ABSTRACT
Vacuum‐assisted closure (VAC) therapy is a sophisticated system that maintains a closed, humid, sterile and isolated environment. Wound infection is considered a relative contraindication. The objective of this study is to extend the indications for VAC therapy to include infected wounds by demonstrating its ability to increase the antibiotic concentration in the damaged and infected tissues. Patients who presented with ulcers infected with daptomycin‐sensitive bacteria were eligible to be enrolled in this prospective study. They were given antibiotic therapy with daptomycin with a specific protocol. A biopsy of the lesion was carried out to detect tissue concentration of the drug at time 0. Afterwards, the patients were subjected to VAC therapy. At the end of VAC therapy, a second lesion biopsy was performed and analysed to detect tissue concentration of the drug at time 1. A control group was enrolled in which patients followed the same protocol, but they were treated with traditional dressings. Fisher's exact test was used to compare the two groups. The results highlighted a significant increase in the concentration of antibiotics in the study group tissue; the improvement was sensibly lower in the control group. Statistical differences were not found between the two groups. The preliminary analysis of the data showed an important increase of antibiotic concentration in the tissue after VAC therapy. Despite the encouraging data, it is necessary to broaden the sample of patients and perform the same study with other antibiotics.
Keywords: Chronic wound, Daptomicin, Negative pressure wound therapy, Vacuum Assisted Closure therapy
Introduction
The practice to expose a wound to negative atmospheric pressure for a long period of time in order to promote wound healing was first described by Fleischmann et al. in 1993 1. Vacuum‐assisted closure (VAC) therapy is a sophisticated system that produces a humid, closed, sterile and isolated environment 1, 2, 3. The negative pressure accelerates the healing process by removing exudates, reducing bacterial load, increasing the growth of granulation tissue, favouring the contraction of the lesion margins and promoting angiogenesis 4, 5, 6. VAC therapy is applied in many fields: traumatic and dehiscent surgical wounds, acute and chronic wounds, partial‐thickness burns (grade I and II), sternal osteomyelitis 7, 8, 9 and mediastinitis 10, 11, 12, 13, 14, 15, 16.
There are few published articles regarding the use of VAC therapy on infected wounds 17. Infection is considered one of the contraindications to negative pressure wound therapy (NPWT) 17. This contraindication is related to the possibility of promoting bacterial growth as a result of leaving the VAC dressing on the bed of an infected wound for several days. Despite this notion, it has not limited the use of this device by many centres for infected wounds.
The aim of this study is to extend the indication for the use of NPWT in infected wounds by demonstrating the ability of VAC therapy to increase the antibiotic concentration in damaged and infected tissues. The rationale of this study is related to the known capacity of VAC therapy to activate the process of neoangiogenesis, increasing the concentration of capillaries in the tissue in which it is applied and enhancing the delivery of the antibiotic to the injury site. The demonstration of increased local concentration of the antibiotic could then provide evidence to support the use of VAC therapy in infected sites and alleviate previous reservations about relative contraindications.
Materials and methods
This was a prospective study carried out by the Department of Plastic and Reconstructive Surgery of Policlinico Umberto I in Rome, Italy. The study was initiated in June 2014, and 33 patients were enrolled until June 2016. Each patient was pre‐counselled and later provided informed consent. Patients presenting with infected ulcers of any aetiology and of any grade were eligible for this study. Inclusion criteria were the presence of infection in the wound and the sensitivity of the isolated bacteria to daptomycin, demonstrated with bacterial culture and antibiogram. All the patients who demonstrated resistance to daptomycin were excluded from the study. Of the 33 patients enrolled in the study, 15 patients presented with post‐traumatic wounds (Figures 1 and 2); 11 patient presented with vascular lesions and 7 patients with diabetic ulcers. Patient information was collected and included sociodemographic data (age, gender, educational level, employment status and marital status), any comorbidities and present diagnosis. Comorbidities were assessed by reviewing the patient's medical record and any tobacco/alcohol/ recreational drug consumption.
Figure 1.
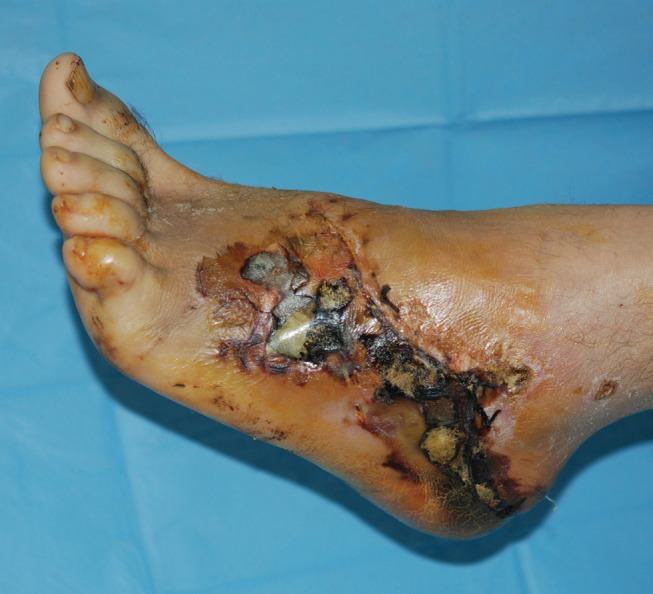
Preoperative image in a patient with post‐traumatic wound with necrotic area.
Figure 2.
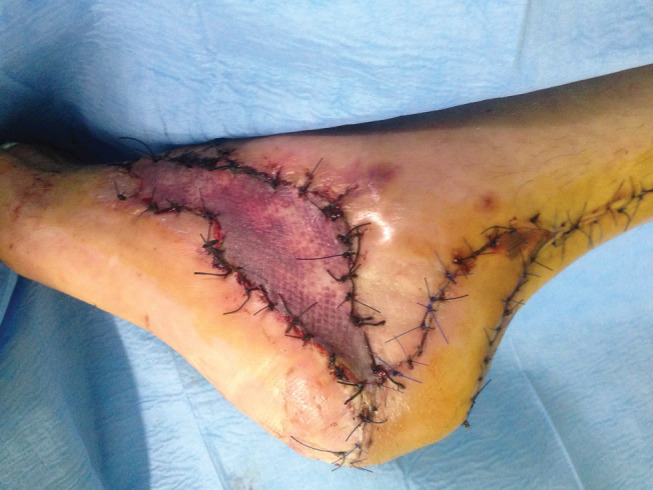
Postoperative image of the lesion after two cycles of VAC therapy and reconstruction with local flap and skin graft.
Patients included in the study were divided into two groups. In the first group, all patients received 750 mg/day of daptomycin for 2 days and 500 mg/day for the following days. On the second day, a biopsy of the lesion was carried out with the dual purpose of performing a deep culture test and detecting tissue concentration of the drug (T0) by means of chromatography coupled with mass spectrometry. Following the first biopsy, patients were continued on antibiotic therapy and started on VAC therapy (KCI Licensing, Inc., San Antonio, TX). The device was set to exert a negative pressure of 125 mmHg. Two cycles of 3 days each of VAC therapy were performed. At the end of the second cycle (after 6 days of VAC therapy and 8 days of antibiotic therapy), a second lesion biopsy was performed and analysed with subsequent chromatography to measure the antibiotic concentration (T1). In the second group, all patients underwent the biopsies and chromatography, but VAC therapy was not applied. In this control group, all patients were treated with traditional dressings. Statistical analyses using Fisher's exact test were performed to compare the two groups.
Chemical analysis
Ultrapure water for HPLC‐MS/MS analysis was obtained from Milli‐Q Plus system (Millipore, Bedford, MA). Methanol RS‐plus grade, ammonium acetate, formic and acetic acid were supplied by Sigma‐Aldrich (Milwaukee, WI). Ofloxacin was used as an internal standard and was supplied by Sigma‐Aldrich.
The mixtures' working standards (stock solution) were prepared in methanol and stored at −20°C.
Pre‐treatment of sample–solvent extraction and SPE purification
The sample of fabric supplied is accurately weighed (about 50 mg) and subjected to cryogenic grinding using a CryoMill (Retsch, Dusseldorf, Germany) at −196°C, 30 Hz for 3 minutes after the addition of the internal standard. A total of 500 µl of methanol was then added to the ground sample. The extraction was carried out by sonication for 16 minutes and then centrifuged at 8500 g for 5 minutes.
The supernatant was diluted with 4·5 ml of acetate buffer (pH 5·2, 5 mM) and then loaded on a StrataX SPE cartridge (Phenomenex, Torrance, CA). The following procedure was performed:
Activation of the cartridge with 1 ml of MeOH.
Conditioning of the cartridge with 1 ml acetate buffer/methanol (90/10, v:v).
Loading of the sample onto the cartridge.
Washing of the cartridge with 1 ml water/methanol (90/10, v:v).
Elution of the analytes with 500 µl of 5 mM formic acid in methanol.
The elutant was transferred to autosampler vials, and 6 µl was injected into the chromatographic system.
LC‐MS/MS
The chromatographic system consisted of a Series 200 micro LC Pump (Perkin Elmer, Nerwalk, CT) equipped with a Series 200 autosampler with a 20‐µl loop and a vacuum degasser.
A Kinetex column C18 (100 × 2·10 mm ID) from Phenomenex, packed with core shell particles of an average diameter of 2·6 microns and porosity 100 Å, was used for the chromatographic separation. The mobile phases used were methanol (A) and water (B), both containing 5 mM formic acid; the elution of the analytes was performed using the following gradient:
B at 100% for 0·3 minutes.
Increase A from 0% to 100 % in 1 minute.
A at 100% for 2·1 minutes.
The flow rate of the mobile phase in the column was 350 µl/minute; only a fraction of 200 µl/minute was transferred to the ion source of the mass spectrometer.
For the identification and quantification of analytes, the HPLC was coupled with a triple quadruple tandem mass spectrometer API 2000 (ABSciex, Toronto, Canada) equipped with a TurboIonSpray source operating in positive ionisation (PI).
The mass calibration of each quadrupole, which acts as a mass filter (Q1 and Q3), has been realised during infusion at a flow rate of 10 pl/minute with a standard solution of polypropylene glycol (PPG ) containing several molecular cuts, provided by the manufacturer. The monitored ions [59, 175·1, 384·3, 616·5, 906·7, 1254·9, 1545·1 and 1778·3 Dalton (Da)] calibrate the instrument on the entire range of masses, from 20 to 1800 m/z, as well as adjust the resolution of quadrupoles Q1 and Q3, checking that the width at half of the height of the corresponding mass peaks was equal to 0·7 ± 0·1 Da.
The working mode was multi‐reaction‐monitoring (MRM), selecting two pairs of transition precursor ion/ion fragments for the analyte. The data processing was performed using Analyst 1·5 software (PE Sciex). The calibration curve was prepared in methanol, and quantification was performed using the internal standard method.
The chromatographic profiles are recorded in a single period in total ion current (TIC) as the sum of the two MRM transitions selected, optimised for maximum sensitivity allowed by the instrument (Table 1). Nitrogen, coming from a nitrogen generator (Parker Balston, model 75A74, Haverhill, MA) that is connected to a compressor (Jun‐ Air 4000‐40 M, Bromsgrove, UK), was used as the curtain gas (P = 20 psi) and collisionally activated dissociation (CAD) gas (fragmentation gas at a pressure of 4·10−5 torr), while air was used as drying or turbo gas (P = 65 psi) and nebuliser gas (P = 35 psi). The temperature of turbo gas was set to 375°C, and the capillary voltage was set at 5500 V.
Table 1.
LC‐MS/MS parameters for daptomycin and the IS ofloxacin. (Q1: precursor ion mass; Q3: product ion mass; DP: declustering potential; FP: focusing potential; EP: entrance potential; CE: collision energy; CXP: collision exit potential)
| Analytes | Q1 (amu) | DP (V) | FP (V) | EP (V) | Q3 (amu) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Daptomycin | 811 | 49 | 400 | 12 | 241·1 | 49 | 8 |
| 285·2 | 32 | 14 | |||||
| Ofloxacin | 362 | 27 | 400 | 10 | 318 | 27 | 15 |
Results
Of 65 eligible patients, 33 were enrolled in the study; 26 patients were excluded because the bacteria present were not sensitive to daptomycin, and 6 patients were excluded because there was no evidence of infection after deep culture. The mean age was 54·1 years old (range: 28–78 years). In the intervention group, 11 patients were male, and 4 were female. Of these, nine patients had post‐traumatic ulcers, five patients suffered diabetic ulcers, and one patient acquired an ulcer from venous insufficiency. In the control group, 12 patients were male, and 6 were female. The aetiology was diabetic (eight patients), post‐traumatic (eight patients) and venous insufficiency (two patients). In all cases, the presence of infection was confirmed by deep cultures performed 96 hours before establishing the antibiotic of choice. Analysis of the results obtained with the LC‐MS/MS analysis of samples T0 and T1 has highlighted a trend towards significant increase in the concentration of daptomycin in the intervention group, quantifiable in 38% compared with 23% in the control group (Table 2). Fisher's exact test between the two groups was not statistically significant (P = 0·82).
Table 2.
LC‐MS/MS analysis of samples T0 and T1 showed a significant increase (38%) in the concentration of antibiotic in the tissue in the study group, whereas the improvement was lower (23%) in the control group. T0: daptomycin concentration time 0 (first biopsy); T1: daptomycin concentration time 1 (second biopsy)
| Patients | VAC therapy group | Control group | ||
|---|---|---|---|---|
| T0 (µg/g) concentration | T1 (µg/g) concentration | T0 (µg/g) concentration | T1 (µg/g) concentration | |
| 1 | 20·1 | 27·5 | 22 | 264 |
| 2 | 15·5 | 23·2 | 18·6 | 22·4 |
| 3 | 22·2 | 30·6 | 14·3 | 17·8 |
| 4 | 25·2 | 33 | 24·8 | 29·2 |
| 5 | 12·2 | 21·5 | 12·7 | 16·2 |
| 6 | 20·1 | 27·5 | 20·3 | 25 |
| 7 | 16·5 | 22·1 | 12·7 | 18 |
| 8 | 18 | 24·3 | 11·1 | 14·6 |
| 9 | 22·1 | 30·1 | 15·7 | 19·8 |
| 10 | 20·2 | 28·6 | 21·2 | 25·6 |
| 11 | 24·4 | 32·9 | 20·2 | 24·1 |
| 12 | 23·6 | 32·8 | 20·1 | 24·5 |
| 13 | 13 | 19·5 | 15·5 | 20 |
| 14 | 19·3 | 26·4 | 25·2 | 29·4 |
| 15 | 18·7 | 25·4 | 12·8 | 17·3 |
| 16 | 20 | 26·1 | ||
| 17 | 18·8 | 22·5 | ||
| 18 | 15·7 | 19·4 | ||
| Standard deviation | 3·89 | 4·35 | 4·25 | 4·44 |
| Mean average | 18·53 | 25·65 | 16·78 | 20·82 |
Discussion
VAC therapy has become widely used in the medical and surgical fields, and it is indicated for various types of pathology. The mechanisms by which VAC therapy promotes wounds healing are diverse: it removes exudates, reduces bacterial load, increases the growth of granulation tissue, favours the contraction of the lesion margins and promotes angiogenesis 4, 5, 6. By inducing the contraction and the approximation of lesion margins, the NPWT applies a mechanical stress to the tissues causing cell micro deformation that facilitates cell division and proliferation 18. In a recent study, Yang SL et al. 19 highlighted, by immunohistochemical and western blot analysis, a greater expression of basic fibroblast growth factor (bFGF) and ERK 1/2 (extracellular signal‐regulated kinase) in a group of patients with diabetic foot ulcers treated with VAC therapy compared with the control group treated with traditional dressings. Several studies show an increase in the rate of formation of granulation tissue richly vascularised and an increase of capillary bed at the site of application of the NPWT. A study by Gyo Seo Sang et al. 20 shows an increase in the number of endothelial progenitor cells (EPCs) during treatment with NPWT, while an experiment conducted by Labler L et al. 21 detected an increase in the concentrations of VEGF and IL‐8 through measurements using ELISA and histological typing. Furthermore, continuous cleaning of the wound can reduce the bacterial load in the wound and removes inflammatory mediators that inhibit healing. Interestingly, a study published by Liu D et al. 22 showed an increase of the expression of the genes of the IL‐1β and IL‐8 in the early stages of inflammation and a decrease in those of TNF–α in full‐thickness dorsal ulcers in rabbit models. The ulcer model was inoculated with a known strain of Staphylococcus aureus, and NPWT was applied. The increase of cytokines was confirmed by histology that showed a significant increase in the number of neutrophils and a reduction in bacterial load compared to the sample inoculated. The exudate that accumulates in the injured tissues can compress the microcirculation and mechanically reduce the blood flow in the wound; however, its removal may reduce the oedema of the tissue and increase blood flow in the damaged area.
Immunocytochemical techniques also demonstrated that there is an increase in the density of lymphatic vessels in response to the application of NPWT 23.
Although there are many indications for VAC therapy, a number of contraindications exist, such as osteomyelitis that has not treated surgically; active sepsis; coagulopathies; Marjolin ulcers; allergies to one of the components required for NPWT treatment; fistulae that have not been explored; treatment with anticoagulants; wounds with exposed blood vessels, nerves or vital organs; and the presence of necrotic tissue within the wound. Besides these hard clinical contraindications, there are also some relative contraindications. Falling into this category is the use of VAC therapy in grossly infected wounds. Hesitation to accept the use of NPWT for grossly infected wounds stems from the nature of NPWT dressings, in that they stay on for a prolonged period of time, and the risk of bacterial proliferation may be increased.
Currently, there is no quality evidence in the literature that validates the use of NPWT in the course of infection or not 17. However, there are several publications in which VAC therapy has been used in diseases that assume the presence of infection with positive results. On the other side, cases of toxic shock syndrome during the course of NPWT have been reported 24. In a recent study, Mouses et al. 25 analysed in a sample of eight patients the effects of VAC therapy on increasing local concentrations of vancomycin. They sampled the exudate taken from the canister of the device for vancomycin concentration after 3 days of combined treatment with VAC therapy and systemic antibiotic therapy. Our study analyses the antibiotic concentration in wounded and infected tissue before and after the application of NPWT. Thus, we evaluate the capability or incapability of VAC therapy to modify the concentrations of antibiotic at the site of application. The experiment was performed to validate or not the use of this method for grossly infected ulcers. Our study differs from the previous study in the methodology used for the assessment of antibiotic concentrations. In order to accurately measure tissue concentrations of the antibiotic, a biopsy procedure at the base of the ulcer was chosen. We believe that indirect measurements from the exudate content in the canister may be less accurate. Preliminary results show that there was an increase of nearly 40% in local concentrations of antibiotic at the end of two cycles of VAC therapy, compared with a control group in which the improvement was considerably lower at 23%.
NPWT likely induces an increase in the concentration of local antibiotic through two different mechanisms. The first is the removal of excess exudate. NPWT reduces the mechanical obstruction to blood flow in tissues, thus promoting the delivery of antibiotics and white blood cells. The second mechanism is the induction of increased amounts of granulation tissue, which is richly vascularised tissue.
Only small and heterogeneous studies reported on the relationship between VAC usage and the bacterial load. Overall, no definite conclusions can be made about the effect VAC therapy may have on antibiotic concentration or bacterial load 17.
In theory, NPWT may function to reduce bacterial load by increasing local concentrations of antibiotic. Daptomycin is a new lipopeptide antibiotic that has been shown in vitro to possess concentration‐dependent bactericidal activity. Also, it has a rapid dermal penetration of 68·4% after 24 hours from the first intravenous administration of a dose of 4 mg/kg 26. Furthermore, daptomycin is able to carry out its bactericidal action even within the cells as it has been demonstrated to possess a penetration capacity of 60% in neutrophils and macrophages 25, 27.
Normally, substances with a molecular weight less than 5000 are able to freely cross the capillary wall. The mechanism through which daptomycin (PM 1619·7086 g/mol) migrates from the blood compartment to the interstitial space is not yet known. However, it has been hypothesised that this migration occurs through passive diffusion according to the gradient concentration. Consequently, the fourth mechanism by which the NPWT acts to increase local antibiotic concentrations is accomplished by the negative pressure applied on the interface that increases interstitial filtration according to the pressure gradient, non‐specifically for daptomycin.
However, further studies with a greater number of patients and a longer follow‐up period are required in order to improve our knowledge. It could be particularly be interesting to obtain biopsies at more time points in order to investigate how antibiotic concentrations change over time.
Conclusion
VAC therapy is a practice‐changing technology in the medical and surgical fields, and it is indicated in the treatment of numerous pathologies. The absence of objective and repeatable studies about the use of this device during the course of infection has prompted us to study the changes in local antibiotic delivery during VAC therapy. The preliminary analysis of the data showed an increase of about 40% of antibiotic concentration at the end of two cycles of therapy. However, despite the encouraging data, it is necessary to broaden the sample of patients and perform the same type of study with other antibiotics commonly used for infected ulcers.
Acknowledgements
All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence this study.
References
- 1. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 2. Sibbald RG, Mahoney J. A consensus report on the use of vacuumassisted closure in chronic, difficult‐to‐heal wounds. Ostomy Wound Manage 2003;49:52–66. [PubMed] [Google Scholar]
- 3. Concepts K. Incorporated (KCI) V.A.C. Therapy Clinical Guidelines. San Antonio: KCI, 2004. [Google Scholar]
- 4. Stal S, Serure A, Donovan W, Spira M. The perioperative management of the patient with pressure sores. Ann Plast Surg 1983;11:347–56. [DOI] [PubMed] [Google Scholar]
- 5. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment. Clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 6. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment. Animal studies and basic foundation. Ann Plast Surg 1997;38:553–61. [DOI] [PubMed] [Google Scholar]
- 7. Sansone F, Mossetti C, Bruna MC, Oliaro A, Zingarelli E, Flocco R, Del Ponte S, Casabona R. Transomental titanium plates for sternal osteomyelitis in cardiac surgery. J Card Surg 2011;26:600–3. [DOI] [PubMed] [Google Scholar]
- 8. Scholl L, Chang E, Reitz B, Chang J. Sternal osteomyelitis: use of vacuum‐assisted closure device as an adjunct to definitive closure with sternectomy and muscle flap reconstruction. J Card Surg 2004;19:453–61. [DOI] [PubMed] [Google Scholar]
- 9. Cowan KN, Teague L, Sue SC, Mahoney JL. Vacuum‐assisted wound closure of deep sternal infections in high‐risk patients after cardiac surgery. Ann Thorac Surg 2005;80:2205–12. [DOI] [PubMed] [Google Scholar]
- 10. Tarzia V, Carrozzini M, Bortolussi G, Buratto E, Bejko J, Comisso M, Mescola V, Penzo V, Guarino M, De Franceschi M, Pagnin C, Castoro M, Guglielmi C, Testolin L, Bottio T, Gerosa G. Impact of vacuum‐assisted closure therapy on outcomes of sternal wound dehiscence. Interact Cardiovasc Thorac Surg 2014;19:70–5. [DOI] [PubMed] [Google Scholar]
- 11. Karaca S, Kalangos A. Vacuum‐assisted closure (VAC)‐Instill® with continuous irrigation for the treatment of Mycoplasma hominis mediastinitis. Int Wound J 2014;31:595–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu AW, Rippel RA, Smock E, Jarral OA. In patients with post‐sternotomy mediastinitis is vacuum‐assisted closure superior to conventional therapy? Interact Cardiovasc Thorac Surg 2013;17:861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sjögren J, Mokhtari A, Gustafsson R, Malmsjö M, Nilsson J, Ingemansson R. Vacuum‐assisted closure therapy for deep sternal wound infections: the impact of learning curve on survival and predictors for late mortality. Int Wound J 2008;5:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sjögren J, Malmsjö M, Gustafsson R, Ingemansson R. Poststernotomy mediastinitis: a review of conventional surgical treatments, vacuum‐assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm. Eur J Cardiothorac Surg 2006;30:898–905. [DOI] [PubMed] [Google Scholar]
- 15. Mokhtari A, Petzina R, Gustafsson L, Sjögren J, Malmsjö M, Ingemansson R. Sternal stability at different negative pressures during vacuum‐assisted closure therapy. Ann Thorac Surg 2006;82:1063–7. [DOI] [PubMed] [Google Scholar]
- 16. Salazard B, Niddam J, Ghez O, Metras D, Magalon G. Vacuum‐assisted closure in the treatment of poststernotomy mediastinitis in the paediatric patient. J Plast Reconstr Aesthet Surg 2008;61:302–5. [DOI] [PubMed] [Google Scholar]
- 17. Patmo AS, Krijnen P, Tuinebreijer WE, Breederveld RS. The effect of vacuum‐assisted closure on the bacterial load and type of bacteria: a systematic review. Adv Wound Care 2014;3:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 19. Yang SL, Han R, Liu Y, Hu LY, Li XL, Zhu LY. Negative pressure wound therapy is associated with upregulation of bFGF and ERK1/2 in human diabetic foot wounds. Wound Repair Regen 2014;9:548–54. [DOI] [PubMed] [Google Scholar]
- 20. Seo SG, Yeo JH, Kim JH, Kim JB, Cho TJ, Lee DY. Negative‐pressure wound therapy induces endothelial progenitor cell mobilization in diabetic patients with foot infection or skin defects. Exp Mol Med 2013;45:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labler L, Rancan M, Mica L, Härter L, Mihic‐Probst D, Keel M. Vacuum‐assisted closure therapy increases local interleukin‐8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 2009;66:749–57. [DOI] [PubMed] [Google Scholar]
- 22. Liu D, Zhang L, Li T, Wang G, Du H, Hou H, Han L, Tang P. Negative‐pressure wound therapy enhances local inflammatory responses in acute infected soft‐tissue wound. Cell Biochem Biophys 2014;70(1):539–47. [DOI] [PubMed] [Google Scholar]
- 23. Labanaris AP, Polykandriotis E, Horch RE. The effect of vacuum‐assisted closure on lymph vessels in chronic wounds. J Plast Reconstr Aesthet Surg 2009;62:1068–75. [DOI] [PubMed] [Google Scholar]
- 24. Gwan‐Nulla DN, Casal RS. Toxic shock syndrome associated with the use of the vacuum‐assisted closure device. Ann Plast Surg 2001;47:552–4. [DOI] [PubMed] [Google Scholar]
- 25. Ida Y, Matsumura H, Onishi M, Ono S, Imai R, Watanabe K. Measurement of vancomycin hydrochloride concentration in the exudates from wounds receiving negative pressure wound therapy: a pilot study. Int Wound J 2014;13(2):204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wise R, Gee T, Andrews JM, Dvorchik B, Marshall G. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob Agents Chemother 2002;46:31–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baltch AL, Ritz WJ, Bopp LH, Michelsen PB, Smith RP. Antimicrobial activities of daptomycin, vancomycin, and oxacillin in human monocytes and of daptomycin in combination with gentamicin and/or rifampin in human monocytes and in broth against Staphylococcus aureus. Antimicrob Agents Chemother 2007;51:1559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


