Abstract
A Pseudomonas aeruginosa quorum‐sensing system, which produces N‐(3‐oxododecanoyl)‐l‐homoserine lactone (3‐oxo‐C12‐HSL) and N‐butanoyl‐l‐homoserine lactone (C4‐HSL), regulates the virulence factors. In our previous study, 3‐oxo‐C12‐HSL, encoded by lasI gene, was shown to promote wound healing. However, the effect of C4‐HSL, encoded by rhlI gene, remains to be elucidated. We addressed the effect of C4‐HSL on wounds in P. aeruginosa infection. Wounds were created on the backs of Sprague–Dawley SD rats, and P. aeruginosa PAO1 (PAO1) or its rhlI deletion mutant (ΔrhlI) or lasI deletion mutant (ΔlasI) was inoculated onto the wound. Rats were injected intraperitoneally with anti‐C4‐HSL antiserum or treated with C4‐HSL at the wound surface. PAO1 inoculation led to significant acceleration of wound healing, which was associated with neutrophil infiltration and TNF‐α synthesis. These responses were reversed, except for TNF‐α production, when ΔrhlI was inoculated instead of PAO1 or when rats were co‐treated with PAO1 and anti‐C4‐HSL antiserum. In contrast, the healing process and neutrophil infiltration, but not TNF‐α synthesis, were accelerated when C4‐HSL was administered in the absence of PAO1. This acceleration was not affected by anti‐TNF‐α antibody. These results suggest that C4‐HSL may be involved in the acceleration of acute wound healing in P. aeruginosa infection by modifying the neutrophilic inflammation.
Keywords: N‐butanoyl‐l‐homoserine lactone, Pseudomonas aeruginosa, Quorum sensing, Wound healing
Introduction
The wound healing process in skin consists of three stages, including the activation of inflammatory pathways leading to infiltration of leukocytes into the wound site, tissue degradation and tissue formation 1. Neutrophils quickly infiltrate the wound tissues, which are affected by interleukin (IL)‐1β, tumour necrosis factor (TNF)‐α, interferon (IFN)‐γ and certain pathogen‐associated molecular patterns (PAMPS), including bacterial components from Pseudomonas aeruginosa 1, 2. Although neutrophils are reported to inhibit some aspects of the repair process 3, they are intimately involved in natural wound healing, especially bacterial‐contaminated wounds 4. Previously, we demonstrated that P. aeruginosa inoculation promoted skin wound healing by inducing neutrophil infiltration, in which TNF‐α played a critical role 4. Macrophages were recruited after the infiltration of neutrophils and they supported the ongoing repair process, including the promotion and resolution of inflammation upon exposure to proinflammatory cytokines, lipopolysaccharides (LPS) or other microbial products 5.
P. aeruginosa is often isolated from chronic wounds as a primary causative agent of infection and the presence of this bacterium appears to be involved in the delay of wound repair 6. Acute infection with this bacterium leads to the production of proinflammatory cytokines and chemokines, such as TNF‐α, IL‐1α, IL‐1β and IL‐8, and a massive influx of neutrophils into the wound tissues 6. P. aeruginosa is rarely isolated from acute wounds, and thus, this bacterium appears to have different effects on the healing process between acute and chronic wounds 1, 6. Recent studies from our and other laboratories have demonstrated that quorum‐sensing (QS) molecules secreted from P. aeruginosa trigger the initiation of inflammatory responses 2, 7, 8, 9. Two different QS molecules, N‐3‐oxododecanoyl homoserine lactone (3‐oxo‐C12‐HSL) and butanoyl‐homoserine lactone (C4‐HSL), are encoded by the las and rhl genes, respectively. They are generated when the growth of this bacterium reaches a threshold level 10. A hierarchical relationship exists between the Las and Rhl systems, where the Las system controls the Rhl system, as shown by direct upregulation of rhlR transcription by the 3‐oxo‐C12‐HSL–LasR, receptor of its HSL, complex 11.
3‐Oxo‐C12‐HSL plays a critical role in regulating bacterial virulence 12 and modulating several mammalian cells including neutrophils 8, 13 and macrophages 13. In addition, we demonstrated that 3‐oxo‐C12‐HSL was involved in the wound healing process by inducing TNF‐α production 2. In contrast, although C4‐HSL is also involved in the regulation of bacterial virulence 14, 15, it is not fully understood how this QS molecule affects the host immune responses. In our previous investigation, we determined that inoculation with a P. aeruginosa PAO1 strain promotes only the acute phase of wound healing by inducing a ‘moderate’ and ‘evanescent’ inflammatory response 4.
In the present study, we used a rat model with deep partial‐thickness wounds to examine the involvement of C4‐HSL in acute skin wounds that healed following infection with P. aeruginosa. We also addressed the role of this QS molecule in leukocyte accumulation and TNF‐α synthesis. We demonstrated that C4‐HSL helped to promote the wound healing process, as shown by increased wound re‐epithelialisation, epidermal cell proliferation and neo‐vascularisation and by accelerated inflammatory responses, such as the infiltration of neutrophils and macrophages, but not TNF‐α synthesis.
Materials and methods
Animals
Male Sprague–Dawley (SD) rats (200–300 g, 7–10 weeks of age) were obtained from Clea Japan, Inc. (Tokyo, Japan) and maintained under a 12‐hour light/12‐hour dark cycle. Food and water were made available ad libitum. All handling of the animals was performed under anaesthesia that was induced by pentobarbital (40 mg/kg; NEMBTAL, Dainippon Sumitomo Pharma, Osaka, Japan). At the end of the experiments, the animals were euthanised by an overdose of pentobarbital. All experimental protocols described in the present study were approved by the Ethics Review Committee for Animal Experimentation of Tohoku University.
Bacteria
P. aeruginosa strain PAO1 and its rhlI‐deletion mutant strain KG7002 (ΔrhlI) and lasI‐deletion mutant strain KG7001 (ΔlasI) 16 were stored in a Microbank storage system (Pro‐Lab Diagnostics Inc., Richmond Hill, ON, Canada) at −80°C. These bacteria were inoculated onto a sheep blood agar plate and incubated at 37°C overnight, and colonies were cultured in Brain Heart Infusion (BHI) broth (Eiken Chemical Co., Ltd, Tokyo, Japan) at 37°C for 24 hours and washed three times in normal saline. After the final suspension was mixed, bacterial counts were performed by measuring absorbance at 600 nm. In each experiment, a quantification culture was performed to confirm the inoculation dose.
Wound creation and tissue collection
Dorsal hair of the SD rats was shaved to fully expose the skin, which was then disinfected using 70% ethanol. Deep partial‐thickness dermal wounds preserving the cutaneous muscle were created on the backs of the rats using a 6‐mm diameter biopsy punch (Biopsy Punch, Kai Industries Co., Ltd, Gifu, Japan) and scissors under sterile conditions. Ten deep, partial‐thickness wounds were made on the back of each rat. Wounds were separated from each other by at least 5 mm. A suspension (11 µl) of P. aeruginosa PAO1, ΔrhlI or ΔlasI was applied to the base of the wounds at 3·5 to 5·5 × 107 cfu/wound in an individual rat. P. aeruginosa solution was prepared in every experiment to keep their viability, and therefore, it is not possible to prepare them at exactly the same inoculum dose. Uninoculated wounds in other rats were used as controls. Wounds were covered with a polyurethane foam dressing (Tegaderm Transparent Dressing, 3M Health Care, St. Paul, MN) and the whole trunk of the rats was covered with an elastic adhesive bandage (Hilate, Iwatsuki, Tokyo, Japan) to protect the wound from contamination and mechanical irritation. The day when the wounds were made was designated as day 0. Tissues were harvested from the wound sites at days one and three post‐wounding by excising the area with an 8‐mm diameter biopsy punch.
Reagents, antibodies and antiserum
C4‐HSL was purchased from Santa Cruz Biotechnology (Dallas, TX). 3‐Oxo‐C12‐HSL was synthetically produced by Wako Pure Chemical Industries (Osaka, Japan), dissolved in dimethyl sulfoxide (DMSO) at 3 mg/ml and then diluted with PBS to a final dose of 0·1% DMSO for in vivo use. Wounds were created using the above‐mentioned methods, and just after wounding, a 10 µl suspension of 10 μM C4‐HSL, 3‐oxo‐C12‐HSL or control DMSO was applied to the base of the wounds. Anti‐TNF‐α monoclonal antibody (mAb) was purified from the culture supernatants of hybridoma cells (clone MP6‐XT2.2‐11) using a protein G column kit (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). To neutralise the TNF‐α biological activity, rats were injected intraperitoneally with 1 mg of a mAb against this cytokine one day before wound creation. Rat IgG (ICN Pharmaceuticals, Inc., Aurora, OH) was used as the control Ab. To neutralise the biological activity of C4‐HSL or 3‐oxo‐C12‐HSL, rabbit antiserum against each compound, prepared as previously described 17, was injected intraperitoneally at a dose of 3 ml/kg, 18 hours before wound creation. Non‐immune rabbit serum was used as a control.
Histology and immunohistochemistry
The wound tissues were collected using an 8‐mm diameter biopsy punch (Biopsy Punch, Kai Industries Co., Ltd, Gifu, Japan) and dissected in the caudocranial direction at the centre of the wound. The tissues were fixed with a 4% paraformaldehyde‐phosphate buffer solution and embedded in paraffin. Sections 3 µm thick were harvested from the central portion of each wound and stained with haematoxylin–eosin (HE), according to the standard method. Epithelial migration and the extent of re‐epithelialisation were measured in these HE‐stained sections by measuring the distance from the normal wound margin to the edge of the migrating epithelium at the centre of the wound. The re‐epithelialisation index was determined based on the percentage of new epithelium present in the total wound (n = 6 per group). For immunohistochemistry, sections were treated with anti‐PCNA antibody (1:400 dilution; Dako, Tokyo, Japan) or anti‐CD31 antibody (1:100 dilution; Santa Cruz Biotechnology, Dallas, TX) after endogenous peroxidase and non‐specific binding were blocked. They were then incubated with peroxidase‐conjugated secondary antibodies (simple stain rat MAX‐P®, Nichirei Co., Tokyo, Japan). To analyse keratinocyte proliferative activity in the neo‐epidermis, the incidence of PCNA‐positive basal nuclei in 500 cells between the wound margin and the extremity of the neo‐epidermis was determined. The number of PCNA‐positive basal cells is presented as a percentage of these 500 cells. The vascular density in the granulation tissue in five random fields (each 0·2 mm2) was determined by counting the number of CD31‐positive vessels. All analyses were performed under blinded conditions. Control sections were treated with isotype‐matched irrelevant IgG in place of the first antibodies.
Preparation of leukocytes in the wound tissues
One group of rats was sacrificed 24 hours after wounding. Tissue samples from the wounds were collected from three rats in each group, as described below. The area was excised using a biopsy punch (8 mm in diameter) and teased apart using a stainless‐steel mesh in RPMI1640 medium (Nipro, Osaka, Japan) supplemented with 10 mM HEPES, 10% foetal calf serum (FCS) (BioWest, Nuaillé, France), 1 mg/ml collagenase and 1 mg/ml hyaluronidase (Sigma‐Aldrich, St. Louis, MO). The pellets were washed, then suspended in 4 ml of 40% Percoll solution (Pfizer, Inc., Täby, Sweden), added to 80% Percoll solution and centrifuged at 579 × g for 20 min at 20 °C. Pellets were washed three times with RPMI1640 supplemented with 1% FCS. Approximately 1 × 105 cells were applied onto a glass slide at 40 × g for 3 min using a Cytofuge‐2 centrifuge (Statspin Inc., Norwood, MA) and stained using the Diff‐Quik technique. The total number of neutrophils, lymphocytes and macrophages was estimated by multiplying the total leukocyte number by the proportion of each fraction in 500 cells.
RNA extraction and quantitative real‐time RT‐PCR
Total cellular RNA was extracted from the wound tissues using ISOGEN (Wako Pure Chemical, Osaka, Japan) Japan), and first‐strand cDNA was synthesised using PrimeScript first‐strand cDNA synthesis kit (TaKaRa Bio Inc., Otsu, Japan), according to the manufacturer's instructions. Quantitative real‐time PCR was performed in a volume of 20 µl using gene‐specific primers and FastStart essential DNA green master mix (Roche Applied Science, Branford, CT) in a LightCycler nanosystem (Roche Applied Science).
The primer sequences used for amplification are shown in Table 1. Reaction efficiency with each primer set was determined using standard amplifications. Target gene expression levels and that of the glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene as a reference were calculated for each sample using the reaction efficiency. We confirmed that expression of GAPDH was not affected by P. aeruginosa inoculation. The results were analysed using a relative quantification procedure and are presented as expressions relative to GAPDH expression.
Table 1.
Primer sequences for real‐time PCR
| Gene product | Forward primer | Reverse primer |
|---|---|---|
| TNF‐α | CCCAGACCCTCACACTCAGAT | TTGTCCCTTGAAGAGAACCTG |
| IL‐1β | CACCTTCTTTTCCTTCATCTTTG | GTCGTTGCTTGTCTCTCCTTGTA |
| IL‐6 | AGCCCACCAGGAACGAAAGTCA | TGGCTGGAAGTCTCTTGCGGA |
| IL‐17A | TTCCATCCATGTGCCTGATGCTG | TCGGCGTTTGGACACACTGAAC |
| CXCL‐1 | TGGAGAAAGAAGATAGATTGC | TTCTTCCCGCTCAACACCTTC |
| CXCL‐2 | CCTGGAAAGGAAGAACATGGG | ACCTCCCAACTACATAAGTAA |
| CXCL‐3 | TGGTCAAGAAGTTTGTCTCAACCC | CACAGGGAGGGGCTCTTCAGTA |
| GAPDH | TAGAGACAGCCGCATCTTCTTGTG | GCCAAATCCGTTCACACCGAC |
GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; IL‐1β, interleukin (IL)‐1β; TNF‐α, tumour necrosis factor.
Measurement of cytokine concentrations
Wound tissues were collected from three rats in each group and homogenised with RPMI1640 medium supplemented with 10 mM HEPES, 10% FCS, 1 mg/ml collagenase and 1 mg/ml hyaluronidase, and the concentration of TNF‐α in the supernatants was measured using an enzyme‐linked immunosorbent assay (ELISA) kit (Endogen, Cambridge, MA). The results are expressed as the average value present in one wound. The detection limit was 5·1 pg/ml.
Statistical analysis
Data analysis was performed using a statistical software package (SPSS 13.0 J for Windows, SPSS Japan, Tokyo, Japan). Data are expressed as the mean ± standard deviation (SD). Statistical analysis between groups was performed using the Mann–Whitney U‐test, Student's t‐test or one‐way ANOVA. A P value <0·05 was considered to indicate statistical significance.
Results
Effect of P. aeruginosa PAO1 or ΔrhlI inoculation on wound healing, accumulation of neutrophils and TNF‐α expression
Previously, we reported that inoculation of P. aeruginosa PAO1 promoted the healing process at day 3, but not at other time points, after wound creation 4. In the present study, we asked how deletion of either rhlI or lasI gene or both in P. aeruginosa influenced the wound healing effect caused by this bacterium on day 3. We created deep partial‐thickness wounds, preserving the cutaneous muscle, in the backs of rats and histologically evaluated the process of re‐epithelialisation. As shown in Figure 1A, re‐epithelialisation was promoted on day 3 after wounding, as shown by the leading edges (arrows) of epidermis, which tended to extend farther in the PAO1‐inoculated group than in the uninoculated group; these findings were not observed when wounds were inoculated with ΔrhlI or ΔlasI instead of PAO1. To quantitatively evaluate re‐epithelialisation, the distance between the leading edges of the epidermis on either side of the wound was measured in the histological sections at day 3 after wounding, and the re‐epithelialisation extent was calculated as the ratio of the distance between the leading edges to the distance between the wounded margins (arrowheads). As shown in Figure 1B, the re‐epithelialisation extent was significantly greater in the PAO1 group than in the ΔrhlI, ΔlasI and uninoculated groups on day 3. As alternate indicators of wound healing, we evaluated the expression levels of proliferating cell nuclear antigen (PCNA) in the epidermis and CD31 in the dermal layer, which indicated epidermal cell proliferation and neo‐vascularisation, respectively. As shown in Figure 1C and D, the expression levels of both indicators were significantly greater in the PAO1 group than in the ΔrhlI, ΔlasI and uninoculated groups. In addition, we confirmed that there were no differences in the amounts of bacteria in the wounds on day 3 among the groups and no other bacterial strain was detected in the wounded tissue after inoculation with P. aeruginosa PAO1, rhlI deletion or lasI deletion. Thus, these data demonstrate that P. aeruginosa PAO1 is involved in the promotion of the acute phase of wound healing.
Figure 1.
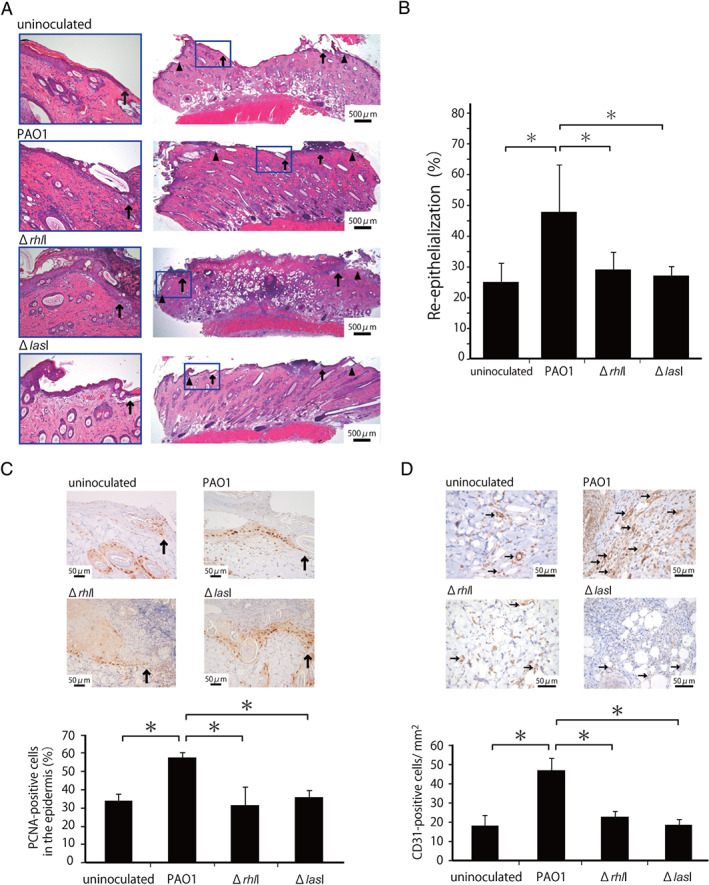
Effect of the rhlI‐defect on skin wound healing promoted by Pseudomonas aeruginosa. Wounds were created and a suspension of PAO1 or ΔrhlI or ΔlasI was inoculated into the wounds. Uninoculated wounds were created in other rats. (A) Representative histological views of skin wounds on day 3. Arrowheads and arrows indicate the original wound edges and re‐epithelialised leading edges, respectively. (B) Re‐epithelialisation extent was calculated on day 3 (n = 6). (C) Cellular proliferation in the epidermis at the wound edges. Arrows indicate proliferating cell nuclear antigen (PCNA)‐positive microvessels. The percentages of PCNA‐positive cells among the 500 cells in the epidermis on day 3 (n = 3). (D) Microvessel counts on day 3. Arrows indicate CD31‐positive microvessels. The vascular density/mm2 was determined by counting the positive vessels within six visual fields (n = 3). Each column represents the mean ± standard deviation (SD). *P < 0·05.
Neutrophil accumulation at the wound sites after P. aeruginosa PAO1 or ΔrhlI inoculation
In our previous study, local neutrophil infiltration at the wound site reached a peak level at 24 hours after P. aeruginosa inoculation 4, and lasI gene contributed to neutrophil infiltration 2. In the next series of experiments, we addressed the question of whether the P. aeruginosa rhlI gene affected neutrophil infiltration. To quantitatively evaluate the cellular inflammatory responses, leukocytes were isolated from the wound tissue homogenates, and the total number of leukocytes and the proportions of neutrophils, macrophages and lymphocytes were morphologically analysed at 24 hours after wounding. As shown in Figure 2A, the number of total leukocytes and neutrophils was significantly greater in the PAO1 group than in the ΔrhlI, ΔlasI and uninoculated groups. There was no significant difference in the numbers of macrophages and lymphocytes between the four groups.
Figure 2.
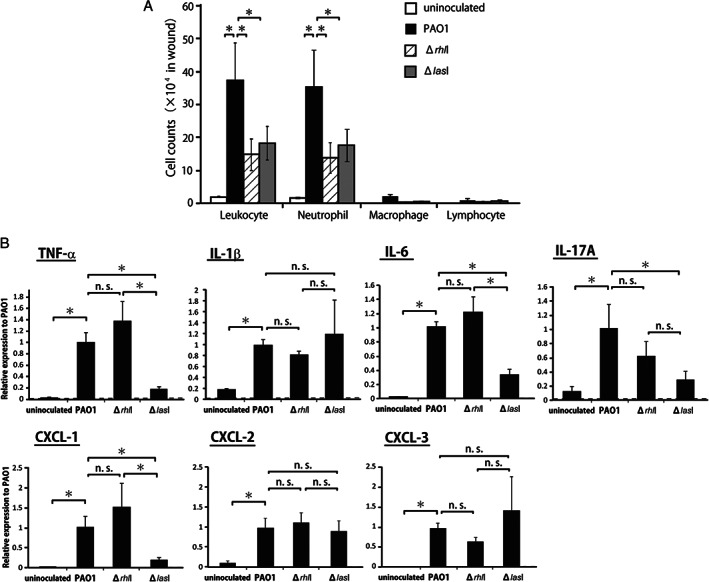
Cellular inflammatory response and expression of inflammatory cytokines after inoculation with Pseudomonas aeruginosa PAO1, ΔrhlI or ΔlasI. (A) Numbers of leukocytes, neutrophils, macrophages and lymphocytes in the wound tissues from rats inoculated with P. aeruginosa PAO1, ΔrhlI, ΔlasI or uninoculated rats were analysed at 24 hours following wound creation. (B) Tumour necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6, IL‐17A, CXCL‐1, CXCL‐2 and CXCL‐3 expression in wounds was measured at 12 hours following wound creation. Each column represents the mean ± standard deviation (SD). *P < 0·05; NS, not significant.
Inflammatory cytokine and chemokine expression after inoculation with P. aeruginosa PAO1, ΔrhlI or ΔlasI
Inflammatory cytokines and chemokines play a central role in leukocyte accumulation in the wounded tissues. Therefore, we next examined the expression of TNF‐α, IL‐1β, IL‐6, IL‐17A, and CXCL‐1, CXCL‐2 and CXCL‐3. As shown in Figure 2B, cytokine and chemokine mRNA expression was strongly induced at 12 hours post‐wounding in the PAO1 group compared with that in the uninoculated group. The increase in TNF‐α, IL‐6, IL‐17A and CXCL‐1 mRNA expression was significantly attenuated when the ΔlasI strain was inoculated instead of the PAO1 strain. However, attenuation was not observed when the ΔrhlI strain was used. Similar results were obtained when TNF‐α production in the wounded tissues was measured using ELISA (Figure S1, Supporting information). However, IL‐1β, CXCL‐2 and CXCL‐3 mRNA expression was comparable among the PAO1, ΔrhlI and ΔlasI groups.
Contribution of C4‐HSL to the wound healing promoted by P. aeruginosa PAO1 inoculation
To define the role of C4‐HSL in the wound healing promoted by P. aeruginosa PAO1, we first examined the effects of anti‐C4‐HSL antiserum on the wound healing process. As shown in Figure 3A–D, treatment with anti‐C4‐HSL antiserum significantly reduced the re‐epithelialisation, epidermal cell proliferation and neo‐vascularisation almost to the levels in the uninoculated group on day 3 after wounding, which was similar to the results observed using anti‐3‐oxo‐C12‐HSL antiserum. Non‐immune rabbit serum did not show these effects. In addition, we confirmed that there was no difference among the groups in the bacterial counts in the wounds on day 3.
Figure 3.
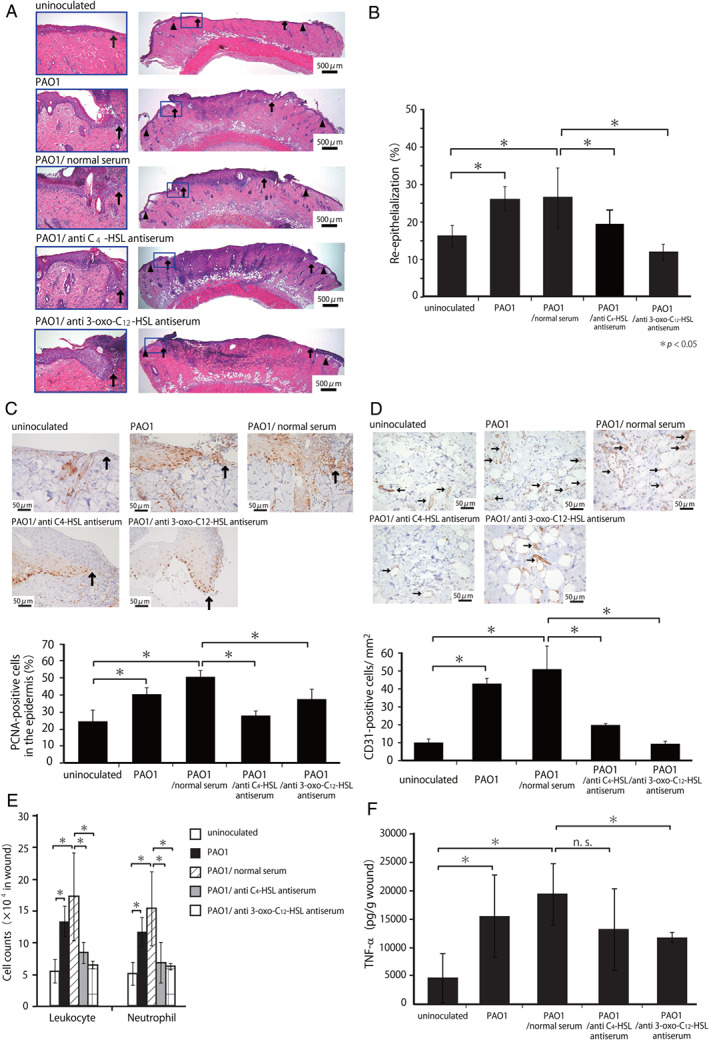
Effect of anti‐C4‐HSL antiserum on wound healing promoted by Pseudomonas aeruginosa PAO1 inoculation. Rats were injected with anti‐C4‐HSL antiserum, anti‐3‐oxo‐C12‐HSL antiserum or preimmune serum. (A) Representative histological views on day 3. Arrowheads and arrows indicate the original wound edges and re‐epithelialised leading edges, respectively. (B) Re‐epithelialisation extent on day 3. (C) Cellular proliferation in the epidermis on day 3. Arrows indicate PCNA‐positive microvessels. The percentages of PCNA‐positive cells among the 500 cells in the epidermis were calculated (n = 3). (D) Microvessel counts on day 3. Arrows indicate CD31‐positive microvessels. The vascular density/mm2 was determined by counting the numbers of positive vessels within six visual fields (n = 3). (E) The number of leukocytes and neutrophils was analysed at 24 hours. (F) TNF‐α concentrations were measured at 24 hours. Each column represents the mean ± standard deviation (SD). *P < 0·05; NS, not significant.
To evaluate the inflammatory responses, leukocytes were isolated from the wound tissues on day 1, and the number of leukocytes and neutrophils was counted. As shown in Figure 3E, treatment with anti‐C4‐HSL or anti‐3‐oxo‐C12‐HSL antiserum significantly reduced the increase in the number of leukocytes and neutrophils induced by PAO1 inoculation, whereas a similar reduction was not observed by treatment with non‐immune rabbit serum. In addition, as shown in Figure 3F, treatment with anti‐3‐oxo‐C12‐HSL antiserum significantly reduced the increase in TNF‐α synthesis in the wound skin homogenates caused by PAO1 inoculation, whereas such effects were not detected by treatment with anti‐C4‐HSL antiserum or non‐immune rabbit serum. These results suggested that C4‐HSL and 3‐oxo‐C12‐HSL may contribute to the wound healing processes through a different mechanism than that promoted by inoculation with P. aeruginosa.
C4‐HSL promotes wound healing
To further address the possibility that C4‐HSL may contribute to the wound healing processes promoted by P. aeruginosa inoculation, we examined how treatment with 10 μM C4‐HSL affected the re‐epithelialisation and inflammatory responses during wound healing. As shown in Figure 4A–D, the re‐epithelialisation, epidermal cell proliferation and neo‐vascularisation were significantly enhanced in the C4‐HSL‐treated group compared to the control group, which was similar to the results obtained in the 3‐oxo‐C12‐HSL‐treated group compared to the control group. We then examined the effects of C4‐HSL treatment on the inflammatory responses during the wound healing processes. As shown in Figure 4E, the number of leukocytes and neutrophils in the wound tissues on day 1 was significantly higher in the C4‐HSL‐treated group than that in the control group. In contrast, local TNF‐α synthesis in the wound skin homogenates was not affected by C4‐HSL administration (Figure 4F). To further address the role of TNF‐α, we examined the effect of the neutralising anti‐TNF‐α mAb on the wound healing processes promoted by C4‐HSL. As shown in Figure 5A and B, treatment with anti‐TNF‐α mAb did not affect the re‐epithelialisation promoted in rats receiving C4‐HSL, when compared with treatment with control IgG.
Figure 4.
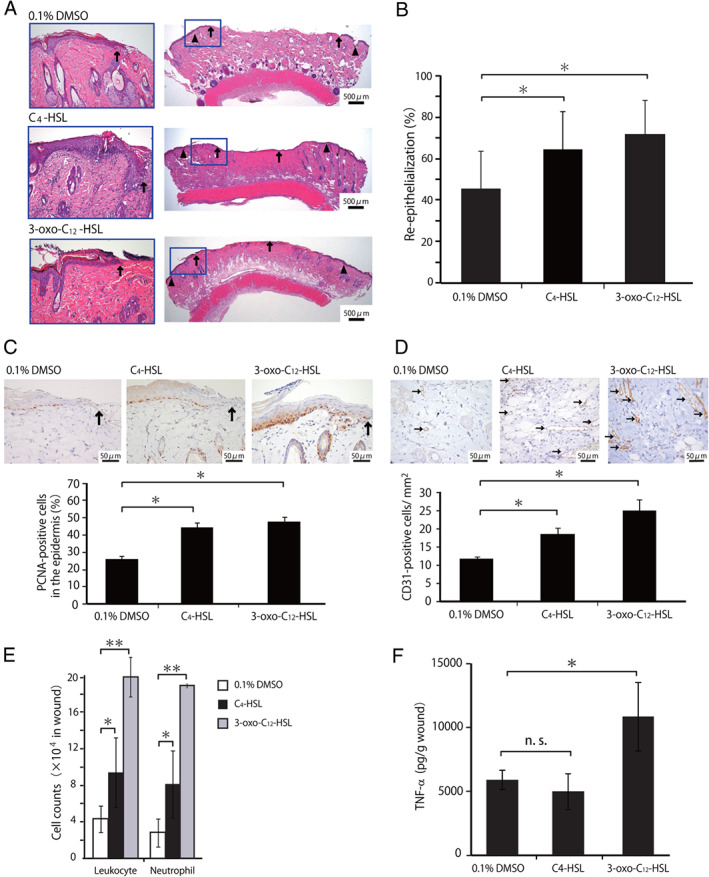
Effect of C4‐HSL administration on the wound healing. Wounded rats received C4‐HSL, 3‐oxo‐C12‐HSL or DMSO at the wound surface. (A) Representative histological views of wounds on day 3. Arrowheads and arrows indicate the original wound edges and re‐epithelialised leading edges, respectively. (B) Re‐epithelialisation extent on day 3. (C) Cellular proliferation in the epidermis on day 3. Arrows indicate PCNA‐positive microvessels. The percentages of PCNA‐positive cells among the 500 cells in the epidermis were calculated (n = 3). (D) Microvessel counts on day 3. Arrows indicate CD31‐positive microvessels. The vascular density/mm2 was determined by counting the numbers of positive vessels within six visual fields (n = 3). (E) The number of leukocytes, neutrophils was analysed at 24 hours (**P < 0·01). (F) TNF‐α concentrations in wounded tissue homogenates were measured at 24 hours. Each column represents the mean ± standard deviation (SD). *P < 0·05; NS, not significant.
Figure 5.
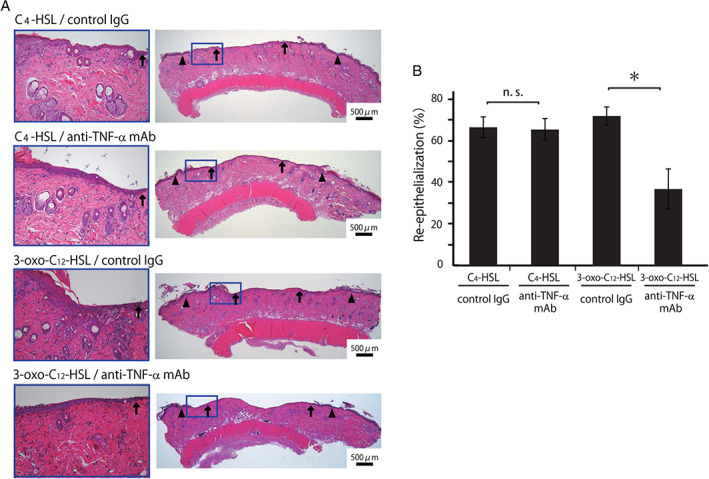
Effect of anti‐TNF‐α mAb on wound healing promoted by C4‐HSL. Rats were treated with anti‐TNF‐α mAb or control IgG on day 1 before wound creation. Wounded rats received C4‐HSL or 3‐oxo‐C12‐HSL at the wound surface. (A) Representative histological views of wounds on day 3. Arrowheads and arrows indicate the original wound edges and re‐epithelialised leading edges, respectively. (B) Re‐epithelialisation extent on day 3. NS, not significant.
Discussion
In the present study, we demonstrated that C4‐HSL, a bacterial QS signalling molecule, was intimately involved in the acceleration of skin wound healing in P. aeruginosa infection, which was associated with the increased neutrophil infiltration. This is the first report that shows the involvement of C4‐HSL from P. aeruginosa in skin wound healing by modulating the inflammatory responses.
Previously, we demonstrated that P. aeruginosa infection accelerated wound repair in skin through secretion of 3‐oxo‐C12‐HSL that is encoded by the lasI gene, which triggered neutrophil infiltration and TNF‐α synthesis, and promoted wound healing processes such as re‐epithelialisation and generation of new vessels 2. P. aeruginosa produces two distinct QS molecules, 3‐oxo‐C12‐HSL and C4‐HSL. In the current study, we focused on C4‐HSL, which is synthesised by an autoinducer synthase that is encoded by the P. aeruginosa rhlI gene. It has been reported that 3‐oxo‐C12‐HSL affects the host responses by stimulating a variety of cells 18, triggering the NF‐κB translocation into the nucleus 9 and inducing apoptosis in these cells 13. However, few reports have shown the effect of C4‐HSL on host responses.
Many investigators have reported that the rhlI gene product is involved in the regulation of P. aeruginosa virulence factors, including rhamnolipid 19, 20, elastase 15, 20, hydrogen cyanide 15 and pyocyanin 19, 20. Consistent with these findings, an rhlI‐deficient mutant strain of P. aeruginosa was reported to show lower virulence, as indicated by the reduced mortality in a burn injury model 21, and the lower incidence of bacteremia and reduced mortality in a mouse model of pneumonia 22. In the current study, P. aeruginosa inoculation promoted neutrophil accumulation and accelerated repair of skin wounds. However, these effects were abolished when mutant strains of P. aeruginosa were defective in either lasI or rhlI. Although Imamura et al. previously reported that the rhlI gene product was involved in neutrophil accumulation in pneumonia 23, the precise mechanism remains to be determined. A variety of chemoattractants are known to recruit neutrophils in P. aeruginosa‐infected tissues, including P. aeruginosa‐derived fMet‐Leu‐Phe (FMLP) and rhamnolipid and host‐derived C5a, leukotriene B4 and IL‐8 1, 24, 25. In addition, neutrophil‐derived TNF‐α also contributes to neutrophil accumulation and the wound healing process in P. aeruginosa infection 4. Recently, we demonstrated that lasI is involved in TNF‐α synthesis in P. aeruginosa infection during wound healing 2; this was in contrast with the current data, which indicates that the rhlI deficiency did not affect TNF‐α production (Figure 2B, Figure S1). In addition, lasI deficiency led to a reduction in the expression of CXCL‐1, IL‐6 and IL‐17A, which are chemokines and cytokines that are related to neutrophil attraction, in the wounded tissues during P. aeruginosa infection. This reduction was not observed in infection with the rhlI‐deficient strain. These findings suggest that P. aeruginosa‐derived factors that are regulated by rhlI, such as rhamnolipid, rather than by host factors, may contribute to the neutrophil accumulation. Trevani et al. indicated that DNA from other Gram‐negative bacteria, Escherichia coli and Acinetobacter baumannii, but not mammalian DNA, induced human neutrophil activation 26, and these responses were induced through a TLR9‐independent and MyD88‐dependent pathway 27. These observations suggested that DNA from P. aeruginosa may also be involved in neutrophil attraction in the current study.
In addition to the experiments with anti‐C4‐HSL antiserum, the current study demonstrated that C4‐HSL administration at the wound surface promoted wound healing, as evaluated by the increase in re‐epithelialisation, epidermal cell proliferation and neo‐vascularisation. The direct effects of C4‐HSL on wound healing have not yet been reported, and leukocyte accumulation after C4‐HSL administration was first identified in the current study. These were the same results as those for anti‐3‐oxo‐C12‐HSL antiserum and 3‐oxo‐C12‐HSL administration, but the effect on TNF‐α synthesis was different between C4‐HSL and 3‐oxo‐C12‐HSL. It is well known that TNF‐α is directly activated in response to re‐epithelialisation and angiogenesis in skin wound healing 4, 28, but wound healing that is promoted by C4‐HSL is likely to be caused by other mediators, and further research is necessary.
Both 3‐oxo‐C12‐HSL and C4‐HSL contribute to regulation in the production of virulence factors from P. aeruginosa, such as elastase, exotoxin A, alkaline protease and biofilm 10, 29, 30, 31. In contrast, production of rhamnolipid and activation of the type III secretion system are regulated by C4‐HSL, but not by 3‐oxo‐C12‐HSL 14, 15, suggesting that these HSLs act differently on the virulence factors from P. aeruginosa. In a clinical study by Bjarnsholt et al., C4‐HSL and rhamnolipid synthesis was significantly increased in the mucoid type of P. aeruginosa compared with the non‐mucoid type, which was isolated from the lungs of 238 cystic fibrosis patients 32. This suggests that C4‐HSL may regulate the production of alginate, a major component of mucus, in a manner independent of 3‐oxo‐C12‐HSL. Thus, two QS molecules from P. aeruginosa, C4‐HSL and 3‐oxo‐C12‐HSL, may possess distinct biological actions, as shown in the current study.
Many investigations have focused on the effect of 3‐oxo‐C12‐HSL, a P. aeruginosa QS molecule, on the host response. This QS molecule showed various effects on the inflammatory responses, including the production of TNF‐α from neutrophils 2, IFN‐γ from T cells 33 and IL‐8 from human bronchiolar epithelial cells 7 and lung fibroblasts 18. It was also reported that 3‐oxo‐C12‐HSL impaired human airway epithelial cell activation 34 and neutrophil and macrophage apoptosis 13. By contrast, the current study shows, for the first time, the effect of C4‐HSL on the host response. Although 3‐oxo‐C12‐HSL receptors in the host cells remain to be defined, this QS molecule modulates inflammatory pathways involving NF‐κB 35 and binds to peroxisome proliferator activated receptor (PPARγ), a family of nuclear membrane‐associated transcriptional regulators that act as lipid sensors 36, and protease‐activated receptor (PAR), which activates matrix metalloproteases (MMPs) 37. However, it is unclear how C4‐HSL acts on the host cells. To understand the significance of C4‐HSL during the wound healing process in P. aeruginosa infection, further research is necessary to define the C4‐HSL host cell receptor.
In the current study, we used the typically non‐mucoid, wild‐type P. aeruginosa PAO1 strain and its mutants, which is widely used in biofilm research 34, 38. We also previously confirmed the formation of biofilm by P. aeruginosa PAO1 on the wounds, and our results suggested that the acute wound healing process in rats was unaffected by biofilm formation 39. In contrast, in chronic wounds, it has been suggested that microbial biofilms are implicated in both the infection of wounds and the failure of those wounds to heal 40. In the initial report by Davies et al., lasI gene was found to be essential for the creation of mature, differentiated biofilms 38. The involvement of lasI and rhlI gene products with the biofilm formation within the chronic wounds has not yet been demonstrated. Perhaps clinical isolates of P. aeruginosa might be needed for an extended analysis of biofilm formation on the chronic wounds because P. aeruginosa PAO1 is not a particularly virulent wound isolate.
In conclusion, the current study demonstrated that acceleration of acute phase of wound healing in P. aeruginosa infection was mediated by the rhlI product, C4‐HSL, which triggered neutrophil infiltration and the lasI product, 3‐oxo‐C12‐HSL, as reported in our recent study 2. Although the mechanism by which C4‐HSL activates the neutrophil inflammatory response remains to be elucidated, the results presented here suggest the presence of a pathway that is distinct from pathways activated in response to 3‐oxo‐C12‐HSL‐mediated effects. In addition, based on the data indicating that C4‐HSL alone promoted the repair of the wounded tissues, this compound could be a potentially promising therapeutic agent in the treatment of refractory skin wounds in the clinical setting. However, a high microbial burden results in redundant inflammatory responses, such as prolonged infiltration of neutrophils, which is believed to retard the healing of chronic wounds 41. Thus, the beneficial effect of P. aeruginosa infection and C4‐HSL treatment found in the current study may be applicable to acute wounds, but not to chronic wounds. This idea does not conflict with the dominant paradigm of current wound care, in which microbial infection contributes to the development of chronic wounds 6, 41.
Supporting information
Figure S1. Production of TNF‐α at the wound sites after inoculation with Pseudomonas aeruginosa PAO1 or ΔrhlI. TNF‐α concentration in the wound tissue homogenates from rats inoculated with P. aeruginosa PAO1, ΔrhlI, ΔlasI or from uninoculated rats were measured at 24 hours following wound creation. *P < 0·05; NS, not significant.
Acknowledgements
The authors thank Dr. Akio Nakane (Hirosaki University Graduate School of Medicine, Hirosaki, Japan) for his kind gifts of hybridoma cells (clone MP6‐XT2.2‐11). This work was supported in part by a Grant‐in‐aid for Scientific Research (C) (25463284) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors declare that there are no financial conflicts of interest.
References
- 1. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–25. [DOI] [PubMed] [Google Scholar]
- 2. Kanno E, Kawakami K, Miyairi S, Tanno H, Otomaru H, Hatanaka A, Sato S, Ishii K, Hayashi D, Shibuya N, Imai Y, Gotoh N, Maruyama R, Tachi M. Neutrophil‐derived tumor necrosis factor‐α contributes to acute wound healing promoted by N‐(3‐oxododecanoyl)‐l‐homoserine lactone from Pseudomonas aeruginosa . J Dermatol Sci 2013;70:130–8. [DOI] [PubMed] [Google Scholar]
- 3. Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil‐depleted mice. J Leukoc Biol 2003;73:448–55. [DOI] [PubMed] [Google Scholar]
- 4. Kanno E, Kawakami K, Ritsu M, Ishii K, Tanno H, Toriyabe S, Imai Y, Maruyama R, Tachi M. Wound healing in skin promoted by inoculation with Pseudomonas aeruginosa PAO1: the critical role of tumor necrosis factor‐α secreted from infiltrating neutrophils. Wound Repair Regen 2011;19:608–21. [DOI] [PubMed] [Google Scholar]
- 5. Koh TJ, Dipietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjarnsholt T, Kirketerp‐Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10. [DOI] [PubMed] [Google Scholar]
- 7. DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin‐8. J Clin Invest 1995;96:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmermann S, Wagner C, Müller W, Brenner‐Weiss G, Hug F, Prior B, Obst U, Hänsch GM. Induction of neutrophil chemotaxis by the quorum‐sensing molecule N‐(3‐oxododecanoyl)‐l‐homoserine lactone. Infect Immun 2006;74:5687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. IL‐8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N‐3‐oxododecanoyl homoserine lactone is transcriptionally regulated by NF‐kappa B and activator protein‐2. J Immunol 2001;167:366–74. [DOI] [PubMed] [Google Scholar]
- 10. Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa . Microbiol Mol Biol Rev 2012;76:46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum‐sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary‐phase sigma factor RpoS. Mol Microbiol 1996;21:1137–46. [DOI] [PubMed] [Google Scholar]
- 12. Erickson DL, Endersby R, Kirkham A, Stuber K, Vollman DD, Rabin HR, Mitchell I, Storey DG. Pseudomonas aeruginosa quorum‐sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect Immun 2002;70:1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N‐3‐oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun 2003;71:5785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N‐acylhomoserine lactone signal produced by Pseudomonas aeruginosa . Proc Natl Acad Sci U S A 1995;92:1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winson MK, Camara M, Latifi A, Foglino M, Chhabra SR, Daykin M, Chapon V, Salmond GP, Bycroft BW, Lazdunsklt A, Stewart GSAB, Williams P. Multiple N‐acyl‐l‐homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 1995;92:9427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minagawa S, Inami H, Kato T, Sawada S, Yasuki T, Miyairi S, Horikawa M, Okuda J, Gotoh N. RND type efflux pump system MexAB‐OprM of Pseudomonas aeruginosa selects bacterial languages, 3‐oxo‐acyl‐homoserine lactones, for cell‐to‐cell communication. BMC Microbiol 2012;12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyairi S, Tateda K, Fuse ET, Ueda C, Saito H, Takabatake T, Ishii Y, Horikawa M, Ishiguro M, Standiford TJ, Yamaguchi K. Immunization with 3‐oxododecanoyl‐l‐homoserine lactone‐protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol 2006;55:1381–7. [DOI] [PubMed] [Google Scholar]
- 18. Smith RS, Iglewski BH. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Invest 2003;112:1460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ochsner UA, Koch AK, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa . J Bacteriol 1994;176:2044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR‐RhlI, another set of regulators in strain PAO1 with homology to the autoinducer‐responsive LuxR‐LuxI family. J Bacteriol 1995;177:7155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect Immun 1999;67:5854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearson JP, Feldman M, Iglewski BH, Prince A. Pseudomonas aeruginosa cell‐to‐cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun 2000;68:4331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imamura Y, Yanagihara K, Tomono K, Ohno H, Higashiyama Y, Miyazaki Y. Role of Pseudomonas aeruginosa quorum‐sensing systems in a mouse model of chronic respiratory infection. J Med Microbiol 2005;54:515–8. [DOI] [PubMed] [Google Scholar]
- 24. Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol 2006;534:1–11. [DOI] [PubMed] [Google Scholar]
- 25. Alhede M, Bjarnsholt T, Jensen PØ, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Høiby N, Rasmussen TB, Givskov M. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 2009;155:3500–8. [DOI] [PubMed] [Google Scholar]
- 26. Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, Schettini J, Raiden S, Geffner J. Bacterial DNA activates human neutrophils by a CpG‐independent pathway. Eur J Immunol 2003;33:3164–74. [DOI] [PubMed] [Google Scholar]
- 27. Alvarez ME, Fuxman Bass JI, Geffner JR, Calotti PX, Costas M, Coso OA, Gamberale R, Vermeulen ME, Salamone G, Martinez D, Tanos T, Trevani AS. Neutrophil signaling pathways activated by bacterial DNA stimulation. J Immunol 2006;177:4037–46. [DOI] [PubMed] [Google Scholar]
- 28. Frank J, Born K, Barker JH, Marzi I. In vivo effect of tumor necrosis factor alpha on wound angiogenesis and epithelialization. Eur J Trauma 2003;29:208–19. [Google Scholar]
- 29. Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa . J Bacteriol 1997;179:3127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum‐sensing in Gram‐negative bacteria. FEMS Microbiol Rev 2001;25:365–404. [DOI] [PubMed] [Google Scholar]
- 31. Favre‐Bonté S, Köhler T, Van Delden C. Biofilm formation by Pseudomonas aeruginosa: role of the C4‐HSL cell‐to‐cell signal and inhibition by azithromycin. J Antimicrob Chemother 2003;52:598–604. [DOI] [PubMed] [Google Scholar]
- 32. Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK, Rybtke MT, Tolker‐Nielsen T, Givskov M, Høiby N, Ciofu O; Scandinavian Cystic Fibrosis Study Consortium . Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One 2010;5:e10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ritchie AJ, Yam AO, Tanabe KM, Rice SA, Cooley MA. Modification of in vivo and in vitro T‐ and B‐cell‐mediated immune responses by the Pseudomonas aeruginosa quorum‐sensing molecule N‐(3‐oxododecanoyl)‐l‐homoserine lactone. Infect Immun 2003;71:4421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP. Inactivation of a Pseudomonas aeruginosa quorum‐sensing signal by human airway epithelia. Proc Natl Acad Sci U S A 2004;101:3587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Modulation of gene expression via disruption of NF‐kappaB signaling by a bacterial small molecule. Science 2008;321:259–63. [DOI] [PubMed] [Google Scholar]
- 36. Cooley MA, Whittall C, Rolph MS. Pseudomonas signal molecule 3‐oxo‐C12‐homoserine lactone interferes with binding of rosiglitazone to human PPARgamma. Microbes Infect 2010;12:231–7. [DOI] [PubMed] [Google Scholar]
- 37. Eum SY, Jaraki D, Bertrand L, András IE, Toborek M. Disruption of epithelial barrier by quorum‐sensing N‐3‐(oxododecanoyl)‐homoserine lactone is mediated by matrix metalloproteinases. Am J Physiol Gastrointest Liver Physiol 2014;306:G992–G1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell‐to‐cell signals in the development of a bacterial biofilm. Science 1998;280:295–8. [DOI] [PubMed] [Google Scholar]
- 39. Kanno E, Toriyabe S, Zhang L, Imai Y, Tachi M. Biofilm formation on rat skin wounds by Pseudomonas aeruginosa carrying the green fluorescent protein gene. Exp Dermatol 2010;19:154–6. [DOI] [PubMed] [Google Scholar]
- 40. Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen 2012;20:647–57. [DOI] [PubMed] [Google Scholar]
- 41. Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol 2010;130:38–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Production of TNF‐α at the wound sites after inoculation with Pseudomonas aeruginosa PAO1 or ΔrhlI. TNF‐α concentration in the wound tissue homogenates from rats inoculated with P. aeruginosa PAO1, ΔrhlI, ΔlasI or from uninoculated rats were measured at 24 hours following wound creation. *P < 0·05; NS, not significant.


