐inflammatory response. Cells derived from amniotic membranes have been shown to down‐regulate the production of certain inflammatory cytokines, including TNF‐α, CXCL10, CXCL9 and CCL5 54, 55. The suppression of TNF‐α is of particular interest as TNF‐α has been found to be elevated in non‐healing DFUs 56. Amniotic membranes have been shown to suppress the production of transforming growth factor beta 1 (TGF‐β1), Interleukin 8 IL‐8 and granulocyte macrophage colony‐stimulating factor by fibroblasts, which are known to be stimulated under inflammatory conditions 57. Another relevant cytokine known to trigger inflammatory responses within chronic wounds, IL‐1β, was found to be significantly reduced in gingival crevicular fluid following the application of amniotic membranes with bone grafting for periodontal pockets in a 30‐patient clinical study 58. In a mouse bleomycin injury model, amnion‐derived epithelial cells have been shown to limit macrophage infiltration into the lungs and to also drive a majority of pulmonary macrophages to the anti‐inflammatory M2 phenotype 59. The shift to M2 polarisation in response to amnion‐derived cells is relevant to DFUs as M2 and M2‐like macrophages induce anti‐inflammatory, regulatory and reparative functions that lead to wound closure 60, 61, 62. While the exact mechanisms responsible for these anti‐inflammatory effects remain unclear, potential sources include the release of interleukins from the membrane, including both interleukin 10 (IL‐10) and IL‐1ra, which were measured in HSAM. IL‐10 is known to counteract the effects of several pro‐inflammatory cytokines, including IL‐6, IL‐1, IL‐8 and TNF‐α 63, and IL‐1ra is a potent inhibitor of IL‐1, shown to suppress the inflammatory response triggered by IL‐1 64. While not measured in this study, we suspect that the presence of heavy chain hyaluronic acid 65, prostaglandin E2 66, and macrophage migration‐inhibitory factor 67, 68 may also play a role in the anti‐inflammatory effects observed with HSAM.
An essential part of resolving chronic wounds is the reduction of proteases within the wound environment to prevent the destruction of growth factors and ECM proteins necessary for wound healing. MMP‐9 has been found to be elevated in non‐healing DFUs 56, and the addition of inhibitors to MMPs (TIMPs) to the chronic wound environment has been shown to be beneficial to wound healing 9, 25, 69. Amniotic membranes have been previously shown to inhibit MMP activity 70, 71, and here, we have demonstrated the presence and the release curves of TIMP‐1 and TIMP‐2 within HSAM. In addition to TIMP‐1 and 2, possible mechanisms behind the protease inhibition observed with amniotic membranes include contributions by type‐1 plasminogen activator inhibitor and TSP‐1 24.
The destruction of essential growth factors and their receptors is another consequence of excessive protease levels within chronic wounds. This consequently leads to cellular dysregulation, especially with fibroblasts and keratinocytes. For fibroblasts, a marked decrease in cellular migration, proliferation and ECM deposition has been observed 72, 73. Here, we have demonstrated that CM from HSAM significantly improves both fibroblast proliferation and migration. Fibroblasts were also shown to migrate into and deposit ECM proteins within HSAMs. The likely cause of this proliferative and migratory response of fibroblasts to CM is from the release of FGF, TGF‐β1, TFG‐β3 and IGF‐1 from the HSAM, which have been shown to lead to the activation and migration of fibroblasts and stimulate their proliferation 42. Keratinocytes along the borders of chronic wounds have also been found to demonstrate abnormal behaviour, including failure to differentiate and migrate 17. Here, we have demonstrated that HSAM contains several growth factors know to stimulate keratinocyte migration and proliferation, including epidermal growth factor (EGF), platelet‐derived growth factor BB (PDGF‐BB) and TGF‐β1. More importantly, we have shown that growth factors/cytokines found within CM from HSAM are active and can promote both proliferation and migration of keratinocytes. In a recent study by Zhao et al., human amniotic epithelial cells were found to improve keratinocyte migration and proliferation. This increase in keratinocyte migration and proliferation was found to be primarily the result of EGF and keratinocyte growth factor KGF produced by these cells, which, in turn, activated the extracellular signal‐regulated kinases ERK, jun N‐terminal kinase JNK and protein kinase B AKT pathways in keratinocytes 74. Fibroblasts and keratinocytes are known to interact in the wound environment 35, 75. Suppression of fibroblast proliferation and migration in chronic wounds have been shown to result in changed cytokine and protease release profiles 76, 77, and these changes result in deleterious impacts on keratinocyte proliferation and migration 73. We expect the combined effects of HSAM CM on both fibroblasts and keratinocytes to promote significant improvements clinically in chronic wound healing.
Prolonged hypoxia in the wound environment can prevent wounds from healing normally; therefore, angiogenesis is critical to restore oxygen and nutrients to the injured tissue 7. Because many patients with chronic wounds, especially DFUs, have underlying vascular disease, lack of effective angiogenesis can be particularly problematic in healing these patients. In addition to these underlying issues, there are several anti‐angiogenic factors present within chronic wounds, including inflammatory factors, proteases and cell death mediators, which deter pro‐angiogenic processes 22. We expect that HSAM may mitigate both the prolonged pro‐inflammatory response and high protease content within the wound to create an environment more favourable to angiogenesis. HSAM contains numerous angiogenic growth factors, including FGF, vascular endothelial growth factor (VEGF), PDGF‐BB, angiopoietin (ANG), placental growth factor (PLGF), APL4 and TSP1. The results from in vitro tube formation assays reflected the activity of high levels of angiogenic growth factors; these results support the hypothesis that the application of HSAMs to chronic wounds may promote angiogenesis resulting in improved wound healing and closure.
Placental‐derived membranes have a long history in wound care 78, 79; however, most current commercially available membranes are processed in such a way that they do not maintain native viable cell populations. While these membranes have shown positive effects on healing in chronic wound care 51, 80, the absence of a viable cell population prevents these grafts from dynamically responding to the chronic wound environment 81. Numerous studies have shown that the various cell types populating the amniotic membrane, which are present within HSAM, produce factors that act to reduce inflammation, improve cell proliferation and migration and improve angiogenesis 54, 55, 59, 66, 67. Recent clinical data suggest that cell containing amniotic grafts may be more effective in treating chronic wounds compared to non‐viable placental‐derived alternatives 82. Future studies will focus on how the cell populations within HSAM respond to chronic wound environments through in vitro models in the context of inflammation and in vivo studies utilising an ischaemic animal model, which more closely mimics the chronic wound environment 83.
Conclusion
HSAMs offer a new treatment alternative to promote healing in chronic wounds; these membranes deliver a native ECM, growth factors and cytokines and a viable cell content. In this study, we have focused on the overall activity of the grafts by primarily using media conditioned by the grafts to study mechanisms of action. We have reported concentrations of numerous growth factors and cytokines known to contribute to wound healing and detailed their released from HSAM over 7 days. Furthermore, we have shown that HSAM CM significantly increases fibroblast and keratinocyte proliferation and migration, suggesting its potential to improve healing in chronic wounds where the normal activity of these cells is known to be suppressed. Finally, this study shows that HSAM promotes increased tube formation in HUVECs suggesting a potential mechanism for HSAM to promote and improve angiogenesis. In sum, we believe hypothermically stored amniotic grafts are a compelling option for promoting pro‐healing activities spanning various functions, including reducing inflammation and protease activity as well as promoting angiogenesis and cellular responses.
Acknowledgements
The authors acknowledge the support of the University of Alabama at Birmingham's Pathology Core Research lab, particularly Dr. Dezhi Wang, for their assistance with histology and immunohistochemistry. This study was supported and funded by NuTech Medical, Birmingham, AL. JPM, JBV and KCM are employees of NuTech Medical.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report: estimates of diabetes and its burden in the United States. Atlanta: Centers for Disease Control and Prevention, 2014. [Google Scholar]
- 2. Boulton AJM, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 3. Brantley JN, Verla TD. Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv Wound Care 2015;4:545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2014;37:651–8. [DOI] [PubMed] [Google Scholar]
- 5. Fortington LV, Geertzen JHB, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg 2013;46:124–31. [DOI] [PubMed] [Google Scholar]
- 6. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Margolis DJ, Parsons NB. Economic outcomes among Medicare patients receiving bioengineered cellular technologies for treatment of diabetic foot ulcers. J Med Econ 2015;18:586–95. [DOI] [PubMed] [Google Scholar]
- 7. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–43. [DOI] [PubMed] [Google Scholar]
- 8. McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care 2013;2:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP‐1 to TIMP‐1 is a predictor of wound healing. Diabet Med 2008;25:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lobmann R, Schultz G, Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 2005;28:461–71. [DOI] [PubMed] [Google Scholar]
- 11. McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen 2012;20:125–36. [DOI] [PubMed] [Google Scholar]
- 12. Grazul‐Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, Abdullah KM. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787–800. [DOI] [PubMed] [Google Scholar]
- 13. Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg 1998;25:341–56. [PubMed] [Google Scholar]
- 14. Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care 2013;22:407–8, 410–2. [DOI] [PubMed] [Google Scholar]
- 15. Loot MA, Kenter SB, Au FL, van Galen WJ, Middelkoop E, Bos JD, Mekkes JR. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF‐I, bFGF and PDGF‐AB compared to controls. Eur J Cell Biol 2002;81:153–60. [DOI] [PubMed] [Google Scholar]
- 16. Hoke GD, Ramos C, Hoke NN, Crossland MC, Shawler LG, Boykin JV. A typical diabetic foot ulcer keratinocyte protein signaling correlates with impaired wound healing. J Diabetes Res 2016;2016:1586927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem 2008;56:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brem H, Tomic‐Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, Galiano RD, Tomic‐Canic M. Molecular pathogenesis of chronic wounds: the role of beta‐catenin and c‐myc in the inhibition of epithelialization and wound healing. Am J Pathol 2005;167:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006;117(7 Suppl):S35–41. [DOI] [PubMed] [Google Scholar]
- 21. Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003;23:117–45. [DOI] [PubMed] [Google Scholar]
- 22. Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics 2013;13:2670–81. [DOI] [PubMed] [Google Scholar]
- 23. Bao P, Kodra A, Tomic‐Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Litwiniuk M, Bikowska B, Niderla‐Bielińska J, Jóźwiak J, Kamiński A, Skopiński P, Grzela T. Potential role of metalloproteinase inhibitors from radiation‐sterilized amnion dressings in the healing of venous leg ulcers. Mol Med Rep 2012;6:723–8. [DOI] [PubMed] [Google Scholar]
- 25. Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013;10:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steed DL, Trumpower C, Duffy D, Smith C, Marshall V, Rupp R, Robson M. Amnion‐derived cellular cytokine solution: a physiological combination of cytokines for wound healing. Eplasty 2008;8:e18. [PMC free article] [PubMed] [Google Scholar]
- 27. Chen H‐C. Boyden chamber assay. Methods Mol Biol 2005;294:15–22. [DOI] [PubMed] [Google Scholar]
- 28. Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 2010;5:628–35. [DOI] [PubMed] [Google Scholar]
- 29. DeCicco‐Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L, O'Neill RC, Morin A, Wiest JS. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J Vis Exp 2014;91:e51312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater 2015;103:1133–40. [DOI] [PubMed] [Google Scholar]
- 31. Kruse FE, Joussen AM, Rohrschneider K, You L, Sinn B, Baumann J, Völcker HE. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol 2000;238:68–75. [DOI] [PubMed] [Google Scholar]
- 32. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 33. Hao Y, Ma DH‐K, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000;19:348–52. [DOI] [PubMed] [Google Scholar]
- 34. Akita S, Akino K, Hirano A. Basic fibroblast growth factor in scarless wound healing. Adv Wound Care 2013;2:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 36. Matsumoto K, Hashimoto K, Yoshikawa K, Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocyte growth factor. Exp Cell Res 1991;196:114–20. [DOI] [PubMed] [Google Scholar]
- 37. Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL‐1 receptor antagonist impaired wound healing along with aberrant NF‐kappaB activation and a reciprocal suppression of TGF‐beta signal pathway. J Immunol 2006;176:5598–606. [DOI] [PubMed] [Google Scholar]
- 38. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 39. O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol 1997;29:63–78. [DOI] [PubMed] [Google Scholar]
- 40. Schreml S, Szeimies R‐M, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol 2010;63:866–81. [DOI] [PubMed] [Google Scholar]
- 41. Ries C. Cytokine functions of TIMP‐1. Cell Mol Life Sci 2014;71:659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin P. Wound healing – aiming for perfect skin regeneration. Science 1997;276:75–81. [DOI] [PubMed] [Google Scholar]
- 43. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 44. Pastar I, Stojadinovic O, Tomic‐Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int 2008;17:105–12. [PubMed] [Google Scholar]
- 45. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18. [DOI] [PubMed] [Google Scholar]
- 46. Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, Higashiyama S, Hashimoto K. Heparin‐binding EGF‐like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 2005;118(pt 11):2363–70. [DOI] [PubMed] [Google Scholar]
- 47. Freedberg IM, Tomic‐Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol 2001;116:633–40. [DOI] [PubMed] [Google Scholar]
- 48. Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc 2000;5:40–6. [DOI] [PubMed] [Google Scholar]
- 49. Massee M, Chinn K, Lim JJ, Godwin L, Young CS, Koob TJ. Type I and II diabetic adipose‐derived stem cells respond in vitro to dehydrated human amnion/chorion membrane allograft treatment by increasing proliferation, migration, and altering cytokine secretion. Adv Wound Care 2016;5:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perepelkin NM, Hayward K, Mokoena T, Bentley MJ, Ross‐Rodriguez LU, Marquez‐Curtis L, McGann LE, Holovati JL, Elliott JA. Cryopreserved amniotic membrane as transplant allograft: viability and post‐transplant outcome. Cell Tissue Bank 2016;17:39–50. [DOI] [PubMed] [Google Scholar]
- 51. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013;10:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pachuau L. Recent developments in novel drug delivery systems for wound healing. Expert Opin Drug Deliv 2015;12:1895–909. [DOI] [PubMed] [Google Scholar]
- 53. Ashraf A, Lee PH, Kim K, Zaporojan V, Bonassar L, Valentini R, Spangenberger A, Weinzweig J. Effect of sustained‐release PDGF and TGF‐beta on cyclophosphamide‐induced impaired wound healing. Plast Reconstr Surg 2009;124:1118–24. [DOI] [PubMed] [Google Scholar]
- 54. Magatti M, De Munari S, Vertua E, Nassauto C, Albertini A, Wengler GS, Parolini O. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant 2009;18:899–914. [DOI] [PubMed] [Google Scholar]
- 55. Magatti M, Caruso M, De Munari S, Vertua E, De D, Manuelpillai U, Parolini O. Human amniotic membrane‐derived mesenchymal and epithelial cells exert different effects on monocyte‐derived dendritic cell differentiation and function. Cell Transplant 2015;24:1733–52. [DOI] [PubMed] [Google Scholar]
- 56. Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012;61:2937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Solomon A, Wajngarten M, Alviano F, Anteby I, Elchalal U, Pe'er J, Levi‐Schaffer F. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy 2005;35:941–8. [DOI] [PubMed] [Google Scholar]
- 58. Kumar A, Chandra RV, Reddy AA, Reddy BH, Reddy C, Naveen A. Evaluation of clinical, antiinflammatory and antiinfective properties of amniotic membrane used for guided tissue regeneration: A randomized controlled trial. Dent Res J (Isfahan) 2015;12:127–35. [PMC free article] [PubMed] [Google Scholar]
- 59. Tan JL, Chan ST, Wallace EM, Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant 2014;23:319–28. [DOI] [PubMed] [Google Scholar]
- 60. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013;229:176–85. [DOI] [PubMed] [Google Scholar]
- 61. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacLeod AS, Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care 2016;5:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin‐10 and related cytokines and receptors. Annu Rev Immunol 2004;22:929–79. [DOI] [PubMed] [Google Scholar]
- 64. Dinarello CA, Wolff SM. The role of interleukin‐1 in disease. N Engl J Med 1993;328:106–13. [DOI] [PubMed] [Google Scholar]
- 65. He H, Li W, Tseng DY, Zhang S, Chen SY, Day AJ, Tseng SC. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter‐alpha‐inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem 2009;284:20136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rossi D, Pianta S, Magatti M, Sedlmayr P, Parolini O. Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One 2012;7:e46956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci 2005;46:900–7. [DOI] [PubMed] [Google Scholar]
- 68. Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol 1998;160:5693–6. [PubMed] [Google Scholar]
- 69. Cook H, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP‐1, TIMP‐2, and MMP‐2 activity. J Invest Dermatol 2000;115:225–33. [DOI] [PubMed] [Google Scholar]
- 70. Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res 2000;70:329–37. [DOI] [PubMed] [Google Scholar]
- 71. Litwiniuk M, Grzela T. Amniotic membrane: new concepts for an old dressing. Wound Repair Regen 2014;22:451–6. [DOI] [PubMed] [Google Scholar]
- 72. Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen 1993;1:181–6. [DOI] [PubMed] [Google Scholar]
- 73. Widgerow AD. Chronic wounds – is cellular “reception” at fault? Examining integrins and intracellular signalling. Int Wound J 2013;10:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao B, Liu JQ, Zheng Z, Zhang J, Wang SY, Han SC, Zhou Q, Guan H, Li C, Su LL, Hu DH. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res 2016;365:85–99. [DOI] [PubMed] [Google Scholar]
- 75. Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing epithelial–mesenchymal interactions in tissue repair. J Invest Dermatol 2007;127:998–1008. [DOI] [PubMed] [Google Scholar]
- 76. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence – implications for treatment. Int Wound J 2005;2:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc 1998;3:1–5. [PubMed] [Google Scholar]
- 78. Gruss JS, Jirsch DW. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J 1978;118:1237–46. [PMC free article] [PubMed] [Google Scholar]
- 79. Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns 1989;15:339–42. [DOI] [PubMed] [Google Scholar]
- 80. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 2014;11:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Duan‐Arnold Y, Gyurdieva A, Johnson A, Uveges TE, Jacobstein DA, Danilkovitch A. Retention of endogenous viable cells enhances the anti‐inflammatory activity of cryopreserved amnion. Adv Wound Care 2015;4:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Johnson E, Marshall J, Michael G. A comparative outcomes analysis evaluating clinical effectiveness in two different human placental membrane products for wound management. Wound Repair Regen 2016. epub ahead of print (https://www.ncbi.nlm.nih.gov/pubmed/?term=27997744) [DOI] [PubMed] [Google Scholar]
- 83. Roy S, Biswas S, Khanna S, Gordillo G, Bergdall V, Green J, Marsh CB, Gould LJ, Sen CK. Characterization of a preclinical model of chronic ischemic wound. Physiol Genomics 2009;37:211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


