Abstract
Low recruitment rates for randomised controlled trials (RCTs) are a common issue. Information on barriers and facilitators to recruitment for RCTs may inform researchers on how to improve the recruitment rate. The aim of this qualitative project was to identify barriers and facilitators to participant recruitment for a randomised double‐blinded placebo‐controlled trial on the clinical effectiveness of aspirin as an adjunct to compression therapy in healing chronic venous leg ulcers. We have conducted interviews with health professionals and project workers to understand their perspective on low recruitment rate, barriers to, and facilitators of recruitment. NVivo 11 software was used for data management and coding. Thematic analysis was applied as a method of data analysis. Although strict recruitment criteria were the main barrier, there were other recruitment barriers that should be considered when planning RCTs. We have further developed a framework of factors influencing the recruitment rate. The main recruitment barriers, including study‐related, participant‐related, practitioner‐related, collaboration‐related, ethics‐related, practice‐related, and health system‐related barriers, should be considered for inclusion in the “Other Information” section of Consolidated Standards of Reporting Trials Statement to improve the quality of reporting and ensure the strategic planning of future RCTs.
Keywords: barriers, enablers, RCTs, recruitment rate, venous leg ulcers
1. INTRODUCTION
Venous leg ulcer (VLU) management should be based on evidence.1, 2 Well‐designed randomised controlled trials (RCTs) are considered the “gold standard” in clinical research because they provide high‐quality evidence related to the management of specific conditions,3 including VLUs.4 Unfortunately, recruitment issues for participation in the RCTs experienced by health professionals are not uncommon.5, 6, 7 These issues are related to researchers’ inability to recruit the required number of participants or to retain the recruited participants in the study.8, 9 Findings from a systematic review10 of discontinued RCTs indicate that 76% of the discontinued RCTs were related to poor recruitment. Discontinued RCTs waste research and clinical resources.11
Principal investigators tend to overestimate the number of the participants who meet the eligibility criteria for their study.12 Slow‐progressing recruitment may increase the duration of a study and incur extra costs, requiring extra recruitment time and additional funding.12 Low recruitment rate and small sample size may reduce the quality of findings because of type II errors.12 Although the Consolidated Standards of Reporting Trials (CONSORT) Statement on how to conduct a rigorous RCT,13 including on wound management,14 requires researchers to report on why a particular RCT was ended or stopped, they do not contain a specific recommendation to include the recruitment barriers and facilitators.
The barriers to participant recruitment in RCTs in various health fields reported to date are classified into 4 major groups; these are: participant‐related, health professional‐related, practice‐related, and study‐related.6 These groupings were confirmed by a recent systematic review of barriers and facilitators to RCTs in chronic wound management conducted by Bugeja and associates.15 The main facilitators to patient recruitment for RCTs in other health fields were reported as follows: support provided by the project researchers to health professionals and health professionals’ perceived benefits of the study results for their practice.6, 16 Unfortunately, in the field of chronic wound management, study facilitators/enablers to participant recruitment in RCTs were not identified.15
Qualitative studies can provide a deeper insight on the barriers and facilitators of recruitment for RCTs and inform training initiatives.17, 18, 19 French and Stavropoulou16 developed a framework of factors influencing recruitment for RCTs based on data obtained from qualitative interviews with nurses on perceived factors that influenced recruitment. They included 4 factors from Foster's6 classification of barriers related to participants, practitioners, practice, and to the study itself and 1 additional factor—the research team factor. A review of qualitative studies aimed to elicit RCT recruitment barriers in various other health care fields reported that the main barrier was the difficulty to combine a research and a clinical role as experienced by practitioners.19 To date, however, there are no qualitative studies on the barriers and facilitators to participant recruitment for RCTs on VLU management. Our research project aimed to identify barriers and facilitators to participant recruitment for the ASPirin in Venous Leg Ulcer healing (ASPiVLU) study, which is a randomised, double‐blinded, placebo‐controlled trial on the clinical effectiveness of aspirin as an adjunct to compression therapy in healing chronic VLUs.
1.1. ASPiVLU study background
The ASPirin in Venous Leg Ulcer healing (ASPiVLU) trial is investigating the efficacy and safety of a daily dose of 300 mg of aspirin as an adjunct to compression therapy to treat VLUs.20 The primary outcome of this trial is time to healing within 12 weeks. Secondary outcomes are ulcer recurrence, wound pain, quality of life and well‐being, adherence to study medication, adherence to compression therapy, serum inflammatory markers, hospitalisations, and adverse events at 24 weeks. Participants are eligible if (1) they are aged 18 years and older, (2) have 1 or more leg ulcers in the presence of chronic venous insufficiency (CVI) as confirmed by clinical assessment and/or duplex ultrasound; (3) the target ulcer (largest ulcer if more than 1) is separated from the other ulcers by at least 1 cm; (4) the target ulcer has been present for at least 6 weeks or the patient has a prior history of venous ulceration; (5) the target ulcer area is ≥1 to ≤20 cm2 as measured by digital planimetry techniques; (6) ankle brachial pressure index (ABPI) measure of ≥ 0.7 mm Hg or systolic toe pressure ≥ 50 mm Hg to exclude arterial insufficiency; and (7) if the potential participant is capable to provide informed consent (as per clinicians’ judgement).20 Participants are excluded if they are currently using aspirin, have aspirin intolerance, have a contraindication to taking aspirin or to participating in the trial (as per clinicians’ judgement), are concurrently using any other antiplatelet or anticoagulation therapy, or are pregnant or breastfeeding.20
The wound clinic medical practitioner and/or wound clinic nurse assesses the eligibility of patients as outlined in the inclusion/exclusion criteria. At the subsequent visit, the wound clinic nurse confirms eligibility, obtains consent, and performs the baseline assessment including: demographics (age, ethnicity, smoking status, and employment status), physical examination, medical history including current medication use, and target ulcer assessment (general features, wound size and duration). Following this, the participant is randomised. An electronic data capture (EDC) system was used for this study. The REDCap EDC system is a secure, web‐based system that is free to institutional partners (www.projectredcap.org). Each user has his or her own unique password to access the system with user‐specific permissions and capabilities, which are centrally allocated. The ASPiVLU recruiters were provided and trained to use the Samsung tablet to enter data. ASPiVLU research nurses were assigned to clinical sites to do pre‐consultation screening and identify the eligible patients. Clinical consultants were paid per recruit.
The ASPiVLU researchers aim to recruit 268 participants to provide 90% power to detect a moderate difference between participants randomised to aspirin versus placebo for the primary end‐point (time to healing of target ulcer within 12 weeks). ASPiVLU participants are followed for 24 weeks from randomisation. In those healed within the treatment period, target ulcer recurrence is assessed monthly. As the participant recruitment rate for this RCT was lower than expected, the ASPiVLU research team decided to conduct a qualitative research project to identify the recruitment barriers and facilitators. In this article, we present the main barriers and facilitators to the ASPiVLU recruitment and develop a framework of factors that influence the recruitment rate. We also discuss the clinicians’ suggestions on how to improve the recruitment rate.
2. METHODS
2.1. Study design
We conducted a qualitative study, involving wound clinic specialists, general practitioners, wound clinic nurses, and research nurses from 4 clinical sites of the ASPiVLU study with the aim to identify barriers and facilitators to ASPiVLU participant recruitment. The selected methods were semi‐structured face‐to‐face and telephone interviews, subject to health professionals’ preference and availability. Health professionals were invited to share their experience of participant recruitment for the ASPiVLU study. They were encouraged to share their ideas on recruitment facilitators and how to eliminate recruitment barriers in future studies to maximise recruitment success.
2.2. Recruitment
During regular visits to the wound clinics located in Melbourne, the ASPiVLU researchers distributed information sheets about the research project and invited health professionals who facilitated recruitment for the ASPiVLU study to participate in the interviews. Contact details and phone numbers of health professionals who provisionally agreed to participate had been collected for later contact with regards to interview arrangements. The participant explanation letter was emailed to all potential participants prior to the interview. The potential participants were asked to contact the researchers should they have any questions or require additional information. Face‐to‐face interviews with health professionals were arranged at a time and place convenient for them. If telephone interviews were preferred, they were arranged at a convenient time. The potential participants were enthusiastic to participate in this study for a number of reasons, including: (1) know the reasons for insufficient recruitment; (2) validate their inability to recruit; (3) have a voice in the recruitment process; and (4) contribute to the improvement of the recruitment process.
2.3. Ethical considerations
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the approval obtained from the (removed for peer‐review purpose) Ethics Committee. Prior to the interview, the participants were asked if they had any questions related to the project or to the interview after reading the participant information statement, and their questions were answered by the researcher. They were reminded about the voluntary nature of participation, their withdrawal rights, and confidentiality issues. All potential participants provided either a signed consent before the face‐to‐face interview began or their verbal agreement prior to the telephone interview, which was audio recorded. Participants’ permission for audio recording was sought prior to the interview.
2.4. Data collection
Data collection took place from August 2017 to November 2017 and comprised 22 interviews (10 face‐to‐face and 12 telephone interviews). Most face‐to‐face interviews with health professionals7 took place in a clinical setting either during the lunch break or another time outside of work hours. One interview with a wound clinic consultant and interviews with the research project officers took place in the (Name withdrawn for a review purpose) University offices. The mean interviewing time was 35 minutes, ranging from 20 to 45 minutes. The participants were reimbursed for participation with a $50 gift card.
2.5. Transcription
All interviews were audio recorded. All audio files were transcribed using professional transcription services authorised by (Name withdrawn for a review purpose) University. All transcripts were compared with the voice files by the first author to ensure data quality.
2.6. Data analysis
Data analysis was conducted concurrently with data collection. All transcripts were uploaded in NVivo 11 software for qualitative data management and analysed, using 3 levels of coding. All repeated concepts and ideas were tagged as codes. Three non‐randomly selected transcripts were coded by 2 independent researchers. Codes were discussed; discrepancies were elaborated; and the coding framework was developed. This framework was used for coding the remaining interview transcripts, although additional codes were occasionally added. Thematic analysis was applied as a method of data analysis. We developed 7 Analysing codes for major themes, including (1) study‐related factors, (2) participant‐related factors, (3) practitioner‐related factors, (4) collaboration‐related factors, (5) ethics‐related factors, (6) practice‐related factors, and (7) health system‐related factors (Figure 1). We further grouped these factors according to 3 major pre‐identified themes, including recruitment barriers, recruitment facilitators, and suggestions of how to avoid barriers and facilitate recruitment (Tables 1, 2, 3).
Figure 1.
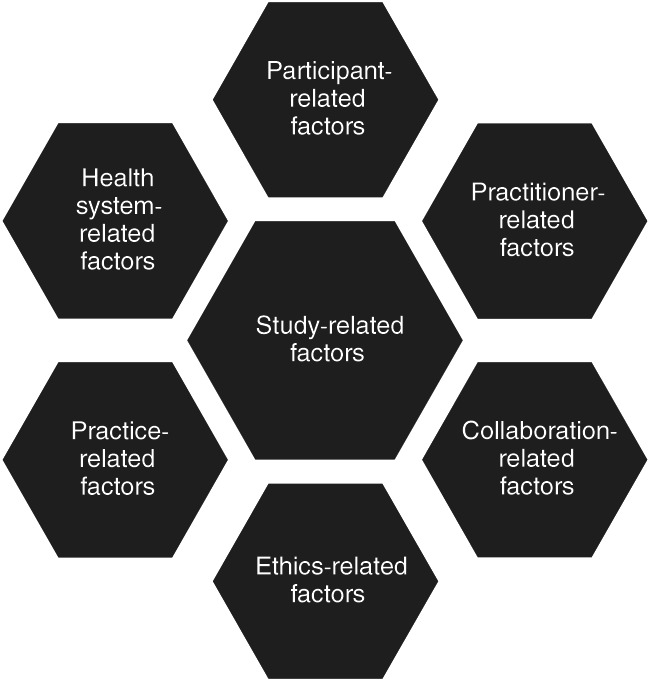
Conceptual framework of factors affecting recruitment for randomised controlled trials (RCTs) (adapted and expanded from French and Stavropoulou16
Table 1.
Barriers to recruitment: Themes and sub‐themes
| Barriers | Sub‐themes |
|---|---|
| Study‐related barriers | Strict recruitment criteria |
Study requirements for the participants
| |
Technology related issues
| |
| Participant‐related barriers | Comorbidities |
Unwilling to participate
| |
| Limited cognitive abilities | |
| Limited English proficiency | |
| Practitioner‐related barriers | Difficulty to combine a research and a clinical role
|
Avoidance of complex project‐related activities after recruitment
| |
Dissatisfaction with the recruitment process
| |
Lack of knowledge and skills
| |
| Forgetfulness | |
| Ethics‐related barriers | Setting up a priority (clinical versus research role) |
| Individual patient risks and benefits | |
| Considering patient priorities | |
| Lack of privacy (shortage of consulting rooms) | |
| Practice‐related barriers | Environmental issues
|
Busy clinic
| |
Administrative issues
| |
| Concurrent other research studies on‐site | |
| Collaboration‐related barriers | Absence of a research nurse on‐site in the beginning of the project |
| Absence of project staff on‐site in the beginning | |
| Irregular reminders about the study | |
| Health system‐related barriers | Nature of employment (full‐time versus part‐time workers; agency nurses) |
| Understaffed clinics | |
| Policies on processing research‐related financial incentives | |
| Hospital systems and internet access | |
| Policies on taking blood samples (research nurse versus pathology staff) |
Safety issue.
Best protocol practice to have 1 cm space between ulcers.
Standard best practice.
There was a simple compression, 3‐layer system, that can be used for those who do not like to wear compression because it is too tight.
Table 2.
Enablers to recruitment: Themes and sub‐themes
| Enablers | Sub‐themes |
|---|---|
| Study‐related enablers | Change to the study protocol |
Revision of study requirements for the participants
| |
| Participant‐related enablers | Patients are willing to participate |
| Patients are enthusiastic about the study | |
| Family support | |
| Practitioner‐related enablers | Routinisation of research activities
|
| Recognising importance of research | |
| Trying to overcome difficulties in the beginning of the project | |
| Ethics‐related enablers | Having a consultation room for project staff to ensure privacy |
| Practice‐related enablers | Busy periods and recruitment
|
Administrative
| |
| Good inter‐professional relationship with pathology staff and pharmacy workers | |
| Collaboration‐related enablers | Availability of a research nurse on‐site |
| Availability of project staff on‐site | |
| Communication with principal investigators | |
| More frequent reminders about the study by project staff | |
| Good relationship between clinical and project staff | |
| Health system‐related enablers | Well‐staffed clinic |
Table 3.
Suggestions for improvement: Themes and sub‐themes
| Suggestions for improvement | Sub‐themes |
|---|---|
| Study‐related suggestions | Careful selection of the recruitment site
|
| Conducting a pilot project | |
| Pre‐screening of patients’ profiles prior to starting the projecta | |
Careful estimation of the recruitment perioda
| |
Simplify technology
| |
| Prepare a research pack for clinical staffa | |
| Participant‐related suggestions | Provision of taxi vouchers for the participantsa |
| Encouraging participants to use compression | |
| Practitioner‐related suggestions | Double check patients’ eligibility after some time |
Regular training sessions for clinicians
| |
| Ethics‐related suggestions | Arranging physical environment to ensure privacy at the time of recruitment |
| Practice‐related suggestions | If busy, make an appointment next week to discuss research participation |
| Collaboration‐related suggestions | Regular recruitment reminders by research staff |
| Health system‐related suggestions | Financial incentives to recruiting nurses rather than to clinics |
| Increasing clinical staff interest in research activities |
Although discussed as suggestions, these activities were conducted by ASPiVLU investigators.
3. RESULTS
3.1. Participants’ characteristics
We interviewed wound clinic consultants,4 general practitioners,3 wound clinic nurses,9 research nurses,4 and research officers2 who facilitated the ASPiVLU study recruitment. The participant characteristics are shown in Table 4.
Table 4.
Participants’ characteristics
| Participant number | Occupation | Gender | Clinical site | Period of work in this clinical site (years) | Frequency of work with people with VLUs | Number of VLU patients per day | Duration of recruitment for the ASPiVLU | Number of participants recruited/facilitated recruitment |
|---|---|---|---|---|---|---|---|---|
| P1 | Research nurse | F | Sites 1–3 | 2 | 2–3 days/week | 4–10 | Since 2015 | 36 |
| P2 | Research assistant | F | Site 3 | 0.5 | Weekly basis | 15 | Since 2017 | 4 |
| P3 | Project manager | F | Sites 1–4 | N/A | N/A | N/A | N/A | N/A |
| P4 | Clinical nurse consultant | F | Site 4 | 10 | Daily basis | 30 | Since 2016 | 2 |
| P5 | Medical doctor | F | Site 3 | 6 | 2 days/week | Day 1‐12, day 2‐10 | Since 2015 | <10 |
| P6 | Wound nurse consultant | F | Site 4 | 6 | 2–3 days/week | 4 | Since 2017 | 4‐7 |
| P7 | Clinical nurse consultant | F | Site 3 | 3 | 3 days/week | 5 | Since 2015 | 5 |
| P8 | Wound study coordinator | F | Site 2 | 9 | Weekly basis | 1‐6 | Since 2015 | 7‐11 |
| P9 | Clinical nurse coordinator | F | Site 3 | 6 | Weekly basis | 4 | Since 2015 | 6‐7 |
| P10 | Registered nurse | F | Site 3 | 7.5 | Weekly basis | 7‐8 | Since 2015 | 10‐20 |
| P11 | Registered nurse | F | Site 2 | 8 | Weekly basis | 5 | Since 2015 | 6 |
| P12 | Registered nurse | F | Site 2 | 11 | 2 days/week | 6‐7 | Since 2015 | 2 |
| P13 | Registered nurse | M | Site 1 | 17 | Weekly basis | 12 | Since 2015 | 5‐6 |
| P14 | Geriatrician | F | Site 4 | 13 | Weekly basis | 3 | Since 2015 | 2 |
| P15 | Registered nurse | F | Site 3 | 0.5 | Weekly basis | 5‐6 | Since 2017 | 5‐6 |
| P16 | Registered nurse | F | Site 2 | 9 | Weekly basis | 10 | Since 2015 | 6‐7 |
| P17 | Registered nurse | F | Site 2 | 10 | Weekly basis | 15 | Since 2015 | 6‐7 |
| P18 | Research nurse | F | Site 3 | 1 | Weekly basis | 4‐6 | Since 2016 | 14 |
| P19 | Clinical nurse consultant | M | Site 2 | 25 | Weekly basis | 0‐6 | Since 2015 | 15 |
| P20 | General practitioner | F | Site 3 | <0.5 | Weekly basis | 2‐3 | Since 2016 | 0 |
| P21 | Wound clinic consultant | M | Site 2 | 20 | Weekly basis | 15‐20 | Since 2015 | 12‐15 |
| P22 | Research nurse, principal investigator | F | Sites 1–4 | N/A | N/A | N/A | Since 2015 | 36 |
VLU, venous leg ulcer.
3.2. Study‐related factors
3.2.1. Barriers
The main barrier to recruitment as indicated by almost all participants was the study exclusion criteria—the current use of aspirin and anticoagulants (Table 1). Participants reported that most people who had been referred to wound clinics had been diagnosed with CVI and were on treatment with either aspirin or other anticoagulants:
“The only difficulty I find is finding the right patient for it. For example, quite a substantial number of people are already on some form of anti‐coagulants which limit a lot of the inclusion criteria already. And also because a lot of patients will come and see us at the chronic wound service at both hospitals already have some form of comorbidity, so I would say, even in general, 70% or 80% of them are already on some form of anticoagulants so they have to be excluded from the study.” (P6, site 4)
Other identified barriers related to the study requirements for the participants include wearing compression therapy for 24 weeks, which is the current standard evidence‐based practice for VLU management,21 and weekly appointments at the wound clinic over 12 weeks or until healed (whichever comes first).
Clinical nurses also discussed barriers related to the use of technology (Table 1). Although the use of technology (The Samsung Galaxy Note October 1, 2014 Edition Android tablet) was not part of the recruitment process, it was supposed to be used immediately after recruitment, such as taking a picture of the wound and uploading it in the REDCap EDC system (www.projectredcap.org), randomising participants, and recording follow‐up visits.
“…we thought it would be really straight forward, because of the physical environment and the wireless connection at [clinical setting]. It was not straight forward… So uploading photographs is a real problem for us because the tablet wouldn't connect to the Wi‐Fi, or it took about half an hour to upload a photo. And that was really problematic. I think what we ended up doing was I think we got a connector so that we could adapt the tablet to the computer and we uploaded it via the computer rather than using Wi‐Fi. And then the other small issue was the IT problem. So because we took a while to recruit people from the initial training, people made mistakes with the passwords and it actually took a couple of weeks for that to get resolved. And because I was emailing [a research nurse] and then [a research nurse] was passing it onto the IT people, and then they were fixing it. By the time it got through that cycle, our IT recognised it as spam and I never got the new reset password.” (P04, site 4)
“But yeah, if I had to complain about anything, it would probably be that the photographic process was time consuming.” (P07, site 3)
3.2.2. Enablers
Some changes to the initial version of the study protocol, particularly to selection criteria, for example, expanding the age limit and including younger people with VLUs, was described as an enabler (Table 2). Revision of study requirements for the participants, including changing the frequency of appointments, was discussed as an enabler. Although some minor changes to the protocol were sought and approved, there were no significant improvements in recruitment rate as confirmed by project nurses.
3.2.3. Suggestions
One of the interesting suggestions was to carefully consider a recruitment site (Table 3). Some participants suggested other recruitment sites, such as general practices and district nursing. In contrast, other participants had rejected this suggestion and criticised the idea because (1) the aetiology of wounds is confirmed in wound clinics and not in other settings; (2) most GP practices are not specialised in wound care; (3) there is the lack of consistency of practice in district nursing; and (4) district nurses do not usually attend to people younger than 65 years.
“I wonder if we're seeing more complex people come to wound clinics, and they tend to have other comorbidities. So, perhaps that would have been a way to improve recruitment, to include more general practice settings, or even community nursing settings. So, I think changing the sites involved, would be something I would change.” (P14, site 4)
“A GP clinic would be very difficult unless it's a GP clinic that just specialises in getting people in to do wound care. I don't know any of those.” (P17, site 2)
“The problem with district nursing is no consistency of practice. We see that here. We send people away with a clear strict protocol and they come back with something different. The way that district nursing has gone in Victoria, which was from being a community service organisation to being a business; they're now saying, “We won't see anybody under 65.” They're just looking for money.” (P19, site 2)
Other suggestions were to either run a pilot project or to carefully assess patients’ files prior to study. However, both of these measures were carefully implemented by the ASPiVLU researchers as indicated in the ASPiVLU study protocol and were confirmed at the time of interviews. An additional suggestion was to involve more recruitment sites and to expand the recruitment period:
“I know [the chief investigator] has done a very good job getting a lot of sites on board, but perhaps even more sites. I think you've got to work on the assumption that most sites are only going to produce about five to ten patients per year, probably closer to five. So you didn't do your arithmetic, if you've got to study with 300 patients you either increase the recruitment period to say three years, so say the recruitment period is three years, let's be worse case scenario, you only get an average of five patients per year per site, that means over three years you're only going to get 15 patients, which means you need 30 sites or 20 sites to get 300.” (P21, site 2)
3.3. Participant‐related factors
3.3.1. Barriers
The main participant‐related recruitment barriers were comorbidities and the lack of willingness to participate (Table 1). Some of these comorbidities were included in the list of exclusion criteria, such as cardiovascular disease and wounds with both venous and arterial components. Other comorbidities were absolute contraindications to the use aspirin.
“I think as part of my role in my screenings some things that might take a lot of extra time as well is clarifying different medical conditions. We had a patient who had pancytopenia which is like a blood condition. We had to discuss a lot with the consultant at the clinic, to determine that that patient wasn't suitable for the study. Its little things like that that are adding up to why we're not recruiting patients [at the expected rate].” (P02, site 3)
“Say because of their age they mostly got mixed venous ulcers and not just straightforward venous. So they'll have a big arterial component and obviously if they've got an arterial component, you would find that they've got cardiac disease, and if they've got cardiac disease, they often have renal disease and then your age‐related dementia… a lot of them have got diabetes, yeah, so it's almost standard to see the package deal rather than just a single venous leg ulcer.” (P15, site 3)
The description of the potential participants’ willingness to participate varied across sites. Some potential participants were enthusiastic; others were not willing to participate, providing various reasons and sometimes provided no reason (Table 1).
“Well the frustration in not being able to get participants, has mostly been because the vast majority have been excluded because they didn't meet the criteria. And we've only had, I believe, two people that we asked to participate who were eligible, who refused.” (P07, site 3)
“I guess there are some issues with people wanting to be stable or have their symptoms managed before being enrolled in the study because they don't want to take other medication. They don't want to start multiple medications at once.” (P05, site 3)
Some potential participants were not recruited because they did not wish to comply with the study requirements. For example, they did not want to use compression or a particular type of compression. Quite a few clinicians mentioned that some people declined because of fear of blood tests.
“…when we say, ‘As part of this study you need to have blood tests,’ that might turn some people off if they're scared of having blood tests.” [P02, site 3]
“Exactly, little things like that, that you have to take a blood sample.” (P19, site 2)
Limited cognitive abilities and limited English proficiency were the exclusion criteria for the ASPiVLU study. However, most recruiters said that these two barriers did not have a significant influence on recruitment.
“Some of our older patients, it's not they necessarily are cognitively impaired of a particularly high level, but often they have a fear factor that “No, no, I don't want to do that,” because they fear that we're going to do something unusual or somehow put them at risk, and many older people have fears of the unknown.” (P19, site 2)
Some people who were in workforce did not want to make work‐related arrangements in order to come for the study‐related appointments. Transport was not an issue for most people, but a few older people declined to participate because they were unable to come for appointments themselves and relied on their family members who gave them a lift to clinic.
3.3.2. Enablers and suggestions
The main enablers were patients’ enthusiasm and willingness to participate in the study alone, with family support for some (Table 2).
“Most of the patients are reluctant, but some of the patients really are desperate. They want to get the wound healing, they are fantastic. … They said, ‘Okay, take me, I will try this.’” (P16, site 2)
“I just feel that the patients that come in, those that are happy to be recruited, understand that they are fortunate to get the treatment that they're getting; and they're prepared to give something back.” (P17, site 2)
The main suggestions (Table 3) from clinicians included encouraging participants to use compression and provision of taxi vouchers for the participants. However, interviews with the project nurses indicated that the participants who were unable to drive were eligible to get taxi vouchers for all project related trips to the wound clinic.
“I did have one patient and she lived a fair way out. So the problem with wound clinics is there's not many of us and so our catchment goes all the way to [suburb] which is quite a hike for people. And so she became quite unreliable in the end; and we ended up giving her taxi vouchers in order for her to attend her appointments so that we could finish the study off. So that was about $60 each way. So it wasn't a problem because we include that as part of our clinic process anyway, but I'm wondering if transport for people who are struggling with transport, would be something to consider as well?” (P04, site 4)
3.4. Practitioner‐related factors
3.4.1. Barriers
There were many staff‐related barriers, which may have affected the recruitment rate (Table 1). The participants stated that it is difficult to combine their research and clinical roles because of increased workload, resulting in limited time for research activities. The priorities were always given to their clinical roles. They did not deny that, in the busy environment of the wound clinic, they may simply forget about the study.
“Look I'll be frank, I have a busy practice in my private rooms; and I sometimes don't think of research recruitment even into my own studies; and it's only when I'm say correcting my own letter later and I think why didn't I talk about research with this patient.” (P21, site 2)
“…when they first talked about the study, it seemed a little bit complicated because we had technology and [had to] learn how to use [it], and we didn't really have any extra time to do that, we just had to try and squeeze it in between the clinical task we had for the day.” (P10, site 3)
Some staff discussed the complexity of the project‐related activities in relation to recruitment, such as measuring the size of the wound, photographing the wound, entering data, and taking a blood sample or related arrangements with pathology staff.
“What I might change is the process, in terms of putting in the data to make it a little bit easier for the clinicians to deal with. Because, I think, if it's too time consuming, if the process is quite complicated, it does create a little bit of reluctance to start because it just takes so much time to have my recruitment, especially to start with, it does give you a little set‐back sometimes.” (P6, site 4)
Some participants were concerned with their insufficient project‐related knowledge and skills. All staff who attended the pre‐recruitment training session were satisfied with the amount of information and training provided by the ASPiVLU project researchers. However, the issue was rather related to occasional, very infrequent use of these skills as the number of patients eligible for recruitment was low.
“…the loading of photographs was probably the trickiest bit because every single time I had to do it, I did have to look at what I call the ‘Idiots Guide’, the little guide that said what you had to do. So that never became a simple process as such. But that could also be, I had gaps between when I would be with one recruit to the next. And it could well just have been that I had lost track of exactly what I had to do.” (P07, site 3)
“…when you're not recruiting a lot of people then the process that ‐ you forget the processes in between times. So, that takes longer because you're not doing it so frequently. The more frequently you do it, the quicker you get. So, when it's a couple of months between, it's sort of, “What do I have to do?” (P09, site 4)
Most of the participants were concerned with their inability to recruit patients and discussed the process of recruitment as stressful, challenging, overwhelming, and frustrating. Most of them tried to recruit without success and have found the recruitment process for the ASPIVLU study dissatisfying. The recruitment process was also described by many participants as time‐consuming. In the absence of a research nurse/project nurse, clinical nurses who facilitated recruitment were unable to find this extra time in a busy clinical environment:
“I think it would be at least probably 15 minutes to do a brief screening if they're eligible. And then if they're eligible, it would take me probably another half an hour to have a conversation with that patient about all the aspects, answer any questions about it. And then if I've recruited them, then it's probably 45 minutes to do all the initial surveys, quality of life assessment and the impact, and upload that data onto the website. And I set them up with their four weekly review appointments to make sure that they don't miss those key dates. And then I would say that for those dates that we have to do the measurements and upload the data, I would allocate an hour of my time to make sure that I've answered all the questions, to make sure that the paperwork is all accurate and we haven't missed anything, and then we upload it to the database as well.” (P04, site 4)
3.4.2. Enablers and suggestions
Routinisation of research activities, such as regular check of patient logs to locate the patients with VLUs and regular pre‐screening of their histories, was identified as the main enabler to recruitment (Table 2). Trying to overcome difficulties, mostly technology‐related but also time related, in the beginning of the project was also discussed as an enabler. Regular training sessions delivered by project staff, both recruitment‐ and technology‐focused, were the main suggestion that will help to overcome these difficulties and solve the problems (Table 3). Other enablers were clinicians’ awareness of the nature of research and their recognition of the importance of research and evidence‐based practice. Better staffing was the only suggestion to reduce the individual clinician's load in a busy practice. With regards to eligibility criteria, the suggestion was to double check patient's eligibility after some time. Some clinicians stated that, because of changes in the wound size and the distance between the wounds, some of their patients became eligible to participate and were recruited for the study.
3.5. Collaboration‐related factors
3.5.1. Barriers, enablers, and suggestions
Although the participants stated that the absence of a research nurse or other project staff on site in the beginning of the project has contributed to some inconvenience and delays with the recruitment process, this was not the main recruitment barrier (Tables 1 and 2). The main sources of inconvenience were the lack of time, IT problems, and various other problems that commonly occur in the beginning of a project. The availability of a research nurse, a project nurse, and/or a research assistant on site was considered the main enabler by many clinical staff members involved in recruitment.
“…we're really fortunate to have the support from the research team [names] to assist with the process as well, so that actually has helped a lot in terms of recruitment, timewise. The research team, they all have put in a lot of help in terms of going through all these barriers. So all these obstacles eventually are being sorted which is why we eventually could recruit a few more patients after that.” (P06, site 4)
“So what was really great is at the beginning [the project nurse] just came out to clinic on a [a specific day] and hung out with us for a couple of hours, and she helped us physically do the screening with her so we got into the habit of what we were looking for. And then she kind of assisted with the uploading of data and troubleshooting of the uploading of data. And that was really helpful.” (P04, site 4)
Some clinicians, although being appreciative of support provided by the project tem, particularly with screening procedures, mentioned that their support did not impact the number of people recruited because the main reason was related to strict inclusion and exclusion criteria.
“I mean it's been very helpful having the assistance of [names of a research nurse and a research assistant]. So, that's certainly been an advantage, but we still would have recruited the same people, but it's certainly helpful in terms of the workload of the staff to have their assistance with the recruitment and the documentation.” (P09, site 3)
Good relationships between clinical and project staff, team work, and communication with principal investigators were identified as enablers.
“There's also the principal investigator [name] at [site] and he's the one that has to consent a patient if they go into the trial as the leading doctor… He knows a basic amount about the patients that go into the trial. He knows the inclusion criteria… so he's quite good walking between rooms and he might come out of a room and see us and be like, ‘That's the patient for us for a trial there,’ which we might already know anyway but it's nice that he recognizes that.” (P02, site 3)
Both clinical and research staff found recruitment‐related collaboration very effective. More frequent reminders about the study to clinical staff were the only suggestion (Table 3).
3.6. Ethics‐related factors
3.6.1. Barriers
A few participants mentioned that they were carefully considering individual patient's risk–benefit issues (Table 1). Although no participants had described this issue as a major barrier, they mentioned that some patients were excluded because the risks of taking aspirin were outweighing the benefits, as also stated in the exclusion criteria.
“If you haven't found someone that's not on aspirin already or not on any other things already. So you add another medication. It is another potential to harm because you know that adherence of the medication drop, and the polypharmacy issue associated with it. So I agree that that is another consideration. Saying that, I have to say I haven't actually encountered that as a major barrier for that person to be recruited though, but, yes, theoretically… The other thing is also ‐ like when we were talking about bleeding risk, to start someone on aspirin when they are older when they have other comorbidities, I guess that is something also to think about as well, just because of the potential harm. Or if anyone has some reflux on the background, like how comfortable are the clinician to say, “I'm happy for this patient to be on aspirin”? And I guess that that is something to be considered as well. Again, it's the principle of first do no harm. And plus, at the moment, we don't know what's the efficacy or benefit of putting someone on aspirin for venous ulcer.” (P20, site 3)
Prioritising clinical over research roles in a busy clinical environment was another barrier, as discussed below:
Researcher: how difficult/easy for you to combine research work and clinical work?
Participant 10: It is quite difficult, I have to say. It's [research activities] not probably built into normal working day… and our patient schedule list is sort of planned for the day and there's no time allowed, therefore, to take research studies at all. So extra add into the day, and try and squeeze it in.
Researcher: Does it always work?
Participant 10: I suppose the research study takes a lower priority…
Many participants also declared that, when deciding whether to proceed with the recruitment, they were considering their patients’ health state and patient priorities. For example, in some clinical sites, the VLU patients had structured inter‐professional consultations, including a consultation with a nurse, a registrar, a wound consultant, a physiotherapist, and a dietician. Eligible patients were not recruited on the same day if clinicians found that adding a recruitment process to already prolonged consultation would be overwhelming for the patient, delaying the recruited patient's and next patients’ appointments.
“I think it [adding the recruitment process to the regular activities in wound clinics] sometimes becomes quite overwhelming for the patients as well… I see them when they first come to the clinic, so they see a nurse, they see a registrar, they see a consultant, they see a pharmacist, they see a podiatrist and a dietician… they're already going, ‘Oh, it's a lot to deal with.’ And, I think once you bring in some research on top of it… so you do see them walking away with 5000 pieces of paper in their hand going, ‘Oh, I just came in here because I had an ulcer on my leg, I didn't realize that all this other stuff was going on.’” (P12, site 2)
“I think a lot of time when there's a recruitment happening, if I'm aware that it's going to take a long time, I usually will try to continue with it and then if it's affecting my next patient's appointment, if my next patient might be waiting for me, I might have to get another consultant to assist me with it.” (P06, site 4)
Lack of consulting rooms on some clinical sites, where the research nurses/project nurses can discuss the nature of the study and the study requirements with the potential participants, was an ethical issue raised by project nurses. They said that, sometimes, they did not know how to proceed with recruitment in the absence of vacant consulting rooms. No time was set up for the recruitment process, and patients were supposed to leave the room after their consultation and the next patient was invited.
“We don't have many consulting rooms so it's hard for the researchers probably to have their own room to go through patients’ histories and it's probably a bit hard if they just wanted to take the patient somewhere and have a bit of a talk to them outside the waiting – if they're waiting to come in and they just want to ask them a few questions, I don't think that they have a room that they could really take them to so I suppose a shortage of privacy or consulting rooms is a problem so that probably holds it up for them.” (P11, site 2)
3.6.2. Enablers and suggestions
Because of the absence of vacant rooms at some sites, the project nurses just briefly discussed the study with the potential participants and said that they would continue the conversation next time. Transferring the recruitment process to the next appointment, which usually takes less time than the initial appointment, and having a vacant consultation room to maintain privacy were the discussed enablers (Table 2). The participants suggested that having a room for research activities, including the recruitment process, may facilitate recruitment (Table 3).
3.7. Practice‐related factors
3.7.1. Barriers
Main environment‐related barriers were shortage of consulting rooms, which was discussed earlier, and absence of a desktop for nurses at some sites (Table 1). At one site, to enter recruitment‐related data, nurses were supposed to leave their work place and go to the hot desk computers available for hospital staff.
I did not have a desk area to use in the clinic. Eventually, I was able to locate an area in the clinic that I could regularly use… I've just basically used an empty computer near the clinic… I'm an employee of the hospital, I've gone to any hot desk in the hospital that anyone could use if they had hospital logins.” (P08, site 2)
One of the barriers was the profile of patients attending a particular site. As clinicians noted, on some days, they saw many patients who came for a repeat visit rather than new patients.
“I usually don't see the people coming with new wounds; although sometimes I do. I usually see people when they're coming back to have their leg ulcers reviewed.” (P11, site 2)
There were administrative barriers too, such as screening logs not sent on time, the appointments being cancelled, or a particular patient not turning up for an appointment.
“On one day, say we've got 35 patients, five patients would be new patients. Others are all review patients. But the problem that we've actually got them booked in for an appointment and we can sometimes have five people that just don't turn up.” (P17, site 2)
Time pressure that the recruiters experienced in a busy clinical environment was related to shortage of staff. As many participants noted their clinics were “understaffed.” Some people worked part time, and others were on leave; even if the replacement people were available, they were not aware of the study.
“We haven't had huge staff turnover. The nurses at the sites have remained, but people take holidays, people get sick. They have occasions where they're short staffed so they get moved around perhaps. So the fact is that you haven't got somebody potentially in every single clinic.” (P02, site 3)
“Sometimes, if people get sick, we replace with some people who don't know much about the project or the trial. Again if the doctors are not available, people who are replacing them don't know much about the project, so they're not really stress about this.” [P16, site 2]
A low recruitment rate was also related to other concurrent research studies on some sites. If the patients were enrolled in one study, they did not want to participate in the other. Moreover, recruitment for multiple projects was described as overwhelming by some participants.
“At the moment, we're doing three studies, one for [University 1], one for [University 2], one for somewhere else, and then we've got [the name of pharmaceutical company], a pharmaceutical company wanting to trial their new dressing products. It's a lot to think about. And, they all want to see the patients, and they all want to read the notes, and they all want to take up the patients’ time. I definitely have an issue with that; and I think that can become a problem.” (P12, site 2)
3.7.2. Enablers and suggestions
One of the main practice‐related recruitment enablers on a busy clinic day was to make an appointment for the next week to discuss a patient's participation in the study (Table 2). This was also the main suggestion to improve the recruitment rate (Table 3). Another practical suggestion was to book 90‐minute appointments instead of the regular 60 minutes for patients who were eligible to participate.
3.8. Health system‐related factors
3.8.1. Barriers
The issues related to understaffing, the nature of employment, part time versus full time, and replacement of permanent staff by agency nurses, which were discussed as practice‐related barriers above, could be equally allocated to the group of health system‐related factors that influence the recruitment rate (Table 1). In addition to human resource‐related barriers, there were some policies that were discussed as barriers, including a policy on processing research‐related financial incentives, a policy on taking blood samples, and a policy on Internet access by hospital staff. For example, the clinical sites were required to pay a setup fee for pharmacy for every recruited patient upfront. Although this amount of money was later covered by the project funds, some recruiters have found this policy problematic.
“So we have to pay a setup fee for pharmacy, and it's about $600. I mean it's only $50 a year to maintain, but if you only recruit one patient, that's $650. If you recruit 35 patients, its $600 plus the $50 fee. It's a big upfront expense. So, often, we were paying for that out of our operating cost centre…” (P04, site 4)
Another example relates to the policy of collecting blood samples. Although research and clinical nurses were trained to take blood samples, according to the policy at some sites, only pathology staff were eligible to take blood samples, including for the study. This policy and practice involved initial negotiation and additional explanations to be provided to both patients and pathology staff.
“The other thing is we did have initial problem with trying to get the blood test laboratory to understand what sort of blood test we require for research patients, but having said that all this is being sorted out. We expect that there's always an initial hurdle to start with especially when the other departments do not really understand it.” (P06, site 4)
“The challenge in that particular situation was when we were taking the blood but it was going to be stored and it was de‐identified after we took the blood, to a third party. And so, you know, all of the things we train people to do around three forms of ID, whether it's a year, a date of birth and then labelling the blood. We're saying to them, “Yeah take the ID but don't label the blood. Or you label it with a participant number, not the patient name and not the date of birth.” And so that's quite challenging when you spend a lot of time trying to get people to label everything correctly, because it's a bit different to the normal processes.” (P04, site 4)
It is also not a direct barrier to recruitment, as clinicians stated that, on a busy day, they avoid recruiting patients, knowing that the post‐recruitment process is time‐consuming.
3.8.2. Enablers and suggestions
A few participants suggested providing financial incentives to recruiting nurses rather than to site‐specific principal investigators/clinics in order to increase their interest in recruitment (Table 3). However, other participants warned that this type of incentives may not work:
“Some of the nurses told me that six months ago or maybe 12 months ago, they were offered a voucher, if they recruited patients I guess there's a reward. And I know that that [incentive] didn't make a lot of difference because the issue wasn't people finding patients, the issue was patients being eligible and appropriate.” (P05, site 3)
Another suggestion at the system level was to increase clinical staff interest in research activities through training.
“Well, you can only improve it through education and through practice change. So you need to have a large percentage of your undergraduates doing an honours and a PhD before they get to practice. And you need a large amount of research being done that drives nursing practice. Where, if you go out and talk to the average clinician about why are they doing the things they're doing, it's often because they were taught to do them that way, or experience has taught them. They don't sort of say, ‘Well, I've read an article just last week that says I should start thinking about this.’ Often they will, but not as often as I'd like. It's purely education and training. That's what I said – it's going to be several decades before we see any changes.” (P21, site 2)
4. DISCUSSION
Despite some improvements in recruitment reporting, reporting of participants’ eligibility and the reasons for exclusion are frequently incomplete.22 By conducting qualitative interviews with clinical staff involved in ASPiVLU recruitment, we aimed to elicit more details on the barriers that influenced the low recruitment rate for this trial and to identify recruitment enablers. Consistent with other studies, the main reasons for a low recruitment rate for ASPiVLU were strict eligibility criteria and investigators’ “overestimation” of the pool of the potential participants. Overestimation of the pool of the potential participants is a common problem among investigators.12 Difficulty finding participants for RCTs even if there were no issues in a pilot project has been identified by other researchers12, 23 and is known in literature as Lasagna Law24 and Muench's Third Law.25 One of the most crucial factors to be considered when explaining this phenomenon is that the number of eligible patients is not equal to the number of recruited patients. That is, the recruiters do not always have time to recruit eligible patients, and not all eligible patients will be willing to participate for a number of reasons, as we have outlined above. To avoid overestimation, we suggest that future investigators base their estimates on the lowest, rather than the mean, number of participants recruited over a period of time from 1 particular setting in their pilot project. Investigators need to be aware that recruitment for RCTs in the health field usually takes place in a complex environment where numerous factors intersect and impact the recruitment rate,16 as in the case of the ASPiVLU.
Summarising and grouping barriers and enablers to recruitment for this project, we have developed an expanded framework of factors influencing recruitment for RCTs, utilising a basic framework of barriers and enablers for RCTs developed by French and Stavropoulou.16 Study‐related barriers were the most important group of barriers for ASPiVLU recruitment. However, other factors, including patient‐related, practitioner‐related, and practice‐ related barriers, have also contributed to the low recruitment rate. The additional factors included in our framework were ethics‐related factors and health system‐related factors. We summarised all factors in Tables 1 and 2. Our expanded framework takes into account how broader policies and practices at the level of the health systems may influence the recruitment rate. For example, time‐consuming project‐related activities that take place immediately after recruitment, such us arranging pathology rather than taking blood samples by a clinical nurse and contacting pharmacy staff to arrange either placebo or the medicine at some sites rather than having them on site, may also influence clinicians’ decision to avoid recruitment as a matter of saving time for their clinical work. Concurrent recruitment to other studies at the same site should also be considered.26
The investigators need to be aware that the recruiters’ clinical roles will always be prioritised over their research roles and individual patients’ priorities over research priorities, as Elliott and co‐authors19 have also suggested. This phenomenon/role is known as gatekeeping and is widely discussed as a barrier to recruitment to RCTs in other fields.26, 27, 28, 29 Sometimes, gatekeeping can be a controversial role when the potential participants themselves may want to participate in the study and feel rewarded because other people would benefit from the study findings.29 Patients trust their treating health professionals, and if they do not advocate for study participation, then it is unlikely to occur.
A qualitative approach has helped us identify various recruitment enablers and some relevant suggestions on how to improve recruitment rate of RCTs on wound management, although enablers are rarely reported, as the results of the recent systematic review has indicated.15 However, these identified enablers and proposed suggestions should be considered with caution because some of them may not always work. For example, the availability of researchers on‐site was discussed by many participants as the main enabler, although it helped to slightly increase the recruitment rate at 1 site. Project researchers helped with pre‐screening of eligible patients, discussing the nature of the study, randomising patients, entering patients’ details in the system and recording follow‐up visits, which may have reduced the burden of clinical staff in a busy clinical site, but the number of recruited people was only slightly increased because of strict recruitment criteria and other multiple reasons, including patients’ willingness to participate. Similarly, a suggestion to introduce financial rewards for recruiting staff for each recruited patient, although identified as a promising strategy to improve the recruitment rate in a systematic review,8 may not always work if the main reason is related to the lack of patients eligible for an RCT, as in the case of ASPiVLU. Principal investigators of future trials may consider training research nurses to screen and collect data rather than offer payment per recruit.
This study highlights the complexity of recruitment barriers and enablers and the need for clear communication to health professionals and researchers about various factors that may influence recruitment rate and offering annual training for recruiters if a study recruits for more than 1 year. The findings have the potential to improve participant recruitment skills and enhance recruitment rates in future chronic wound RCTs. Most of these findings could be taken into consideration by RCTs in other fields. In addition, we suggest that that the main factors influencing recruitment be considered for inclusion in the “Other Information” section of Consolidated Standards of Reporting Trials Statement to improve the quality of reporting.
ACKNOWLEDGEMENTS
This study was funded by the NHMRC project grant GNT1069329 (The Aspirin in Venous Leg Ulcer Randomised Controlled Trial: [ACTRN12614000293662]) awarded to Carolina D. Weller. The authors acknowledge Mrs Louise Turnour for facilitating the recruitment of health professionals and Dr Jac‐Kee Low for coding some of the interview transcripts.
Conflict of interest
The authors have no conflict of interest to declare.
Team V, Bugeja L, Weller CD. Barriers and facilitators to participant recruitment to randomised controlled trials: A qualitative perspective. Int Wound J. 2018;15:929–942. 10.1111/iwj.12950
Authorship: All persons designated as authors qualify for authorship. Each author has participated sufficiently in the work to take public responsibility for the content.
Funding information National Health and Medical Research Council, Grant/Award Number: GNT1069329
REFERENCES
- 1. Franks PJ, Barker J, Collier M, et al. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care. 2016;25:S1‐S67. [DOI] [PubMed] [Google Scholar]
- 2. Wounds Australia . Standards for Wound Prevention and Management. 3d ed. Osborne Park, WA: Cambridge Media; 2016. [Google Scholar]
- 3. Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the gold standard—lessons from the history of RCTs. N Engl J Med. 2016;374:2175‐2181. [DOI] [PubMed] [Google Scholar]
- 4. Barker J, Weller C. Developing clinical practice guidelines for the prevention and management of venous leg ulcers. Prim Inten. 2010;18:62. [Google Scholar]
- 5. Bucci S, Butcher I, Hartley S, Neil ST, Mulligan J, Haddock G. Barriers and facilitators to recruitment in mental health services: care coordinators’ expectations and experience of referring to a psychosis research trial. Psychol Psychother. 2015;88:335‐350. [DOI] [PubMed] [Google Scholar]
- 6. Foster JM, Sawyer SM, Smith L, Reddel HK, Usherwood T. Barriers and facilitators to patient recruitment to a cluster randomized controlled trial in primary care: lessons for future trials. BMC Med Res Methodol. 2015;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walters SJ, Bonacho dos Anjos Henriques‐Cadby I, Bortolami O, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7:e015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta‐analysis. BMJ Open. 2013;3:e002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glasser SP. Recruitment and retention in clinical research. In: Glasser SP, ed. Essentials of Clinical Research. Cham, Switzerland: Springer International Publishing; 2014:177‐192. [Google Scholar]
- 10. Briel M, Olu KK, von Elm E, et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol. 2016;80:8‐15. [DOI] [PubMed] [Google Scholar]
- 11. Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 12. Thoma A, Farrokhyar F, McKnight L, Bhandari M. How to optimize patient recruitment. Can J Surg. 2010;53:205‐210. [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet. 2010;357:1191‐1194. [PubMed] [Google Scholar]
- 14. Weller C, McNeil J. CONSORT 2010 statement: updated guidelines can improve wound care. J Wound Care. 2010;19:347‐353. [DOI] [PubMed] [Google Scholar]
- 15. Bugeja L, Low JK, McGinnes R, Team V, Sinha SN, Weller C. Barriers and enablers to patient recruitment for randomised controlled trials on treatment of chronic wounds: a systematic review. Int Wound J. 2018;1‐13. 10.1111/iwj.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. French C, Stavropoulou C. Specialist nurses’ perceptions of inviting patients to participate in clinical research studies: a qualitative descriptive study of barriers and facilitators. BMC Med Res Methodol. 2016;16:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open. 2012;2:e000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newington L, Metcalfe A. Factors influencing recruitment to research: qualitative study of the experiences and perceptions of research teams. BMC Med Res Methodol. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol. 2017;72:789‐798. [DOI] [PubMed] [Google Scholar]
- 20. Weller CD, Barker A, Darby I, et al. Aspirin in venous leg ulcer study (ASPiVLU): study protocol for a randomised controlled trial. Trials. 2016;17:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Australian Wound Management Association , New Zealand Wound Care Society . Australian and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers. Osborne Park, Western Australia, Australia: Cambridge Publishing; 2011. [Google Scholar]
- 22. Toerien M, Brookes ST, Metcalfe C, et al. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lasagna L. Problems in publlcatlon of clinical trial methodology. Clin Pharmacol Ther. 1979;25:751‐753. [DOI] [PubMed] [Google Scholar]
- 25. Bearman JE, Loewenson RB, Gullen WH. Muench's Postulates, Laws and Corollaries, or Biometrician's Views on Clinical Studies (Biometric Note 4). Bethesda, MD: Office of Biometry and Epidemiology, National Eye Institute, National Institutes of Health; 1974. [Google Scholar]
- 26. Borschmann R, Patterson S, Poovendran D, Wilson D, Weaver T. Influences on recruitment to randomised controlled trials in mental health settings in England: a national cross‐sectional survey of researchers working for the mental Health Research network. BMC Med Res Methodol. 2014;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gail E, Margaret R, Stephen B, Janet M, Anna M, Chris T. Recruiting patients into a primary care based study of palliative care: why is it so difficult? Palliat Med. 2004;18:452‐459. [DOI] [PubMed] [Google Scholar]
- 28. Patterson S, Mairs H, Borschmann R. Successful recruitment to trials: a phased approach to opening gates and building bridges. BMC Med Res Methodol. 2011;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abernethy AP, Currow DC, Wurzelmann J, et al. Enhancing enrollment in palliative care trials: key insights from a randomized, placebo‐controlled study. J Support Oncol. 2010;8:139‐144. [PubMed] [Google Scholar]


